Introduction
Primary central nervous system lymphoma (PCNSL) is a
rare intracranial neoplasm. Although the incidence of PCNSL has
increased in patients over 60 years of age in the past two decades;
it currently accounts for a mere 2.4-4.5% of all newly diagnosed
brain tumors (1,2). PCNSL is an uncommon subtype of
extranodal non-Hodgkin lymphoma that involves the central nervous
systems, and the majority of tumors are B-cell phenotypic origin.
The risk factor for the development of PCNSL is immunodeficiency,
however, there are also seems to be increase in incidence of PCNSL
in immunocompetent individuals (2).
PCNSL initially presents with various clinical symptoms, such as
focal neurological deficits, neuropsychiatric symptoms, increased
intracranial pressure, and convulsive seizures (3). Despite the varied clinical
presentation, parkinsonism is extremely rare as an initial clinical
symptom of PCNSL.
Here, we report a rare case of PCNSL presenting with
parkinsonism as an initial clinical symptom in an older adult, and
emphasize the difficulty in distinguishing tumor-associated
parkinsonism (TAP) from vascular parkinsonism (VP) during the
initial diagnosis.
Case report
A 75-year-old man without any history of
immunocompromise was referred to our hospital due to bradykinesia,
discrete movement disorder, and low speech for a month prior to
referral. Before admission, the patient developed a broad base gait
disturbance and a pill-rolling tremor, both of which were evident
upon examination at the time of admission. (Fig. 1). Magnetic resonance imaging (MRI)
with fluid-attenuated inversion recovery (FLAIR) showed multiple
hyperintense lesions in the left basal ganglia, thalamus, and
bilateral frontal periventricular white matter (Fig. 2A and B). A computed tomography (CT) showed
low-density lesions in the bilateral periventricular white matter
and a slightly hyperdense lesion in the left thalamus (Fig. 2C). A dopamine transporter single
photon emission computed tomography (DAT SPECT) showed a decrease
in 123I-Ioflupane uptake in the left striatum (Fig. 2D). Considering the chronic ischemic
changes suspected from the MRI and DAT SPECT findings, we
classified his condition as VP and discharged the patient with
frequent follow-up instructions. One month later, the patient's
condition further deteriorated, presenting with impairment of the
postural reflex, a mask-like face, and micrographia. Due to the
rapid progression of VP, he underwent reexamination by MRI,
revealing enlargement of the hyperintense lesions in the left
thalamus and frontal periventricular white matter with
heterogeneous enhancement (Fig.
2E-H). He was subsequently admitted to our department, with a
Karnofsky Performance Status (KPS) at admission of 40. Serological
examinations showed mildly elevated interleukin-2 receptor levels
(657 U/ml) and normal levels of lactate dehydrogenase (161 U/l).
His HIV antibody test results were negative. Because of the rapid
and atypical progression of the disease, we suspected TAP due to
the brain lesion and performed a stereotactic biopsy for the left
frontal periventricular lesion two months after onset of symptoms.
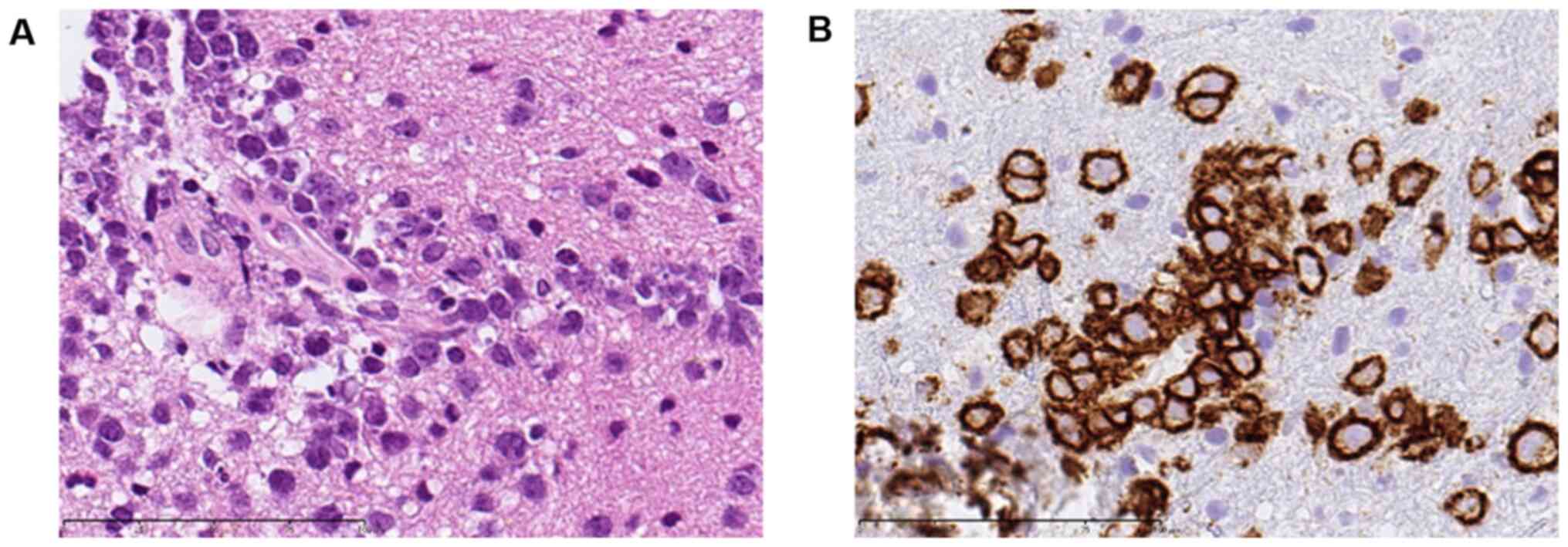
The pathological examination revealed proliferation of medium- to
large-sized atypical lymphoid cells around the small vessels. These
tumor cells strongly expressed CD20, corresponding to a diffuse
large B-cell lymphoma (DLBCL) (Fig.
3). A genetic analysis was not performed due to limitations in
the examination. Given this report, we diagnosed his condition as
TAP in DLBCL, and he immediately underwent corticosteroid therapy.
His condition rapidly improved with treatment, and his KPS
immediately after initial treatment improved to 70. He then
underwent multidrug chemotherapy with rituximab, methotrexate,
procarbazine, and vincristine two weeks after surgery. One month
after treatment, the patient had completely recovered from TAP and
was discharged without any neurological deficits. Follow-up MRI
without contrast enhancement (four months after treatment) revealed
that the lesion in the left thalamus had disappeared and the
periventricular lesion had shrunk (Fig.
4A-D). He received seven cycles of chemotherapy, and ultimately
achieved complete response. Eight months after treatment, his KPS
remained at 70, and he had no recurrence of TAP.
Literature review
PCNSL presents various clinical symptoms, such as
focal neurologic deficit in 70% of patients, behavioral
neuropsychiatric changes in 43%, headache, nausea, and vomit
associated with elevated intracranial pressure in 33%, and seizures
in 14% (3). However, TAP in PCNSL
is extremely rare, and only 5 cases, including ours, have been
reported so far (Table I) (4-7).
TAP in PCNSL is relatively common in older patients, with a mean
age at diagnosis of 74.2 (range, 64–81) years, and no sex
preference, with three male and two female patients. Several
clinical symptoms associated with parkinsonism were described. All
patients presented with bradykinesia and gait disorder, followed by
rest tremor, rigidity and fixed face in 60%, impairment of the
postural reflex in 40%, and abnormal posture in only one case at
initial treatment. Three patients (60%) demonstrated laterality of
clinical symptoms; however, two cases (40%) presented with
bilateral symptoms. Most cases presented with lesions at multiple
sites, including the basal ganglia in 80% (putamen and globus
pallidus in 60%, thalamus in 20%), the periventricular white matter
in 40%, and the corpus callosum in 20% of lesions. Only one case
had a single lesion in the midbrain. Levodopa, which was started as
initial treatment in three cases (60%), was not effective. As TAP
is a rare clinical symptom and course of PCNSL, and because precise
diagnosis is typically delayed (duration: Over seven months), the
prognosis is poor in most cases.
 | Table ISummary of tumor-associated
parkinsonism in PCNSL. |
Table I
Summary of tumor-associated
parkinsonism in PCNSL.
| First author,
year | Case | Age, years/sex | Lesion site | Clinical
symptoms | Response to
Levodopa | Treatment after
diagnosis of PCNSL | Duration from
diagnosis, months | Prognosis (period
from onset) | Refs. |
|---|
| Pramstaller et
al, 1999 | 1 | 75/M | Basal ganglia | Bradykinesia, gait
disorder, abnormal posture, fixed face | No response | MTX, corticosteroid,
radiotherapy | 5 | Dead (7.5
months) | (6) |
| Sánchez-Guerra et
al, 2001 | 2 | 64/F | Basal ganglia, corpus
callosum, periventricular white matter | Tremor, bradykinesia,
rigidity, fixed face | No response | Corticosteroid | 10 | Dead (10 months) | (7) |
| Lin and Hong,
2010 | 3 | 81/M | Midbrain | Tremor, bradykinesia,
rigidity, postural instability, gait disorder, fixed face | Partial response | Declined
treatment | No listed | Transferred to
hospice (not listed) | (4) |
| Nagarajan et
al, 2020 | 4 | 76/F | Basal ganglia | Bradykinesia,
rigidity, gait disorder | Not used | MTX as initial
treatment, radiotherapy after recurrence | 8 | Recurrence (4
months), maintain remission (8 months) | (5) |
| Present study | 5 | 75/M | Basal ganglia,
periventricular white matter | Tremor, bradykinesia,
postural instability, gait disorder | Not used | R-MPV | 2 | Maintain remission (8
months) | - |
The first case of brain tumor with parkinsonism was
reported in 1953, after then, several cases of TAP, which caused by
meningioma, glioma, cavernoma, and other tumors, have sporadically
been reported (8-11).
Whereas, to the best of our knowledge, there are only 5 cases of
TAP in PCNSL in the literatures as previously mentioned. Therefore,
those clinical features are not well known, and TAP in PCLNS may be
underestimated, clinicians should be aware of the risk of TAP in
PCNSL.
Discussion
The clinical features of TAP in PCNSL are not well
known because of their rare nature; however, it is important for
diagnosis and treatment. Parkinson's disease (PD), which presents
as bradykinesia with one additional symptom, such as rigidity,
resting tremor, and postural instability, are well known. These
tend to be unilateral initially, and present as persistent
asymmetrical symptoms. In the beginning, symptoms are evident on
one side of the body with the contralateral symptoms appearing
within a few years (12). A
previous report also described the clinical features of VP. The
predominant manifestation is described as lower body parkinsonism,
including gait disturbance and postural instability, which is
similar to that observed in patients with PD. The progression of
symptoms is variable in VP with an acute onset in some cases. These
clinical symptoms commonly remain stable or deteriorate slowly
(13). However, TAP in PCNSL
progresses rapidly compared to PD and VP.
In the present case, the initial manifestation was
bradykinesia and low voice. As parkinsonism progressed, clumsiness,
gait disturbance, and pill rolling movement appeared within month
after initial onset. Unilateral symptoms were observed in only
three cases, and may be associated with multiple lesions at
diagnosis in most cases. In addition, diurnal fluctuations of
symptoms were not observed in the present case, which may have led
to the distinction between PD and TAP. Levodopa was not
administered in the present case. Generally, an excellent response
to levodopa is a supportive prospective criterion in PD (12). However, it has been demonstrated to
be ineffective or only partially responsive in other TAP cases.
Thus, in the case of rapid progression of parkinsonism or
ineffective levodopa, we should suspect the possibility of TAP of
PCNSL in older patients with basal ganglia lesions.
It is also challenging but critical to distinguish
between cerebral infarction (CI) and PCNSL in the early stages of
disease. CI commonly occurs in the basal ganglia, including the
thalamic area. In particular, older adult patients develop chronic
cerebral ischemia in the white matter of the cerebrum, including
the thalamus, leading to VP (13).
In contrast, brain tumors, such as gliomas, germinomas, and
teratomas, rarely occur and are localized to the thalamic area.
Although PCNSL commonly develops in the periventricular region,
white matter, and corpus callosum, 17% of PCNSLs are found in the
thalamus and basal ganglia (14,15).
It may be difficult to distinguish between PCNSL and CI of
localized lesions in the thalamus using conventional MRI without
contrast reagent. Generally, PCNSL presents as iso- or hypointense
on T1-weighted image (T1WI) and iso- or hyperintense on T2-weighted
image (T2WI)/FLAIR images (15).
Chronic CI, including lacunar and white matter lesions are
hyperintense on T2WI/FLAIR in ordinary cases. Although CE-T1WI
depicts the characteristic homogenous enhancement on PCNSL,
contrast-enhanced imaging is not necessarily used as a routine
examination in cases suspected of CI at primary screening. However,
the PCNSL lesion presents as slightly iso- or hyperdense on CT,
which is related to a very high nuclear/cytoplasmic ratio, in
contrast with CI lesions that show as hypodense (16). In our case, MRI revealed a localized
hyperintensity in the thalamus on FLAIR, thus leading to the
initial diagnosis of VP. Retrospectively, initial CT showed an iso-
to slightly hyperdense thalamic lesions; thus, we should have
suspected the possibility of PCNSL. While rare, we suggest that
PCNSL should be considered as a differential diagnosis in localized
thalamic lesions in older adults, even though it presents in.
The mechanism of TAP in PCNSL remains unclear. Brain
tumors presenting with TAP occur in various locations, such as the
brain stem, basal ganglia, frontal lobe, temporal lobe, cerebellum,
hypothalamus, sella, and pineal regions (8,9,17-19).
Generally, PD is associated with cell loss in the substantia nigra,
whereas only 31% of brain lesions presenting with parkinsonism
include the substantia nigra (20).
Brain tumors disrupt the neuronal circuits, such as the axons of
the presynaptic dopaminergic neuron and the output pathway from
postsynaptic cells of the basal ganglia circuit to the cortex,
either by the mass effect of the tumor itself or by peritumoral
brain edema, leading to the development of parkinsonism (21). In PCNSL, tumor cells invade and
damage the neuronal membranes in the basal ganglia, resulting in
the development of TAP (22). In
the present case, PCNSL located in the periventricular white
matter, basal ganglia, and thalamus, and 123I-Ioflupane
SPECT showed decreased 123I-Ioflupane uptake in the left
basal ganglia, which then developed TAP. Generally, PD causes
distinctive neuropathological brain changes, such as the formation
of abnormal proteinaceous spherical bodies called Lewy bodies, and
drug response becomes limited with disease progression (12). However, TAP recovered following
treatment for PCNSL. Thus, PCNSL induces reversible dysfunction of
neuronal circuits in the thalamus, basal ganglia, and cortex of the
cerebrum and midbrain during invasion within the perivascular
space, triggering the development of TAP. Due to the high tumor
proliferation of PCNSL, TAP progresses rapidly. Although a precise
diagnosis of TAP may be difficult at initial diagnosis, TAP should
be suspected and diagnosed as early as possible, and appropriate
treatment for tumors should be administered before the physical
manifestations of TAP become irreversible.
In conclusion, Parkinsonism is rarely an initial
manifestation of PCNSL. In contrast, VP is common among older
adults. Due to its rarity, it is often challenging to distinguish
between TAP and VP at initial consultation. TAP should be suspected
if the clinical symptoms of parkinsonism progress rapidly, and
thus, physicians should consider repeat MR examination under close
observation in these cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RO and KS drafted the manuscript and wrote the final
paper. YN and JY made substantial contributions to conception and
design. YN and JY drafted parts of the manuscript, revised the
content critically and provided constructive feedback. KS and YN
performed the surgery. RO and KS analyzed all images. KS and JY
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Oral and written informed consent was obtained from
the patient for the publication of the case details and any
associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schlegel U: Primary CNS lymphoma. Ther Adv
Neurol Disord. 2:93–104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grommes C and DeAngelis LM: Primary CNS
lymphoma. J Clin Oncol. 35:2410–2418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin CM and Hong K: Cerebral infratentorial
large B-cell lymphoma presenting as Parkinsonism. Tohoku J Exp Med.
220:187–190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nagarajan E, Yerram SY, Digala LP and
Bollu PC: Primary central nervous system lymphoma presenting as
parkinsonism with atypical MRI findings and elevated 14-3-3
protein. J Neurosci Rural Pract. 11:492–494. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pramstaller PP, Salerno A, Bhatia KP,
Prugger M and Marsden CD: Primary central nervous system lymphoma
presenting with a parkinsonian syndrome of pure akinesia. J Neurol.
246:934–938. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sánchez-Guerra M, Cerezal L, Leno C, Diez
C, Figols J and Berciano J: Primary brain lymphoma presenting as
Parkinson's disease. Neuroradiology. 43:36–40. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Al-Janabi WSA, Zaman I and Memon AB:
Secondary parkinsonism due to a large anterior cranial fossa
meningioma. Eur J Case Rep Intern Med. 6(001055)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Connolly ID, Johnson E, Lummus S and
Hayden Gephart M: Massive intradural chondroma masquerading as
lower body parkinsonism. Cureus. 10(e2099)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ertan S, Benbir G, Tanriverdi T, Alver I
and Uzan M: Parkinsonism caused by cavernoma located in basal
ganglion. Parkinsonism Relat Disord. 11:517–519. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Margulies ME: Parkinsonism and brain
tumor. J Nerv Ment Dis. 117:550–552. 1953.PubMed/NCBI
|
|
12
|
Sveinbjornsdottir S: The clinical symptoms
of Parkinson's disease. J Neurochem. 139:318–324. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Korczyn AD: Vascular
parkinsonism-characteristics, pathogenesis and treatment. Nat Rev
Neurol. 11:319–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Linn J, Hoffmann LA, Danek A and Brückmann
H: Differential diagnosis of bilateral thalamic lesions. Rofo.
179:234–245. 2007.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
15
|
Renard D, Castelnovo G, Campello C, Bouly
S, Le Floch A, Thouvenot E, Waconge A and Taieb G: Thalamic
lesions: A radiological review. Behav Neurol.
2014(154631)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haldorsen IS, Espeland A and Larsson EM:
Central nervous system lymphoma: Characteristic findings on
traditional and advanced imaging. AJNR Am J Neuroradiol.
32:984–992. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Choi KH, Choi SM, Nam TS and Lee MC:
Astrocytoma in the third ventricle and hypothalamus presenting with
parkinsonism. J Korean Neurosurg Soc. 51:144–146. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duron E, Lazareth A, Gaubert JY, Raso C,
Hanon O and Rigaud AS: Gliomatosis cerebri presenting as rapidly
progressive dementia and parkinsonism in an elderly woman: A case
report. J Med Case Rep. 2(53)2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yasuhara T, Agari T, Kambara H, Ichikawa
T, Kurozumi K, Ono S, Miyoshi Y and Tokunaga K: Isao Date.
Parkinsonism related to brain tumors: A case report and review of
the literature. Open Neurosurgery J. 2:4–7. 2009.
|
|
20
|
Joutsa J, Horn A, Hsu J and Fox MD:
Localizing parkinsonism based on focal brain lesions. Brain.
141:2445–2456. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JI, Choi JK, Lee JW and Hong JY:
Intracranial meningioma-induced parkinsonism. J Lifestyle Med.
4:101–103. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Merrill S, Mauler DJ, Richter KR,
Raghunathan A, Leis JF and Mrugala MM: Parkinsonism as a late
presentation of lymphomatosis cerebri following high-dose
chemotherapy with autologous stem cell transplantation for primary
central nervous system lymphoma. J Neurol. 267:2239–2244.
2020.PubMed/NCBI View Article : Google Scholar
|