Introduction
Lung cancer, as the leading cause of deaths due to
cancer worldwide, maksitself a global public health problem
(1-3).
In 2017, it was estimated that there was 22.25 million new cases of
lung cancer and 15.69 million new deaths in the United States
(4). With the rapid increase in
industrialization and aging population in China, more patients have
been diagnosed with lung cancer in recent years (5). Approximately 80% of all lung cancer
cases are attributable to an aggressive and highly invasive tumor:
non-small cell lung cancer (NSCLC) (6,7). Due
to the lack of sensitive screening tests for early diagnosis of
lung cancer, and the ineffectiveness of the current treatments for
advanced and metastatic cancer, the 5-year survival rate of NSCLC
patients is less than 15% (7,8). Thus,
it is imperative to develop early detection strategies to
facilitate a timely diagnosis of primary or recurring cancers. This
requires a better understanding of the molecular causes and
evolution of the disease to enable the design of more effective
treatments and improve the prognosis of lung cancer.
Vascular endothelial growth inhibitor (VEGI), an
anti-angiogenic cytokine that inhibits the neovasculature, is a
potential therapeutic target for the angiogenesis-related diseases,
especially malignant neoplasms. Previous studies (9-14)
have demonstrated that VEGI expression is down-regulated in many
solid tumors, including mammary tumors, bladder cancer, malignant
melanoma, prostate cancer, kidney cancer, colorectal cancer, and
renal cell carcinoma. Moreover, its down-regulation is associated
with low patient survival rates. Several studies (15,16) on
the role of the recombinant human VEGI found that systemic
administration of VEGI significantly inhibited the growth of tumors
and markedly prolonged the survival duration in the Lewis lung
cancer murine tumor model, implying that VEGI possess anti-tumor
effects in lung cancer. However, the expression and role of VEGI in
NSCLC remains unknown. Here, the expression of VEGI protein in
human NSCLC tissues and its role in NSCLC progress was evaluated
and explored.
Materials and methods
Patients and tissue specimens
One hundred and fifty NSCLC specimens, including 75
adenocarcinoma (AC) and 75 squamous cell carcinoma (SCC), were
obtained from patients undergoing surgical resection due to lung
cancer between July 2008 and February 2016 at the department of
Cardiothoracic Surgery, of the Affiliated Hospital of Xuzhou
Medical University (Jiangsu, China). All patients had definite
pathological diagnosis after surgery. Patients who received
adjuvant therapy were excluded. Written informed consent was
obtained from all patients before tissue acquisition. Staging was
performed after pathological examination of the formalin-fixed
specimens according to the current seventh edition of the TNM
classification. The clinical and biological data from the patients
are listed in Table I. All patients
were followed up every 3-6 months for two years post-operatively.
After the second year, every patient was followed-up on a yearly
basis until death or the last date of follow-up (November 1st,
2017). The median follow up period for the population studied was
54.5 months (range, 20-89 months). This study gained approval from
the Ethics Committee of the Affiliated Hospital of Xuzhou Medical
University.
 | Table IAssociation of VEGI expression with
the clinicopathological characteristics of 150 patients with
NSCLC. |
Table I
Association of VEGI expression with
the clinicopathological characteristics of 150 patients with
NSCLC.
| | | VEGI expression | |
|---|
| Characteristics | Case, n (%) | Negative/weak | Moderate/high | P-value |
|---|
| Sex | | | | 0.738 |
|
Male | 109 (72.7) | 74 | 35 | |
|
Female | 41 (27.3) | 29 | 12 | |
| Age, years | | | | 0.164 |
|
≤60 | 61 (40.7) | 38 | 23 | |
|
>60 | 89 (59.3) | 65 | 24 | |
| Smoking | | | | 0.487 |
|
Yes | 116 (77.3) | 78 | 38 | |
|
No | 34 (22.7) | 25 | 9 | |
| Histopathological
type | | | | 0.053 |
|
AC | 75 (50.0) | 57 | 18 | |
|
SCC | 75 (50.0) | 46 | 29 | |
| Tumor location | | | | 0.301 |
|
Left | 61 (40.7) | 39 | 22 | |
|
Right | 89 (59.3) | 64 | 25 | |
| Histopathological
grade | | | | 0.566 |
|
I+II | 113 (75.3) | 79 | 34 | |
|
III | 37 (24.7) | 24 | 13 | |
| Lymphovascular
invasion | | | | 0.065 |
|
Yes | 33 (22.0) | 27 | 6 | |
|
No | 117 (78.0) | 76 | 41 | |
| Tumor size | | | | 0.832 |
|
T1 | 40 (26.7) | 28 | 12 | |
|
T2-4 | 110 (73.3) | 75 | 35 | |
| Lymph node
metastasis | | | | 0.057 |
|
Yes | 65 (43.3) | 50 | 15 | |
|
No | 85 (56.7) | 53 | 32 | |
Immunohistochemistry
Immunostainings were performed as per the standard
protocols. Briefly, de-paraffinized in xylene, rehydrated using
graded alcohols firstly, 4 µm thick tissue sections, then, were
incubated in acitrate buffer at a pH of 6.1 for 15 min to retrieve
the antigen. To remove the endogenous peroxidase activity and
reduce background non-specific staining, sections were treated with
freshly prepared 3% hydrogen peroxide for 10 min and incubated with
10% goat non-immune serum for 20 min. Thenceforth, after incubation
with the goat anti-VEGI polyclonal antibody (1:100, cat. no.
sc-23185, Santa Cruz Biotechnology, Inc.) or mouse anti-CD31
monoclonal antibody (1:80, 3528, Cell Signaling Technology) at 37˚C
for 1 h (h), the sections were incubated with biotin-labeled
secondary antibody (Invitrogen) at 37˚C for 20 min, and then
incubated with HRP-conjugated streptavidin (Invitrogen) at 37˚C for
30 min. The color development was performed with a DAB Substrate
kit (Dako). At last, the sections were counterstained with
dehydrated, cleared and mounted hematoxylin. Appropriate negative
controls were carried out by excluding the primary antibody and/or
replacing it with an irrelevant anti-serum (no data shown).
Evaluation of VEGI expression
The evaluation of the immune histochmical staining
of VEGI was based on the calculation of the percentage and
intensity of the positive cells (per section by two independent
pathologists). On the basis of the percentage of the positive tumor
cells, the immunoreactivity of the tumor cells for VEGI was scored
as follows: 0=no cell stained, 1=1 to 25% of cells stained, 2=26 to
50% of cells stained, 3=51 to 75% of cells stained and 4=more than
75% of cells stained. The intensity of positive tumor cells was
graded as follows: 0 for negative staining, 1 for weak staining, 2
for moderate staining, and 3 for strong staining. Scores for the
intensity and percentage of positive cells were multiplied.
Finally, when the total score was below 2, the classification of
the expression of VEGI was considered as negative/weak, while when
the total score was above 3, it was classified as moderate/high. He
expression of VEGI was negative/weak moderate/high if the total
score was ≥3.
Evaluation of tumor angiogenesis
The immunohistochemical staining of CD31 was used
for microvascular density (MVD) counting as described previously
(17). Briefly, the vessel hot spot
regions with immune reactivity against CD31 were identified under
magnification, x100 and the number of MVD was counted manually in 5
hot spots selected randomly at x400.
Statistical analysis
All statistical analyses were achieved with SPSS
v16.0 software (SPSS, Inc.). Chi-square test was used to assess the
correlation between VEGI protein expression and clinicopathological
variables. The Student's t-test was evaluated the difference of MVD
in different group. Survival curves were constructed by means of
the Kaplan-Meier and the differences between the curves were
compared by the log-rank test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of VEGI protein in
NSCLC
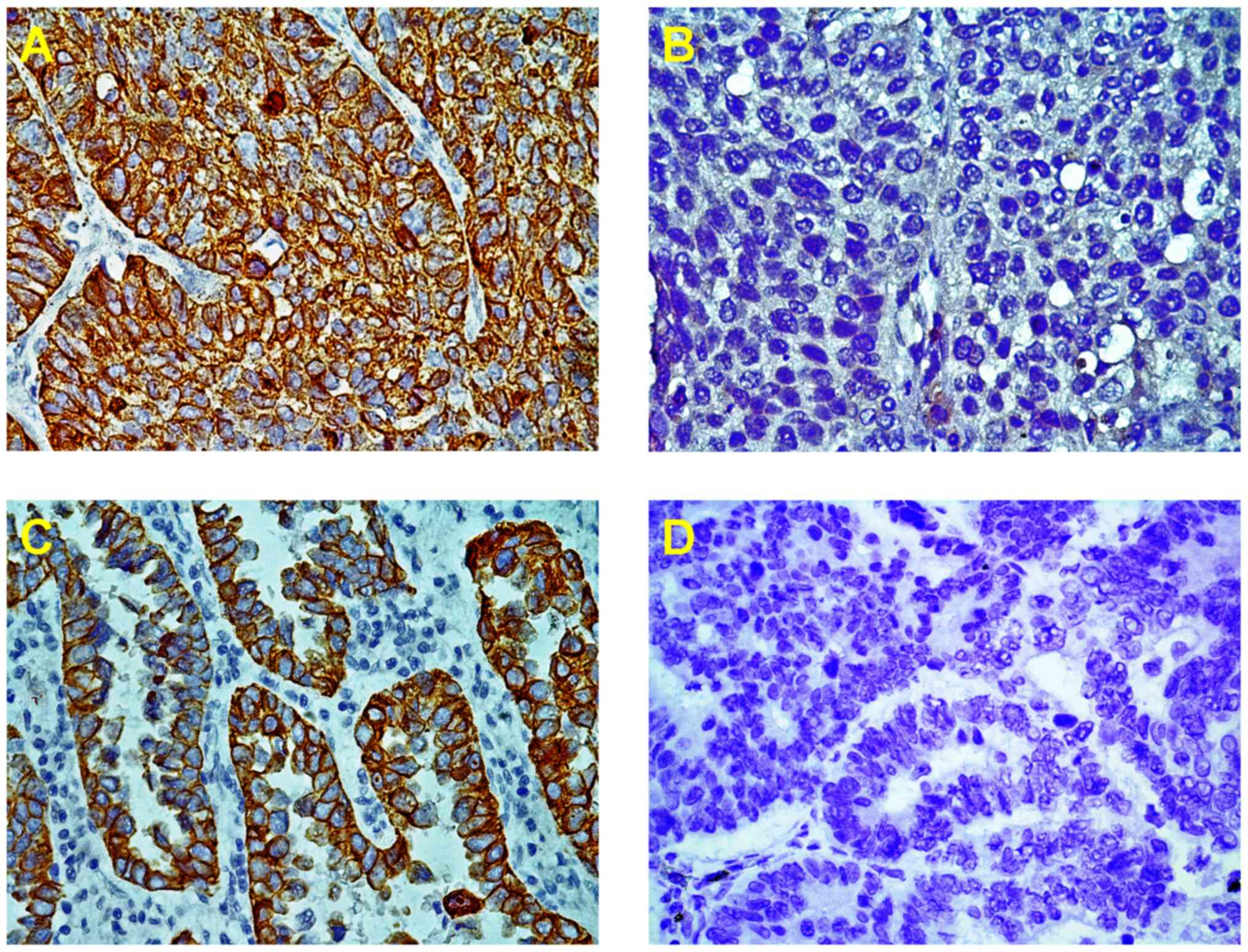
The immunostaining of VEGI protein was represented
as particles in the cytoplasm and the cell membrane which was 31.3%
(47/150) in NSCLC patients (Fig. 1A
and C). However, the VEGI
expression was lost or weak in 103 (68.7%) out of 150 NSCLC
patients, which was significantly higher in AC, 76.0% (57/75), than
in SCC, 61.3% (46/75) (Table
I).
Correlation between VEGI protein
expression and MVD
To investigate whether VEGI exert an inhibitory
effect on tumor-associated blood vessel formation and growth, we
estimated the intratumoral microvessel density (MVD) by measuring
CD31-positive staining, which was mainly detected in vascular
endothelial cell membrane and a lower degree in the cytoplasm. MVD
was significantly lower in VEGI-rich group than in the VEGI-poor
group (P=0.01; Fig. 2), indicating
that VEGI may pose an inhibitory effect on tumor-associated blood
vessel formation and growth, at least partially.
Correlation between VEGI protein
expression and the clinicopathological parameters
The relationship between the VEGI expression and the
clinicopathological features was analyzed in 150 NSCLC patients
(Table I). Significant correlation
between VEGI expression and clinicopathological parameters was not
observed. Nevertheless, the down-regulation of VEGI tended to be
associated with the histopathological type (P=0.059),
lymphovascular invasion type (P=0.065) as well as the lymph node
metastasis type (P=0.057), but this trend was likely due to the
association with AC.
Subsequently, the association of VEGI with
clinicopathological variables classified according to their
histology type was investigated (Table
II). In AC patients, the down-regulation of VEGI protein was
observed in 91.3% (21/23) of cases with lymphovascular invasion,
and in 88.6% (31/35) of cases with lymph node metastasis. A strong
correlation was found between VEGI expression and lymphovascular
invasion (P=0.039) and lymph node metastasis (P=0.017; Table II). No correlation was observed in
the squamous carcinoma group.
 | Table IIAssociation of VEGI expression with
the clinicopathological characteristics of patients with AC and
SCC. |
Table II
Association of VEGI expression with
the clinicopathological characteristics of patients with AC and
SCC.
| | VEGI expression
(AC) | | VEGI expression
(SCC) | |
|---|
|
Characteristics | Case, n (%) | Negative/weak | Moderate/high | P-value | Case, n (%) | Negative/weak | Moderate/high | P-value |
| Sex | | | | 0.828 | | | | 0.166 |
|
Male | 70 (93.3) | 53 | 17 | | 39 (52.0) | 21 | 18 | |
|
Female | 5 (6.7) | 4 | 1 | | 36 (48.0) | 25 | 11 | |
| Age, years | | | | 0.472 | | | | 0.422 |
|
≤60 | 24 (32.0) | 17 | 7 | | 37 (49.3) | 21 | 16 | |
|
<60 | 51 (68.0) | 40 | 11 | | 38 (50.7) | 25 | 13 | |
| Smoking | | | | 0.625 | | | | 0.330 |
|
Yes | 64 (85.3) | 48 | 16 | | 52 (69.3) | 30 | 22 | |
|
No | 11 (14.7) | 9 | 2 | | 23 (30.7) | 16 | 7 | |
| Tumor location | | | | 0.564 | | | | 0.436 |
|
Left | 29 (38.7) | 21 | 8 | | 32 (42.7) | 18 | 14 | |
|
Right | 46 (61.3) | 36 | 10 | | 43 (57.3) | 28 | 15 | |
| Histopathological
grade | | | | 0.463 | | | | 0.810 |
|
I+II | 55 (73.3) | 43 | 12 | | 58 (77.3) | 36 | 22 | |
|
III | 20 (26.7) | 14 | 6 | | 17 (22.7) | 10 | 7 | |
| Lymphovascular
invasion | | | | 0.039 | | | | 0.924 |
|
Yes | 23 (30.7) | 21 | 2 | | 10 (13.3) | 6 | 4 | |
|
No | 52 (69.3) | 36 | 16 | | 65 (86.7) | 40 | 25 | |
| Tumor size | | | | 0.074 | | | | 0.068 |
|
T1(40) | 21 (28.0) | 13 | 8 | | 19 (25.3) | 15 | 4 | |
|
T2-4(110) | 54 (72.0) | 44 | 10 | | 56 (74.7) | 31 | 25 | |
| Lymph node
metastasis | | | | 0.017 | | | | 0.772 |
|
Yes | 35 (46.7) | 31 | 4 | | 30 (40.0) | 19 | 11 | |
|
No | 40 (53.3) | 26 | 14 | | 45 (60.0) | 27 | 18 | |
Correlation of VEGI tissue expression
status with prognosis of NSCLC patients
To further investigate the clinical significance of
VEGI expression in lung cancer, the follow-up was performed on the
patients. Patients with moderate/high expression of VEGI seemed to
have a longer survival period than those with negative/weak VEGI
expression, and was correlated with disease-free survival (P=0.021;
Fig. 3A). Moreover, analysis of
data from a published study including 1926 lung cancer cases,
available on the kmplotwebsite (http://www.kmplot.com/analysis/index.php?p=background),
revealed that 967 patients who displayed the low expression of VEGI
mRNA in lung tumor tissues had shorter disease free time compared
with the 959 cases whose VEGI mRNA expression levels were high in
lung tumor tissues (Fig. 3B). These
data strongly indicate that the expression of VEGI correlates with
the prognosis of NSCLC patients.
 | Figure 3Kaplan-Meier survival curves according
to VEGI expression in patients with NSCLC. (A) OS and VEGI
expression in 150 patients with NSCLC (log-rank, P=0.021). (B)
Kaplan-Meier plot analysis of 1,926 lung cancer cases on VEGI,
available on the kmplot website (http://www.kmplot.com/analysis/index.php?p=background).
(C) OS and VEGI expression in 75 patients with AC (log-rank,
P=0.011). (D) OS and VEGI expression in 75 patients with squamous
cell carcinoma (log-rank, P=0.692). - indicates complete cases; +
indicates censored cases. VEGI, vascular endothelial growth
inhibitor; NSCLC, NSCLC, non-small cell lung cancer; OS, overall
survival; AC, adenocarcinoma. |
Then, a further analysis was performed within each
NSCLC histopathological type because there was a significant
correlation between VEGI expression and lung AC. In AC, patients
with negative/weak VEGI expression had poorer overall survival than
those with moderate/high VEGI expression (P=0.011; Fig. 3C). In contrast, these effects were
not observed in patients with squamous carcinoma (P=0.692; Fig. 3D).
Discussion
In the present study, we investigated the expression
of VEGI protein in tissue samples obtained from NSCLC patients and
analyzed the correlation between the expression level of VEGI and
the clinicopathological parameters. To our knowledge, this is the
first study to explore the clinical significance of VEGI protein
levels in NSCLC patients.
Accumulating evidence have confirmed that
neovascularization is one of the most critical steps for the
progress and metastasis of lung tumor because angiogenesis provides
oxygen and nutrients which are essential for tumor cell growth,
migration, and invasion. The balance between pro-angiogenic factors
and anti-angiogenic molecules is critical for angiogenesis to
occur. Vascular endothelial growth factor (VEGF), an important
pro-angiogenic factor, is overexpressed in lung cancer tissues and
is closely associated with tumor metastasis and prognosis of NSCLC
patients, whose expression can be regulated by numerous factors
(17). For example, epidermal
growth factor receptor (EGFR) promotes the transcription of VEGF
under both normal oxygen and hypoxic conditions in tumor cells
(18). The expression of VEGF is
reportedly induced by EGF (19). As
a key anti-angiogenic molecule, VEGI also can down-regulate the
expression of VEGF in endothelial cells and has been implicated in
a variety of human tumors (20).
Parr et al (9), carried out
a study to determine the expression profile of VEGI in a cohort of
151 mammary tissue samples with a 6-year median follow-up and found
that VEGI was expressed in the normal mammary tissue but it was
dramatically reduced/absent in the breast tumor tissue samples.
Moreover, VEGI level was positively correlated with the survival
rate of breast cancer patients. A subsequent study (21) on the biological function of VEGI in
breast cancer cell lines found that VEGI inhibited the migration,
adhesion invasion, and vascularization capacity of MDA-MB-231 cells
in vitro and in vivo. Similar results have been
reported in other solid tumors (12,13,22,23)
such as bladder tumor, renal cell carcinoma, and pituitary adenomas
implying that VEGI function as a tumor suppressor which negatively
regulates neovascularization during tumorigenesis and progression
of many solid tumors. Consistent with previous studies, absence or
weak expression of VEGI was observed in 68.7% of NSCLC tissues. And
MVD, a indicator of angiogenesis which can predict tumor growth,
metastasis, and recurrence, was significantly increased in
VEGI-poor group. Taken together, these observations suggest that
VEGI may pose an inhibitory effect on NSCLC progression through
inhibiting the intratumoral angiogenesis, at least partially.
Unfortunately, due to the limited number of samples,
the correlation between VEGI expression and the pathological
parameters of NSCLC patients could be made in this study. However,
a mild association between VEGI expression and the
histopathological type was observed. For instance, the
down-regulation of VEGI was more prevalent in AC than that in
squamous cell carcinoma (SCC). A negative correlation between VEGI
expression and lymphovascular invasion and lymph node metastasis
was observed in lung AC, which may be due to the differential
contribution of the vasculature to the progress of lung cancer
between SCC and AC. Moreover, NSCLC patients with reduced VEGI
protein levels had a poor survival outcome than those with high
levels of VEGI in AC, but this effect was observed in patients with
squamous carcinoma, suggesting that the expression of VEGI protein
may be a new prognostic parameter for AC patients but not SCC
patients.
In summary, the down-regulation of VEGI expression
was observed in NSCLC patients, which was correlated with the lower
MVD, poor prognosis and shorter survival period for NSCLC,
particularly in AC patients. Our results indicate that VEGI may be
a tumor suppressor through inhibiting the intratumoral
angiogenesis, at least partially, in NSCLC and a new prognostic
marker for lung AC.
Acknowledgements
The authors would like to thank Professor Zhiwei Liu
(Jiangsu Key Laboratory of Anesthesia and Analgesia Application
Technology) for her excellent technical assistance.
Funding
The present study was supported by the Department of
Science and Technology, Xuzhou, Jiangsu, China (grant no. KC17089)
and the Jiang Su Provincial Medical Youth Talent (grant no.
QNRC2016781).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and YX performed the research and analyzed the
data. QL and SF analyzed the clinical data and contributed clinical
samples. ZL, HL and JL performed immunohistochemical analysis and
histologic assessment. JL and SL designed the experiments and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Regional
Ethics Review Board of the Affiliated Hospital of Xuzhou Medical
University, China (approval no. 2015-0629). Written informed
consent was obtained from patients.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang S, Fu Y, Wang D and Wang J: Icotinib
enhances lung cancer cell radiosensitivity in vitro and in vivo by
inhibiting MAPK/ERK and AKT activation. Clin Exp Pharmacol Physiol.
45:969–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sen Z, Zhan XK, Jing J, Yi Z and Wanqi Z:
Chemosensitizing activities of cyclotides from Clitoria ternatea in
paclitaxel-resistant lung cancer cells. Oncol Lett. 5:641–644.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lortet-Tieulent J, Soerjomataram I, Ferlay
J, Rutherford M, Weiderpass E and Bray F: International trends in
lung cancer incidence by histological subtype: Adenocarcinoma
stabilizing in men but still increasing in women. Lung Cancer.
84:13–22. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vansteenkiste J, Crinò L, Dooms C,
Douillard JY, Faivre-Finn C, Lim E, Rocco G, Senan S, Van Schil P,
Veronesi G, et al: 2nd ESMO Consensus Conference on Lung Cancer:
Early-stage non-small-cell lung cancer consensus on diagnosis,
treatment and follow-up. Ann Oncol. 25:1462–1474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Parr C, Gan CH, Watkins G and Jiang WG:
Reduced vascular endothelial growth inhibitor (VEGI) expression is
associated with poor prognosis in breast cancer patients.
Angiogenesis. 9:73–81. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang N, Sanders AJ, Ye L and Jiang WG:
Vascular endothelial growth inhibitor in human cancer (Review). Int
J Mol Med. 24:3–8. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Vascular endothelial growth inhibitor, expression in
human prostate cancer tissue and the impact on adhesion and
migration of prostate cancer cells in vitro. Int J Oncol.
35:1473–1480. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang N, Sanders AJ, Ye L, Kynaston HG and
Jiang WG: Expression of vascular endothelial growth inhibitor
(VEGI) in human urothelial cancer of the bladder and its effects on
the adhesion and migration of bladder cancer cells in vitro.
Anticancer Res. 30:87–95. 2010.PubMed/NCBI
|
|
13
|
Wu L, Li X, Ye L, Shayiremu D, Deng X,
Zhang X, Jiang W, Yang Y, Gong K and Zhang N: Vascular endothelial
growth inhibitor 174 is a negative regulator of aggressiveness and
microvascular density in human clear cell renal cell carcinoma.
Anticancer Res. 34:715–22. 2014.PubMed/NCBI
|
|
14
|
Zhao Q, Liu T, Hong B, Wang F, Zhou C, Du
X, Chen S, Deng X, Duoerkun S, Li Q, et al: Vascular endothelial
growth inhibitor, a cytokine of the tumor necrosis factor family,
is associated with epithelial-mesenchymal transition in renal cell
carcinoma. Appl Immunohistochem Mol Morphol. 26:727–733.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou W, Medynski D, Wu S, Lin X and Li LY:
A new isoform of TNFSF15, specifically eliminates tumor vascular
endothelial cells and suppresses tumor growth. Clin Cancer Res.
11:5595–5602. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liang PH, Tian F, Lu Y, Duan B, Stolz DB
and Li LY: Vascular endothelial growth inhibitor (VEGI; TNFSF15)
inhibits bone marrow-derived endothelial progenitor cell
incorporation into Lewis lung carcinoma tumors. Angiogenesis.
14:61–68. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Y, Mathy NW and Lu H: The role of
VEGF in the diagnosis and treatment of malignant pleural effusion
in patients with non-small cell lung cancer (Review). Mol Med Rep.
17:8019–8030. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karoor V, Le M, Merrick D, Fagan KA,
Dempsey EC and Miller YE: Alveolar hypoxia promotes murine lung
tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer
Prev Res (Phila). 5:1061–1071. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu X, Li W, Deng Q, You S, Liu H, Peng S,
Liu X, Lu J, Luo X, Yang L, et al: Neoalbaconol Inhibits
angiogenesis and Tumor Growth by suppressing EGFR-mediated VEGF
production. Mol Carcinog. 56:1414–1426. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Zhang K, Cai HX, Gao S, Yang GL, Deng HT,
Xu GC, Han J, Zhang QZ and Li LY: TNFSF15 suppresses VEGF
production in endothelial cells by stimulating miR-29b expression
via activation of JNK-GATA3 signals. Oncotarget. 7:69436–69449.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao Y, Ge Z, Zhang Z, Bai Z, Ma X and Wang
Y: Vascular endothelial growth inhibitor affects the invasion,
apoptosis and vascularisation in breast cancer cell line
MDA-MB-231. Chin Med J (Engl). 127:1947–1953. 2014.PubMed/NCBI
|
|
22
|
Jia W, Sander AJ, Jia G, Ni M, Liu X, Lu R
and Jiang WG: Vascular endothelial growth inhibitor (VEGI) is an
independent indicator for invasion in human pituitary adenomas.
Anticancer Res. 33:3815–3822. 2013.PubMed/NCBI
|
|
23
|
Duan L, Yang G, Zhang R, Feng L and Xu C:
Advancement in the research on vascular endothelial growth
inhibitor (VEGI). Target Oncol. 7:87–90. 2012.PubMed/NCBI View Article : Google Scholar
|