Introduction
Rectal neuroendocrine tumors (NETs) are relatively
rare, with an annual incidence of 1.04 cases per 100,000
individuals (1). The incidence of
rectal NETs has increased by almost ten-fold over the past few
decades, which is thought to be due to increased colorectal cancer
screening, recent improvements in detection due to endoscopic
developments, and a greater clinical understanding (2,3). Among
rectal NETs, 93.3-100% are 1 cm or less at diagnosis (4). The World Health Organization (WHO)
classified rectal NETs as low-grade malignant tumors; however, NETs
and adenocarcinoma had similar survival if the tumor had lymph node
(LN) metastasis or distant metastasis (5). Generally, treatment guidelines for
rectal NETs larger than 2 cm or with potential LN metastasis
recommend a formal oncologic low anterior resection (LAR) with
total mesorectal excision (TME). However, rectal NETs in the lower
rectum may metastasize to the lateral lymph nodes (LLNs) along
alternate lymphatic passages outside of the mesorectal envelope,
similar to adenocarcinoma in the lower rectum. Due to their
low-grade malignant potential and very slow growth, metastatic LLNs
are so small that preoperative identification with computed
tomography (CT) and magnetic resonance imaging (MRI) may be
difficult. There are no detailed reports in English about LLN
metastasis from rectal NETs. Currently, the surgical indications
for LLN metastasis from rectal NETs are unclear. Considering the
lack of effective chemotherapy options, optimally timed radical
resection may help improve the prognosis of rectal NETs.
Case report
A 47-year-old man underwent total colonoscopy as a
routine health examination at another hospital 3 years ago. The
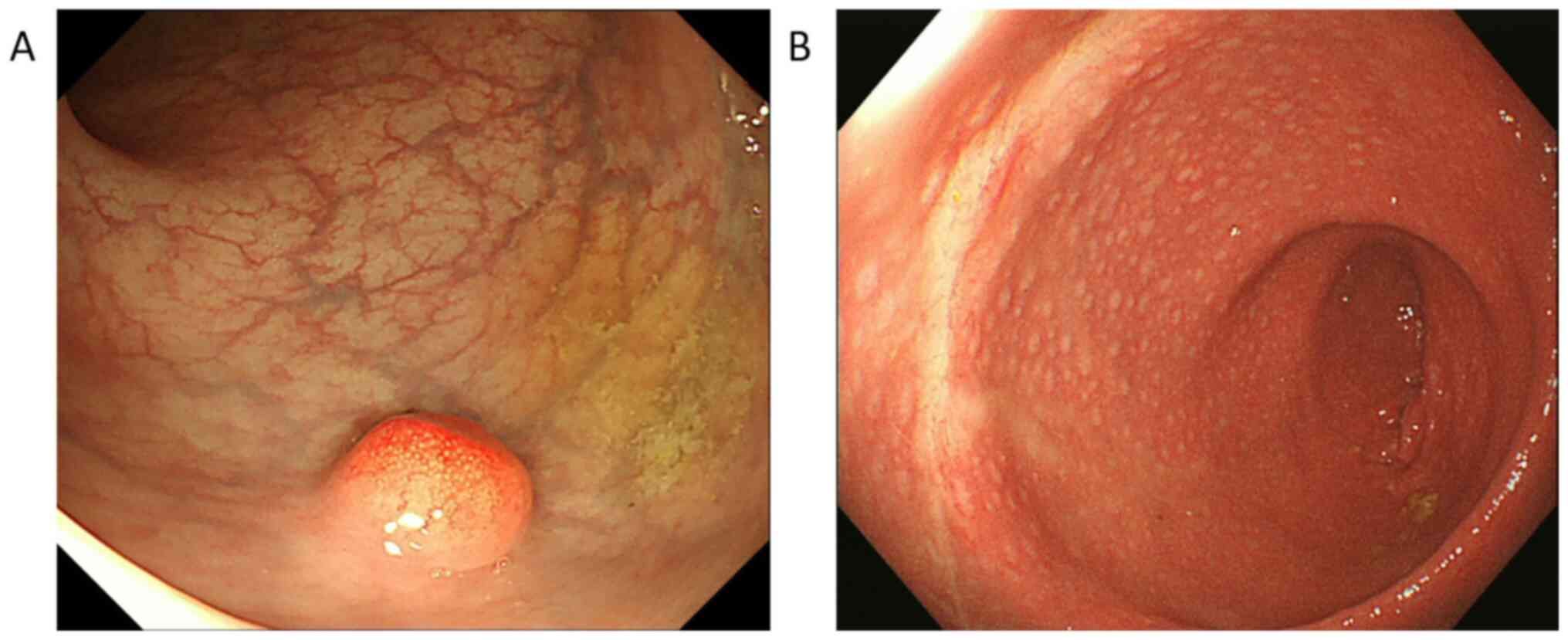
examination revealed a hemispheric submucosal tumor (10 mm in
diameter) in the lower rectum that was located 7 cm from the anal
verge at the anterior side of the rectal wall (Fig. 1A). The lesion did not exhibit a
central depression or ulceration, and the pathological diagnosis
was a NET. Additionally, no signs and symptoms of carcinoid
syndrome were observed. Imaging examinations, including CT and MRI,
were not performed at that time. He underwent endoscopic submucosal
dissection (ESD) at that hospital, and the macroscopic findings of
the resected specimen indicated that the primary tumor was 10 mm in
diameter (Fig. 2A). The
pathological tumor depth was limited to the submucosal layer
(Fig. 2B), and lymphovascular
invasion was detected. A pathological diagnosis of NET G1 was
confirmed according to a Ki-67 index of 1.6% (Fig. 2C). Immunohistochemical analysis for
synaptophysin of the specimens revealed positive immunostaining of
the tumor cells (Fig. 2D). The
tumor margins were clear, and additional surgical resection was not
performed at that time. A CT examination was performed three years
after ESD and revealed LN swelling in the mesorectum and obturator
space on the left side. The patient was referred to our hospital
for surgery. Colonoscopy revealed a scar in the lower rectum after
ESD, and the biopsy detected no evidence of local recurrence
(Fig. 1B). Laboratory data revealed
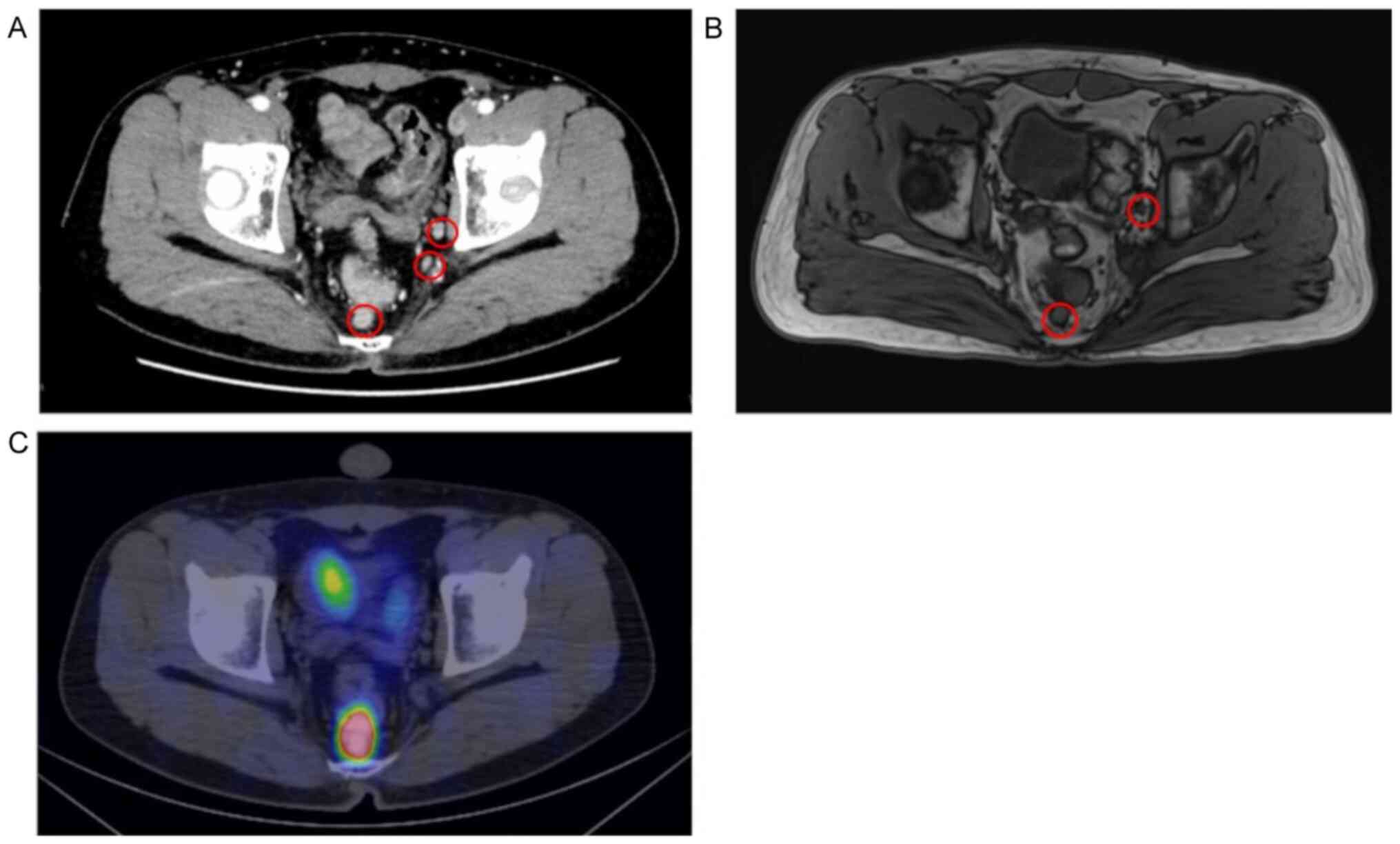
no abnormal findings. Contrast-enhanced CT images and T2-weighted
MRI scans revealed 2 enlarged LNs (maximum size, 12.1 mm) in the
mesorectum and 5 enlarged LLNs (maximum size, 10 mm) on the left
side (Fig. 3A and B). LLNs were not detected on the right
side. 68Ga-DOTATOC positron emission tomography
(68Ga-DOTATOC PET)/CT images revealed high
68Ga-DOTATOC uptake in the mesorectum and no abnormal
uptake on either side of the obturator space (Fig. 3C) or any other distant organs. The
patient was diagnosed with LN metastasis in the mesorectum and LLN
metastasis on the left side from the NET.
Robotic TME and bilateral LN dissection were
performed. The proximal LNs were dissected around the root of the
inferior mesenteric artery (IMA). In the pelvic space, TME was
performed up to the anal canal. Lateral lymphadenectomy was
subsequently performed on both sides as follows. The ureter and
hypogastric nerve were elevated, and the internal iliac vessels
were subsequently cleared from the lymphatic tissue at a safe
distance from the lateral side of the pelvic plexus. The LNs and
fatty tissue were dissected from the obturator space. During the
dissection, the obturator nerve was preserved. Following completion
of the bilateral LN dissection, only the external vessels, internal
iliac vessels and their branches, the obturator nerves, and the
pelvic plexus remained. The operative time was 576 min, and the
intraoperative blood loss volume was 900 ml. The patient recovered
well from surgery. He was discharged on postoperative day 7, and
adjuvant therapy was not performed. His defecation function was
good, with no fecal incontinence. His voiding and sexual functions
were preserved. The macroscopic and microscopic findings of the
resected specimen indicated that there was no residual tumor in the
rectum. Two of the 18 LNs in the mesorectum contained metastases
from the NET, and the LLNs on the left side (which included 13 LNs)
were all negative. In contrast, one of the 6 LLNs on the right side
contained metastasis from the NET. The patient was followed up with
chest and abdominal CT every 6 months. At the one-year follow-up,
no local recurrence and distant metastasis had been found.
Discussion
Rectal NETs are relatively rare. Due to increased
screening with colonoscopy, the incidence of rectal NETs has
increased in the past few years. In the National Comprehensive
Cancer Network guidelines, rectal NETs >2 cm with invasion into
the muscularis propria or LN metastases should be treated with LAR
(6). In the European Neuroendocrine
Tumor Society guidelines and the North American Neuroendocrine
Tumor Society consensus guidelines, patients with rectal NETs >2
cm and 1- to 2-cm NETs with muscular invasion or positive LNs are
recommended to undergo radical resection with LN dissection
(7,8). On the other hand, the surgical
indications for rectal NETs ≤1 cm are still unclear. The incidences
of LN metastasis in tumors of various sizes are 1.0% (≤5 mm), 8.4%
(6-10 mm), 54.5% (11-20 mm) and 66.7% (≥21 mm). Based on the depth
of invasion, the incidences of LN metastasis were 11.7% (limited to
the mucosa or submucosa) and 87.5% (into or through the muscularis
propria) (9). In our case, the
tumor was 10 mm in diameter, and the pathological tumor depth was
limited to the submucosal layer. There was no lymphatic invasion,
but venous invasion was observed. Additional surgical resection
including TME might have been indicated after ESD.
The lymphatic tract of the lower rectum below the
peritoneal reflection consists of two patterns: Along the IMA in
the mesorectum and along the internal iliac artery to the lateral
pelvic floor. Accordingly, not only TME but also LLN dissection on
both sides have been performed in Japan for advanced lower rectal
cancer to achieve better local control (10). For rectal NETs, the indications for
LLN dissection and how this approach contributes to patient
prognosis are still unclear. In our case, the patient was
preoperatively diagnosed with LN metastasis in the mesorectum and
in the lateral pelvic floor on the left side from a rectal NET. We
performed robotic TME and bilateral LN dissection following the
treatment strategy for rectal cancer. However, the 13 resected LLNs
on the left side were all negative, and 1 of the 6 LLNs on the
right side was metastatic. With preoperative imaging examinations,
including CT and MRI, we detected 5 enlarged LLNs on the left side.
However, we did not detect LLNs on the right side. Pathological
specimens, which were harvested from the lateral pelvic space,
showed 13 negative LNs on the left side. In contrast, there was a
metastatic LN (7 mm) and 5 negative LNs on the right side (Fig. 4A). A pathological diagnosis of LLN
metastasis was confirmed according to a Ki-67 index of 2% (Fig. 4C). Immunohistochemical analysis for
synaptophysin and chromogranin A of the specimens revealed positive
immunostaining of the tumor cells (Fig.
4B and D). Ushigome et
al reported that 66% of patients who had LLN metastasis from a
rectal NET had no metastatic LNs in the mesorectum. In this study,
LLN dissection was performed for patients with enlarged LLNs >7
mm on preoperative CT or MRI (11).
Tables I and II show 12 reported cases of LLN
metastasis from rectal NETs (12-22).
Synchronous resection of primary rectal NETs and metastatic LLNs
was performed in 8 cases (Table I),
and heterochronous resection was performed in 4 cases (Table II). Seven patients showed no
metastatic LNs in the mesorectum, and nine patients showed a
primary tumor ≤2 cm in diameter. The tumor invasion depth was
limited to the submucosa in 8 cases. Rectal NETs, even those with a
small size and shallow depth of tumor invasion, can metastasize to
the LLNs. Colorectal cancer develops from the mucosal epithelium;
on the other hand, colorectal NETs develop from Kultschitzky cells
that are located in the deep mucosa (23). The difference in origin may
contribute to the slow metastasis to LLNs in the early stage. The
recurrence interval is relatively long (18-276 m), and patients who
undergo radical resection are expected to achieve long-term
survival. Patients with rectal NETs should be followed up with
imaging examinations over an extended period of time. Considering
the characteristics of low-grade malignant potential and very slow
growth of these tumors, watchful observation without LLN dissection
may be an option for rectal NETs. However, if complete surgical
resection is possible, LLN dissection may be an important treatment
option.
 | Table IReported resection cases of rectal
NETs with LLN metastasis. |
Table I
Reported resection cases of rectal
NETs with LLN metastasis.
| Case no. | First author,
year | Age/sex | Tumor size, mm | Depth of
invasion | Lymphovascular
invasion | Number/maximum size
(metastatic LLN), mm | Metastatic LN in the
mesorectum | Prognosis | (Refs.) |
|---|
| 1 | Tokoro et al,
2006 | 53/F | 20 | Muscularis
propria | + | 2/20 | + | 47 m/alive | (17) |
| 2 | Yamada et al,
2007 | 79/F | 8 | Muscularis
propria | - | 1/150 | - | Unknown | (13) |
| 3 | Yamaguchi et
al, 2009 | 44/M | 16 | Submucosa | + | 1/15 | - | 39 m/alive | (15) |
| 4 | Oi et al,
2010 | 46/M | 12 | Submucosa | - | 2/21 | - | 48 m/alive | (18) |
| 5 | Ohno et al,
2005 | 53/F | 10 | Submucosa | + | 1/11 | + | 3 m/alive | (19) |
| 6 | Miyake et al,
2014 | 44/M | 12 | Submucosa | + | 3/55 | + | 19 m/alive | (22) |
| 7 | Beppu et al,
2016 | 59/M | 7 | Submucosa | + | 1/7 | - | 36 m/alive | (20) |
| 8 | The current study,
2020 | 46/M | 10 | Submucosa | + | 1/7 | - | 12 m/alive | |
 | Table IIReported resection cases of LLN
recurrence from rectal NETs. |
Table II
Reported resection cases of LLN
recurrence from rectal NETs.
| Case no. | First author,
year | Age/sex | Tumor size, mm | Depth of
invasion | Lymphovascular
invasion | Number/maximum size
(metastatic LLN), mm | Metastatic LN in the
mesorectum | Recurrence interval
(LLNR) | Prognosis | (Refs.) |
|---|
| 1 | Ichinokawa et
al, 2005 | 57/F | 35 | Muscularis
propria | + | 1/25 | - | 18 m | 44 m/alive | (16) |
| 2 | Nakamoto et
al, 2014 | 70/M | 20 | Submucosa | Unknown | 1/unknown | + | 50 m | 72 m/alive | (12) |
| 3 | Umeda et al,
2016 | 66/M | 7 | Submucosa | - | 1/23 | - | 276 m | 288 m/alive | (14) |
| 4 | Tokumaru et
al, 2020 | 55/M | 14 | Submucosa | - | 1/14 | - | 54 m | 96 m/alive | (21) |
In this case, there were more LLNs on the left side,
and they were larger than those on the right side in the
preoperative examinations; additionally, we diagnosed LLN
metastasis on the left side. However, a metastatic LLN was present
on the right side. On 68Ga-DOTATOC PET-CT, metastatic
LLNs could not be detected. It may be difficult to diagnose LLN
metastasis from rectal NETs with imaging examinations based on size
alone. No evidence-based data on the surgical indications for LLN
metastasis have been published to date. Despite the small sample
size, these findings suggest that radical resection may be
effective for improving prognosis due to the spread of rectal NETs,
their slow growth, their low malignant potential and a lack of
effective chemotherapy options. Rectal NETs are a relatively rare
malignant tumor. A larger sample size and longer observation period
may help establish an optimal treatment strategy for rectal NETs
and LLN metastasis. This case study presented a case of a rectal
NET that metastasized to the LLN. Early-stage rectal NETs can
metastasize to the LLNs despite the characteristics of slow growth
and low malignant potential. It is difficult to detect metastatic
LLNs with preoperative imaging examinations based on size alone due
to the aforementioned characteristics. However, radical resection,
including resection of the metastatic LLNs, may contribute to a
better prognosis, as suggested by the reported cases. It is
exceedingly difficult to determine the surgical indications for
optimally timed LLN dissection. We should keep several options in
mind when planning a treatment strategy for rectal NETs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF collected the patient's clinical data and wrote
the manuscript. KK, SK, SU and HM performed the surgery and
postoperative management. ST made substantial contributions to
conception and design, and revised the manuscript critically. YF
and KK are responsible for confirming the authenticity of the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Kariya Toyota General
Hospital provided approval for the current study due to the written
informed consent obtained (approval no. 642).
Patient consent for publication
The patient provided written informed consent for
the publication of the case details and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fraenkel M, Kim M, Faggiano A, de Herder
WW and Valk GD: Knowledge NETwork. Incidence of
Gastroenteropancreatic neuroendocrine tumours: A systematic review
of the literature. Endocr Relat Cancer. 21:R153–R163.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hallet J, Law CH, Cukier M, Saskin R, Liu
N and Singh S: Exploring the rising incidence of neuroendocrine
tumors: A population-based analysis of epidemiology, metastatic
presentation, and outcomes. Cancer. 121:589–597. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Scherübl H: Rectal carcinoids are on the
rise: Early detection by screening endoscopy. Endoscopy.
41:162–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Konishi T, Watanabe T, Kishimoto J, Kotake
K, Muto T and Nagawa H: Japanese Society for Cancer of the Colon
and Rectum. Prognosis and risk factors of metastasis in colorectal
carcinoids: Results of a nationwide registry over 15 years. Gut.
56:863–868. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kulke MH, Shah MH, Benson AB III,
Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF,
Fanta P, Giordano T, et al: Neuroendocrine tumors, version 1. 2015.
J Nat Comp Cancer Net. 13:78–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ramage JK, De Herder WW, Delle Fave G,
Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W,
Zheng-Pei Z, et al: ENETS consensus guidelines update for
colorectal neuroendocrine neoplasms. Neuroendocrinology.
103:139–143. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Anthony LB, Strosberg JR, Klimstra DS,
Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB III and
Pommier RF: North American Neuroendocrine Tumor Society (NANETS).
The NANETS consensus guidelines for the diagnosis and management of
gastrointestinal neuroendocrine tumors (nets): Well-differentiated
nets of the distal colon and rectum. Pancreas. 39:767–774.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kasuga A, Chino A, Uragami N, Kishihara T,
Igarashi M, Fujita R, Yamamoto N, Ueno M, Oya M and Muto T:
Treatment strategy for rectal carcinoids: A clinicopathological
analysis of 229 cases at a single cancer institution. J
Gastroenterol Hepatol. 27:1801–1807. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ushigome H, Fukunaga Y, Nagasaki T,
Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S and Ueno M:
Difficulty of predicting lymph node metastasis on CT in patients
with rectal neuroendocrine tumors. PLoS One.
14(e0211675)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakamoto T, Koyama U, Nakagawa T, Nakamura
S, Ueda T, Nishigori N, Inoue T, Kawasaki K, Obara S, Fujii H and
Nakajima Y: Four resections of metachronous liver metastases and
lateral lymph node metastases of a rectal carcinoid tumor-a case
report. Jpn J Cancer Chemother. 41:1829–1831. 2014.PubMed/NCBI(In Japanese).
|
|
13
|
Yamada E, Mori A, Nagayama S, Okamoto T,
Koyama T, Ito R and Onodera H: A case of a minute rectal carcinoid
with a huge metastatic obturator lymph node. Jpn J Gastroenterol
Surg. 40:491–496. 2007.(In Japanese).
|
|
14
|
Umeda S, Hishida M, Jinno S, Shimizu M,
Kobayashi H, Nozaki H and Harada T: Lateral lymph node metastasis
of rectal neuroendocrine tumor G1 23 years after transanal
resection. Jpn J Gastroenterol Surg. 49:556–562. 2016.(In
Japanese).
|
|
15
|
Yamaguchi K, Morita T, Okamura K, Kawamura
T and Horita H: A case of rectal carcinoid tumor with a metastatic
lymph node in the right lateral. Jpn Soc Coloproctol. 62:180–184.
2009.(In Japanese).
|
|
16
|
Ichinokawa M, Nakamura Y, Maeyama Y,
Manase H, Taira K and Hishiyama H: A case of a rectal carcinoid
tumor with solitary recurrence to the right lateral lymph node
performed trans-sacral extirpation after systemic chemotherapy. J
Jpn Surg Assoc. 66:3011–3014. 2005.(In Japanese).
|
|
17
|
Tokoro T, Okun K, Hida J, Ishimaru E, Ueda
K, Yoshifuji T, Matsuzaki T, Minami Y and Shiozaki H:
Radiofrequency ablation therapy for multiple liver metastases of
rectal carcinoid-report of a case. Jpn J Gastroenterol Surg.
39:1816–1821. 2006.(In Japanese).
|
|
18
|
Oi K, Fukumoto Y, Nakamura S, Sawata T and
Shimizu T: Rectal carcinoid tumor, 12mm in diameter, with
metastasis to the internal iliac lymph nodes-a case report. J Jpn
Surg Assoc. 71:2398–2401. 2010.(In Japanese).
|
|
19
|
Ohno R, Konshi T, Ueno M, Fukunaga Y,
Nagayama S, Fujimoto Y and Akiyoshi T: A rectal carcinoid tumor
with lateral lymph node metastasis treated by laparoscopic total
mesorectal excision with lateral lymph node dissection: A case
report. Jpn J Endoscopic Surg. 40:200–213. 2005.(In Japanese).
|
|
20
|
Beppu N, Niki M, Kimura F, Matsubara N,
Tomita N, Yanagi H and Yamanaka N: A case of rectal carcinoid, 7 mm
in diameter, with skip metastasis to the lateral lymph node. Mol
Clin Oncol. 4:549–552. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tokumaru Y, Matsuhashi N, Takahashi T,
Imai H, Tanaka Y, Okumura N, Yamaguchi K and Yoshida K: Rectal
neuroendocrine tumor developing lateral lymph node metastasis after
curative resection: A case report. World J Surg Oncol.
18(74)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miyake Y, Hasegawa J, Kim H, Mikata S,
Shimizu J, Kim Y, Hirota M, Soma Y, Miwa H and Nezu R: A case of
rectal carcinoid detected by nodal metastasis. Jpn J Gastroenterol
Surg. 47:357–363. 2014.(In Japanese).
|
|
23
|
Marks C and Lamberty J: The cellular
structure of bronchial carcinoids. Postgrad Med J. 53:360–363.
1977.PubMed/NCBI View Article : Google Scholar
|