Introduction
Breast reconstruction after total mastectomy
provides important psychosocial and quality of life benefits
(1). The incidence of breast
reconstruction is gradually increasing in most developed countries
and there are many types of reconstruction methods. Among them,
latissimus dorsi (LD) flap reconstruction without a prosthetic
implant is very popular in Japan and at our institution. Since
Asian breast cancer patients generally have small to
moderately-sized breasts, the LD flap provides a natural feel and
appearance postoperatively. The LD flap is rotated anteriorly
through the axillary cavity while preserving the thoracodorsal
bundle. It is a relatively easy procedure because it does not
require vascular anastomosis. Additionally, LD flap reconstruction
reportedly tends to have fewer complications than other autologous
tissue reconstructions (2).
However, because the reconstruction involves anatomical changes,
future locoregional recurrence requiring radical surgery can be
challenging. Especially in the case of axillary recurrence in
patients with an LD flap, passage of the flap through the axilla
might interfere with the operation, since it requires careful
dissection to preserve the axillary vein, thoracodorsal bundle and
long thoracic nerve, in a limited surgical field. Because cases of
axillary lymph node recurrence alone are rare, no paper has ever
reported in detail on the technique and course about lymph node
recurrence after LD flap reconstruction. Since we experienced two
cases of axillary lymph node recurrence in breast cancer patients
after LD flap reconstruction, we report these cases along with a
brief summary of patients who underwent immediate LD flap
reconstruction at our institution.
Materials and methods
Patients
The Institutional Review Board of Tokyo Medical and
Dental University approved the retrospective review of the medical
records of patients who had undergone breast cancer surgery with
immediate LD flap reconstruction from February 2005 to December
2018.
Surgical procedure
LD flap reconstruction was mainly indicated in
Tis/T1/T2 and N0/N1 patients. Nipple sparing mastectomy (NSM) and
skin sparing mastectomy (SSM) were indicated in Tis or T1 patients,
and total mastectomy was performed in T2 patients. Patients who
were expected to receive radiation therapy, for example, T3 or
suspicious of multiple lymph node metastasis patients, were not
recommended for breast reconstruction or NSM/SSM. However, the
surgery was performed even under these conditions if desired by the
patient, with their understanding and consent, after explaining
that it was not an indication. Sentinel lymph node biopsy (SNB) was
performed in all N0 patients. The sentinel lymph nodes were
identified using a combination of radioisotope and dye injection
studies. Axillary lymph node dissection (ALND) was performed in N+
patients and positive sentinel lymph node patients.
The LD flap reconstruction performed at our facility
is a so-called extended LD flap reconstruction (3). The extended LD flap, which is
harvested using an oblique skin island design, usually ranges from
7 to 9 cm wide. The plane of dissection continues along the
subcutaneous plane just above the superficial fascia. As much fat
as possible should be harvested from the scapular region and the
supra-iliac region, while avoiding division of the thoracodorsal
nerve to prevent postoperative muscle atrophy. The humeral
attachment of the LD muscle is preserved to prevent tension on the
vascular pedicle. The flap is rotated and passed under a skin
tunnel to the mastectomy site.
Statistical analysis
In this study, locoregional recurrence was defined
as recurrence of breast cancer in ipsilateral regional lymph nodes
or in the skin or subcutaneous tissue of the ipsilateral chest
wall. Locoregional and distant recurrence were confirmed by biopsy
or imaging. The follow-up period commenced on the day of final
surgery and ended with any type of recurrence (event), death
(censored), or on the day of last follow-up (censored). We
calculated the mean duration of survival using the Kaplan-Meier
method. All statistical analyses were performed using EZR software,
which is a modified version of R Commander designed for statistical
functions frequently used in biostatistics (4) (Saitama Medical Center, Jichi Medical
University, http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html),
and a graphic user interface for R (The R Foundation for
Statistical Computing).
Results
Characteristics of patients and
recurrence-free survival
The characteristics of patients who underwent LD
flap reconstruction at our institute are summarized in Table I. We performed immediate LD flap
reconstruction in 72 breast cancer patients who were followed up
for a median period of 84 months (11-158 months). The primary tumor
was pTis in 24%, pT1 in 47%, pT2 in 26%, and pT3 in 3% of the
patients. Regional lymph nodes were pn0 in 81%, pN1 in 12%, pN2 in
3%, pN3 in 1%, and pNX in 3%. The breast resection procedures
performed were total mastectomy (11%), SSM (68%), and NSM (21%).
The axillary operations were SNB alone (65%), ALND (19%), and
SNB+ALND (13%). The remaining 3% of patients did not undergo
axillary surgery. Post-mastectomy radiation therapy was
administered in only 3% of the patients, while 26% of patients
received systemic chemotherapy. Local recurrence in the skin/chest
wall and regional lymph node recurrence occurred in two patients
(2.8%) each. Distant recurrence occurred in six patients (8%). The
5-year and the 7-year recurrence-free survival rate was 94.0 and
89.5% each (Fig. 1). Both regional
lymph node recurrences were level axillary lymph node recurrences.
The course of treatment for these axillary lymph node recurrences
is described in the next section.
 | Table ICharacteristics of patients (n=72) who
underwent mastectomy with latissimus dorsi flap reconstruction for
breast cancer at our institution (Tokyo Medical and Dental
University, Medical Hospital). |
Table I
Characteristics of patients (n=72) who
underwent mastectomy with latissimus dorsi flap reconstruction for
breast cancer at our institution (Tokyo Medical and Dental
University, Medical Hospital).
| Characteristics | Value |
|---|
| Mean age, years
(range) | 46 (28-74) |
| pT, n (%) | |
|
Tis | 17(24) |
|
T1 | 34(47) |
|
T2 | 19(26) |
|
T3 | 2(3) |
| pN, n (%) | |
|
pN0 | 58(81) |
|
pN1 | 9(12) |
|
pN2 | 2(3) |
|
pN3 | 1(1) |
|
pNX | 2(3) |
| TM/SSM/NSM, n
(%) | |
|
TM | 8(11) |
|
SSM | 49(68) |
|
NSM | 15(21) |
| SNB/Ax, n (%) | |
|
SNB | 47(65) |
|
SNB+Ax | 9(13) |
|
Ax | 14(19) |
|
No
surgery | 2(3) |
| PMRT, n (%) | |
|
Yes | 2(3) |
|
No | 70(97) |
| Chemotherapy, n
(%) | |
|
Yes | 19(26) |
|
No | 53(74) |
| Recurrence, n
(%) | |
|
Local | 2(3) |
|
Regional | 2(3) |
|
No | 68(94) |
| Distant recurrence, n
(%) | |
|
Yes | 6(8) |
|
No | 66(92) |
Case 1
A 67-year-old woman who was diagnosed with T1cN0M0
breast cancer (Luminal A type) underwent total mastectomy and SNB.
Since the SN showed no metastasis, axillary lymph node dissection
was omitted. Immediate breast reconstruction with an LD flap was
simultaneously performed. Three years after the surgery, regular
postoperative ultrasonography (US) follow-up during hormonal
therapy showed axillary lymph node enlargement. US-guided
fine-needle aspiration cytology (FNAC) of the axilla was positive
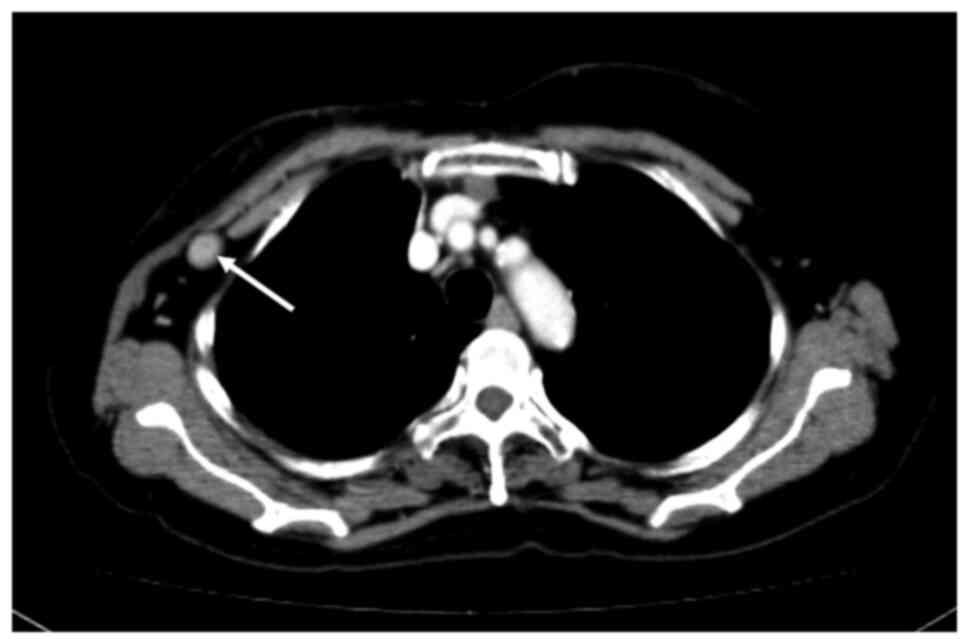
for malignancy. CT showed swelling of a lymph node behind the LD
flap (Fig. 2). Since there was no
metastasis to other organs, axillary lymph node dissection was
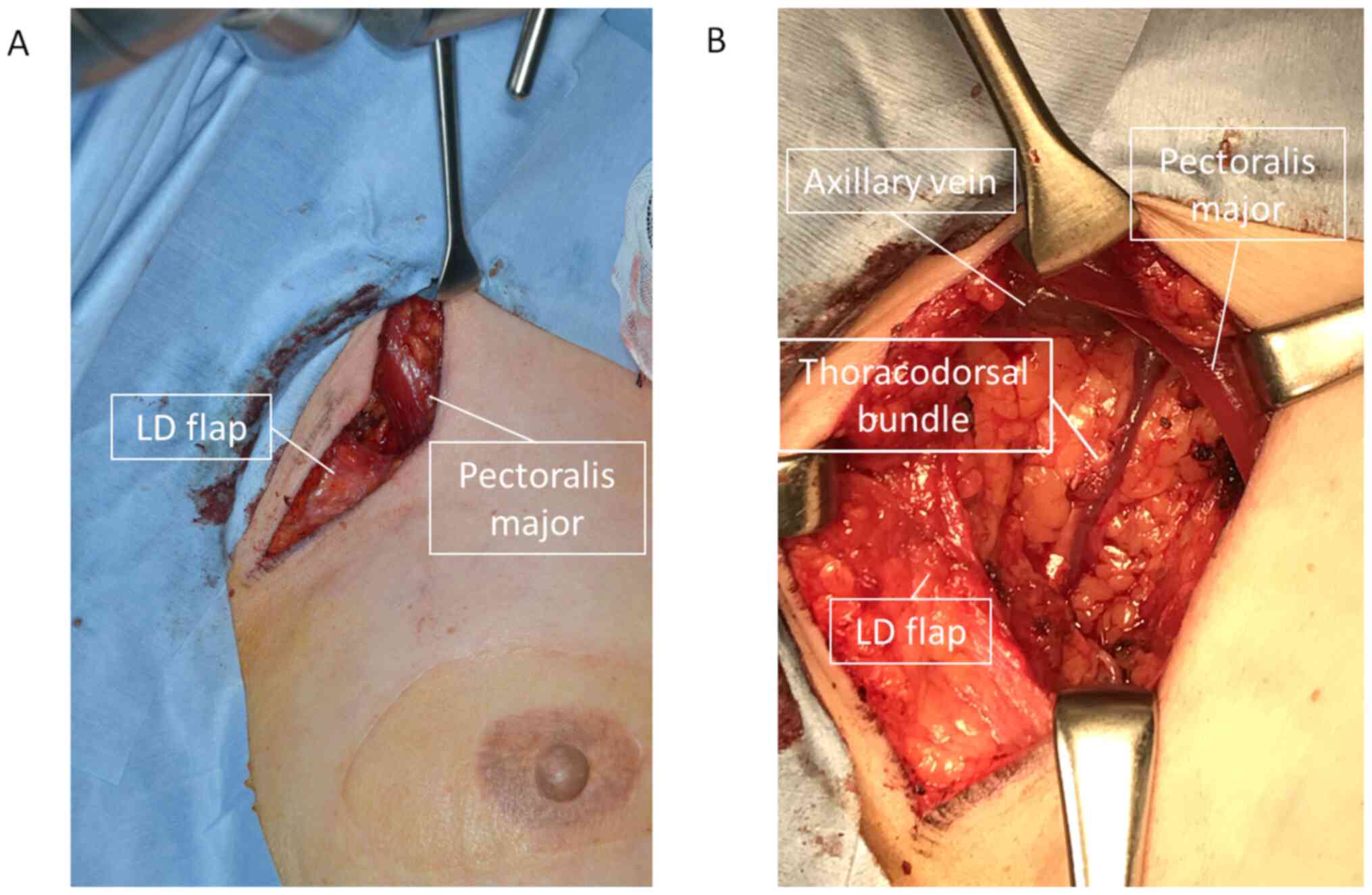
performed. The intraoperative findings are shown in Fig. 3. The skin was incised along the
anterior axillary line, as in the first surgery. When the LD muscle
was retracted caudally, the enlarged lymph node could be
identified. The axillary vein was carefully preserved when cutting
along the side of the pectoralis major and minor muscles. The
thoracodorsal bundle was also identified and preserved. The
surgical field was, however, very limited, because the LD muscle
passed through the caudal part of the field, increasing the level
of difficulty of the procedure. The patient's postoperative course
was uneventful. Pathological evaluation revealed metastasis in only
one of the 14 resected lymph nodes. The preoperative endocrine
treatment regimen was continued postoperatively as adjuvant
therapy. The patient is currently alive and free of disease 14
months after the recurrence.
Case 2
Case 2 was a 55-year-old woman who had previously
undergone total mastectomy and SNB for stage I (T1N0M0 Luminal A
type) breast cancer, at which time there was no metastasis in the
SN. Immediate breast reconstruction with an LD flap was also
performed. Three years after the operation, routine follow-up US
showed lymph node enlargement in the axilla. FNAC was positive for
malignancy. Since there was no metastasis at other sites, axillary
dissection was performed. As in case 1, an enlarged lymph node was
recognized behind the LD muscle passing through the axilla. As in
case 1, the thoracodorsal vein was identified and preserved,
although the surgical field was very limited (Fig. 4). Pathological evaluation indicated
metastasis in only one lymph node, with extra-nodal extension to 11
lymph nodes. Radiation therapy with a total of 50 Gy was given to
the chest wall and supraclavicular region. Endocrine therapy was
switched from tamoxifen to aromatase inhibitors. The patient is
currently alive and free of disease 13 months after recurrence.
Discussion
Many patients who undergo mastectomy are interested
in breast reconstruction after the initial treatment (4). Reconstruction has been shown to
improve the patient's ability to overcome the psychological trauma
in the wake of the primary diagnosis and treatment (5).
Our institution promotes autologous reconstruction
because of the very good aesthetic results obtained, while offering
a better tolerance to radiotherapy than prosthetic reconstruction
(6,7). However, autologous reconstruction has
the disadvantage that the procedure is more complicated than
prosthetic reconstruction. In several types of autologous
reconstructions, creation of an LD flap is regarded as a relatively
safe procedure, because it requires no microsurgical skills
(2). Since this procedure is
particularly suitable for Asians with low breast volume, the number
of such procedures being performed is increasing year by year.
However, since the LD flap usually passes through the axillary
cavity, the concern is that if regional lymph node recurrence
occurs, it will make subsequent surgery more difficult than usual.
It is necessary to preserve the thoracodorsal bundle and pedicle to
avoid atrophy of the LD flap, which requires careful dissection in
a narrow surgical field. We experienced two such cases, both of
which had a good postoperative course. In these two cases, the
enlarged lymph node was behind the LD muscle passing through the
axilla. Axillary dissection could be performed by retracting the
flap caudally. Although the dissection proceeded as normal, it was
noted that the thoracic neurovascular bundle was more anteriorly
shifted than normal in both cases.
The humeral attachment is not resected during LD
flap reconstruction at our institution. If the humeral attachment
is resected at the time of reconstruction, the axillary field of
view is no longer an issue. If it is difficult to secure the visual
field at the time of dissection, as in this case, the LD flap can
be resected, but considering the possibility of muscle atrophy, we
believe it is better to preserve the flap as far as possible.
In both our cases, only one lymph node was enlarged,
without involvement of the surrounding blood vessels or nerves.
Since resection of the flap is likely to be necessary in cases with
many metastatic lymph nodes adherent to one another or to other
structures, breast cancer patients with LD flap reconstruction
should be carefully followed to detect local recurrence at an early
stage.
In the review of previous cases of immediate LD
reconstruction following breast cancer surgery performed at our
hospital, the two cases (2.8%) that had axillary recurrence were
SNB-negative at the time of the first surgery. Distant recurrence
occurred in 6 patients (8%). The 5-year and the 7-year
recurrence-free survival rate was 94.0 and 89.5% each. Previous
reports have reported a 5-year DFS of 85-92% in cases of mastectomy
alone without reconstruction, 90-95% in cases of reconstruction
(8-12).
This is largely consistent with our study. The incidence of
axillary recurrence in SNB-negative breast cancer patients is
reportedly 0.5-1% (13-16).
This data is almost the same as our own, although, since the number
of our cases was small, we need to accumulate more cases in the
future. Due to the low rate of axillary recurrence in patients with
negative sentinel lymph nodes, there are not many reports on
predictive factors for axillary recurrence. Previously reported
cases have pointed to young age and omission of irradiation as
predictors of axillary lymph node recurrence in sentinel
node-negative breast cancers (17,18).
Neither of these factors were present in our two cases. However,
even with SNB with dye and radioisotope injection, as in our
institution, axillary recurrence is sometimes unavoidable. If
axillary lymph node recurrence is detected early, as in our cases,
the foci of recurrence can be resected early, while preserving the
LD flap. Hence, it is important to keep in mind the possibility of
axillary lymph node recurrence during surveillance after LD flap
reconstruction following breast cancer surgery.
In conclusion, we experienced two cases of axillary
lymph node dissection in LD flap reconstruction patients. It should
be noted that if a second surgery is required after LD flap
reconstruction following breast cancer surgery, the field of view
is significantly narrowed by the LD flap, and the thoracodorsal
bundle is anteriorly displaced as compared to normal. In patients
who undergo LD flap reconstruction, careful postoperative follow-up
is necessary to detect axillary lymph node recurrence at an early
stage, to minimize surgical difficulty.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GO performed the surgery, wrote the manuscript,
analyzed the data and performed the statistical analysis. TN, NU
and HM performed the surgery and collected the data. MM and TF were
responsible for the diagnostic imaging and ultrasonography-guided
biopsy. IO was in charge of the pathology evaluations. HU designed
the present study and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tokyo Medical and Dental University Hospital (Tokyo,
Japan). Signed informed consent was obtained from the patients.
Patient consent for publication
Informed consent was obtained from the patients for
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eltahir Y, Werners LL, Dreise MM, van
Emmichoven IA, Jansen L, Werker PM and de Bock GH: Quality-of-life
outcomes between mastectomy alone and breast reconstruction:
Comparison of patient-reported BREAST-Q and other health-related
quality-of-life measures. Plast Reconstr Surg. 132:201e–209e.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bennett KG, Qi J, Kim HM, Hamill JB, Pusic
AL and Wilkins EG: Comparison of 2-year complication rates among
common techniques for postmastectomy breast reconstruction. JAMA
Surg. 153:901–908. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hokin JA: Mastectomy reconstruction
without a prosthetic implant. Plast Reconstr Surg. 72:810–818.
1983.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Korvenoja ML, Smitten K and
Asko-Seljavaara S: Problems in wearing external prosthesis after
mastectomy and patient's desire for breast reconstruction. Ann Chir
Gynaecol. 87:30–34. 1998.PubMed/NCBI
|
|
5
|
Zhong T, McCarthy C, Min S, Zhang J, Beber
B, Pusic AL and Hofer SO: Patient satisfaction and health-related
quality of life after autologous tissue breast reconstruction: A
prospective analysis of early postoperative outcomes. Cancer.
118:1701–1709. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lam TC, Hsieh F and Boyages J: The effects
of postmastectomy adjuvant radiotherapy on immediate two-stage
prosthetic breast reconstruction: A systematic review. Plast
Reconstr Surg. 132:511–518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schaverien MV, Macmillan RD and McCulley
SJ: Is immediate autologous breast reconstruction with
postoperative radiotherapy good practice? A systematic review of
the literature. J Plast Reconstr Aesthet Surg. 66:1637–1651.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park SH, Han W, Yoo TK, Lee HB, Jin US,
Chang H, Minn KW and Noh DY: Oncologic safety of immediate breast
reconstruction for invasive breast cancer patients: A matched case
control study. J Breast Cancer. 19:68–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee SB, Lee JW, Kim HJ, Ko BS, Son BH, Eom
JS, Lee TJ and Ahn SH: Long-term outcomes of patients with breast
cancer after nipple-sparing mastectomy/skin-sparing mastectomy
followed by immediate transverse rectus abdominis musculocutaneous
flap reconstruction: Comparison with conventional mastectomy in a
single center study. Medicine (Baltimore). 97(e0680)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee KT, Mun GH, Lim SY, Pyon JK, Oh KS and
Bang SI: The impact of immediate breast reconstruction on
post-mastectomy lymphedema in patients undergoing modified radical
mastectomy. Breast. 22:53–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ha JH, Hong KY, Lee HB, Moon HG, Han W,
Noh DY, Lim J, Yoon S, Chang H and Jin US: Oncologic outcomes after
immediate breast reconstruction following mastectomy: Comparison of
implant and flap using propensity score matching. BMC Cancer.
20(78)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yi M, Kronowitz SJ, Meric-Bernstam F, Feig
BW, Symmans WF, Lucci A, Ross MI, Babiera GV, Kuerer HM and Hunt
KK: Local, regional, and systemic recurrence rates in patients
undergoing skin-sparing mastectomy compared with conventional
mastectomy. Cancer. 117:916–924. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heuts EM, van der Ent FW, Hulsewé KW,
Heeren PA and Hoofwijk AG: Incidence of axillary recurrence in 344
sentinel node negative breast cancer patients after intermediate
follow-up. A prospective study into the accuracy of sentinel node
biopsy in breast cancer patients. Acta Chir Belg. 108:203–207.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Palesty JA, Foster JM, Hurd TC, Watroba N,
Rezaishiraz H and Edge SB: Axillary recurrence in women with a
negative sentinel lymph node and no axillary dissection in breast
cancer. J Surg Oncol. 93:129–132. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smidt ML, Janssen CM, Kuster DM, Bruggink
ED and Strobbe LJ: Axillary recurrence after a negative sentinel
node biopsy for breast cancer: Incidence and clinical significance.
Ann Surg Oncol. 12:29–33. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
van der Vegt B, Doting MH, Jager PL,
Wesseling J and de Vries J: Axillary recurrence after sentinel
lymph node biopsy. Eur J Surg Oncol. 30:715–720. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Wely BJ, van den Wildenberg FJ,
Gobardhan P, van Dalen T, Borel Rinkes IH, Theunissen EB, Wijsman
JH, Ernst M, van der Pol CC, Madsen EV, et al: ‘Axillary
recurrences after sentinel lymph node biopsy: A multicentre
analysis and follow-up of sentinel lymph node negative breast
cancer patients’. Eur J Surg Oncol. 38:925–931. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hunt KK, Ballman KV, McCall LM, Boughey
JC, Mittendorf EA, Cox CE, Whitworth PW, Beitsch PD, Leitch AM,
Buchholz TA, et al: Factors associated with local-regional
recurrence after a negative sentinel node dissection: Results of
the ACOSOG Z0010 trial. Ann Surg. 256:428–436. 2012.PubMed/NCBI View Article : Google Scholar
|