Introduction
Multilocular Cystic Nephroma (MCN) is an uncommon
clinical entity; it looks like a well-circumscribed encapsulated
mass with numerous locules and septa. The etiology of MCN is
unclear and its histogenesis is arguable. This tumor type has been
nicknamed in the past as multilocular cystic tumor, renal
multilocular cyst, multilocular cystic nephroma, renal cystadenoma
and partial polycystic kidney (1,2) and it
has been considered as a developmental lesion with malignancy
potential. Approximately 200 cases have been described in the
literature (3). The first of these
was reported in 1892 by Edmunds (4): He removed a Cystic Nephroma of the
kidney from an 18-year-old female. Recent advances in diagnostic
imaging have resulted in an increased awareness of this type of
renal tumor, and surgical intervention is an operative method for
treating malignant cystic lesions of the kidney. However,
nephron-sparing surgery may be an option depending on the site and
size of the lesion.
The present study reports a case of a 31-year-old
woman, who was determined to have a Cystic Nephroma while the
underlying cause of a protracted intermittent right renal painful
condition was investigated.
Case report
A 31-year-old woman was admitted to the Division of
Urology with a history of intermittent right-flank pain. She
disclaimed other eliminating complaints and did not exhibit other
significant urological diseases. The physical examination did not
reveal any other significant symptoms except for a mild knocking
pain in the right kidney area. Laboratory examinations revealed
only the presence of microscopic hematuria, and so the urinalysis
showed a mild quantity of red blood cells in the sediment. Urine
cytology was negative for malignancy. To investigate potential
causes of this intermittent right-flank pain the patient underwent
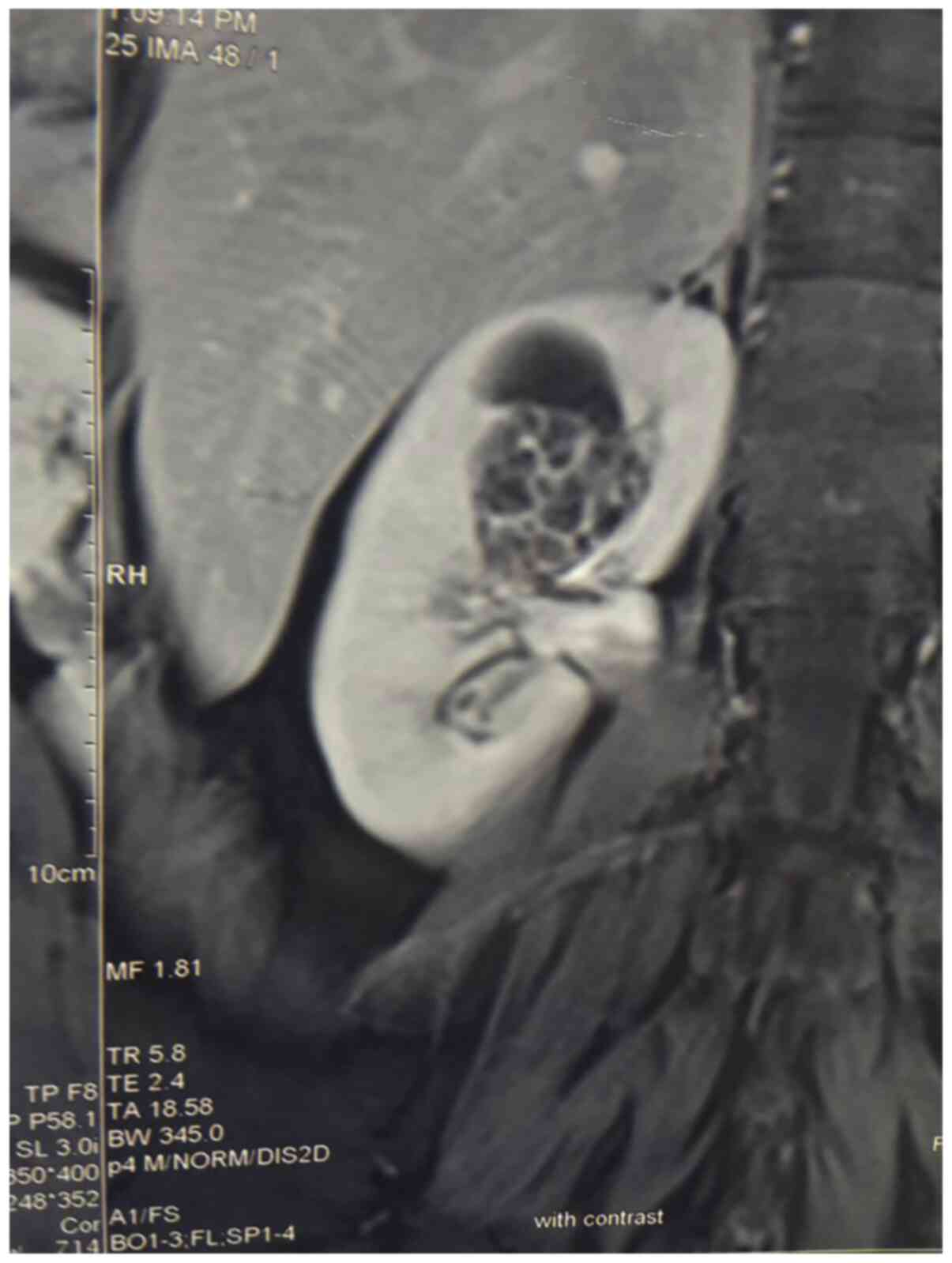
abdominal ultrasound (US) imaging that showed a well-demarcated,
complex cystic growth, with a maximum diameter of ~3 cm in the
upper pole of the right kidney. Ascending pyelography imaging
revealed a large defect in the right renal pelvis. Computed
tomography (CT) confirmed the presence of this growth, which
originated from the renal parenchyma and extended into the right
renal pelvis, as shown in Fig.
1.
In addition, the computed tomography revealed the
presence of calcifications in the cyst without solid components. An
enhanced CT scan showed a cystic lesion. In this case the
obstruction by pelvic herniation of the tumor produced delayed
excretion with hydrocalycosis or no visualization. Distinguishing a
malignant mass from a benign lesion was not possible with the
imaging techniques. Consequently, in order to remove the tumor,
according to the clinical and radiological findings, the
nephron-sparing surgery was performed. By an open right flank
approach the kidney was isolated with its vascular pedicle. The
vascular pedicle was scheletonized and the renal artery was
clamped; opening the upper pole of the kidney, a translucent,
regular mass was noted in the upper calyx as presented in Fig. 2. The mass was completely isolated
and its little pedicle was ligated: The mass was completely
removed, saving the rest of the kidney. Then, the renal parenchima
was sutured with continuous vicryl 000 suture. The entire procedure
was performed in 20 min; then the artery was declamped and the
bleeding was controlled. No lymphadenopathy or metastatic disease
was present. Upper pole partial nephrectomy included the whole
mass, with an area of normal appearing renal tissue. The mass
originated in the renal parenchyma; however, it extended into the
renal pelvis on a pedicle and the tumor bulk was entirely located
within the renal pelvis. No malignancy was highlighted on a frozen
section. The lesion was completely removed as presented in Fig. 3 and the rest of the kidney was
saved.
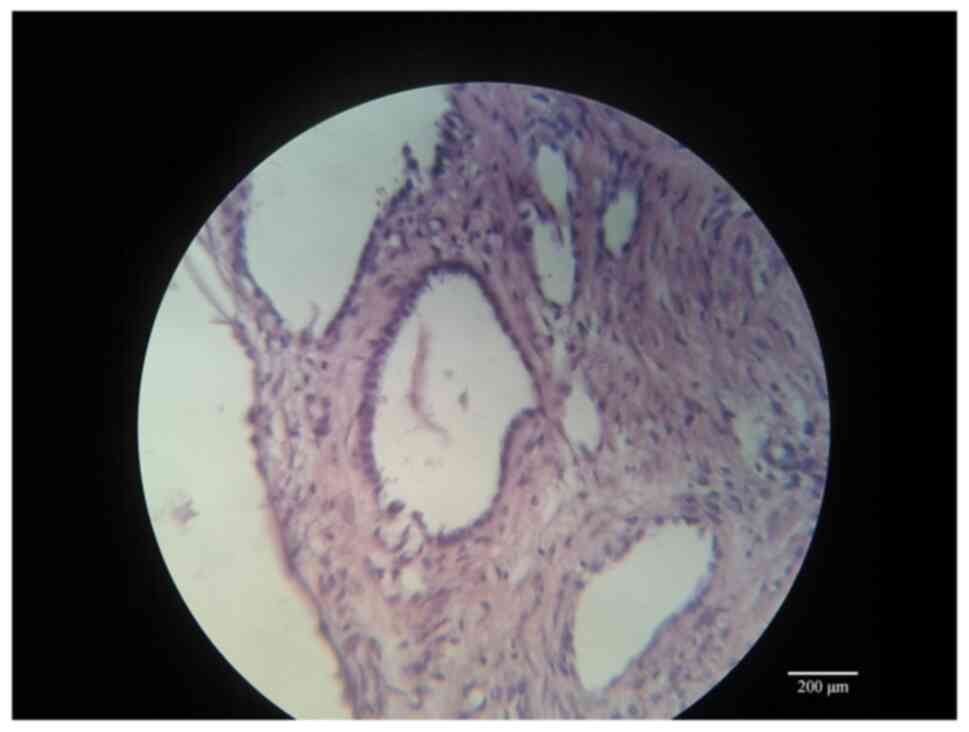
The final histopathological diagnosis was Cystic
Nephroma as shown in Figs. 4,
5 and 6 where large cystic formations with clear
serous content are visible in a moderately vascularized fibrous
stroma. Their lining epithelium is of a single-layered cubic type,
without atypia and mitotic figures. The result, therefore, is a
cystic formation, with a serous content, pure-chambered, with septa
of varying thickness, whose external surface appears smooth and
free of lesions.
The patient was discharged at 5 days post-operation.
The vascular, ureteral, renal pelvis and capsular surgical margins
were not affected by the tumor. After a 4-year follow-up, the
patient was completely asymptomatic, without recurrence and
metastasis.
Discussion
Cystic Nephroma is a rare benign lesion of the
kidney and approximately 200 cases have been described in the
literature. For the first time, this lesion was described in 1892
by Edmunds as ‘cystadenoma of the kidney’ (4). In most cases the lesion is
asymptomatic and discovered incidentally during a radiological
investigation performed for other reasons or sometimes when the
patient presents with non-specific urinary tract symptoms in
adulthood or with an abdominal growth in childhood (5). Several proposed theories explain the
etiology of Cystic Nephroma, considering it as a developmental
defect (6). Others postulated that
it could have neoplastic origin, likely arising from the ureteral
bud. Therefore, the etiology of Cystic Nephroma has always been
controversial (7), with the debate
centering on whether this lesion is neoplastic or developmental in
origin. Those who argue for a developmental origin suggest that
Cystic Nephroma is a form of renal dysplasia, probably related to
polycystic kidney disease, or a result of maldevelopment of the
ureteric bud. According to others Cystic Nephroma arises from
misplaced Mullerian stroma or it is a hamartomatous malformation.
Many currently, like us, believe that Cystic Nephroma is a
neoplasm. In the field of renal neoplasm, the term Cystic Nephroma
has historically been problematic (8). As first deduced and asserted by John
Eble in 1994(9), the term ‘Cystic
Nephroma’ has been used to refer to two apparently distinct lesions
(7). The first, adult Cystic
Nephroma, typically affects adult females (suggesting an
association with circulating hormones) and has been thought by many
to be the highly cystic end of the spectrum of Mixed Epithelial
Stromal Tumor (MEST) (10). In
contrast, pediatric Cystic Nephroma typically affects very young
children (usually below 24 months of age) and has traditionally
been thought to be part of the spectrum of cystic nephroblastic
lesions that includes cystic partially differentiated
nephroblastoma and cystic Wilms tumor. In the 2004 World Health
Organization (WHO) classification of renal neoplasm, pediatric
Cystic Nephroma is not recognized as a distinctive entity (11), instead considering adult Cystic
Nephroma as a separate entity classified under soft tissue tumors
of the kidney (12). Pediatric
Cystic Nephroma is now considered a distinctive entity, associated
with mutations in the DICER1 gene (13). Germline DICER1 mutations have been
identified in young patients with pleuropulmonary blastoma and its
other associated neoplasms, including pediatric Cystic Nephroma;
this constellation of lesions is now termed DICER1 syndrome
(14). Whether adult Cystic
Nephroma should be grouped with MEST remains controversial. Some
authors believe that these are distinctive entities, based upon the
ability to separate lesions into one of these two categories in
most cases (MEST being more solid and complex than Cystic
Nephroma), with different morphology, immunoprofile (frequent
smooth muscle differentiation in MEST and inhibin labeling in
Cystic Nephroma) (15) and etiology
(according to some MEST may not have appeared until after 1950 and
may be linked to exposure to exogenous hormones such as oral
contraceptives) (16). The current
2016 WHO Classification states that ‘on the basis of similar age
and sex distributions, as well as similar immunohistochemical
profile and overlapping histologic features, adult Cystic Nephroma
is now classified within the spectrum of MEST family’ (17). Moreover, although the vast majority
of adult Cystic Nephroma/MEST lack DICER1 mutations, rare cases
currently classified as adult MEST may have DICER1 alterations,
which likely are sporadic. Such lesions probably originated as
Cystic Nephroma in childhood, but remained undetected until adult
life and perhaps underwent morphologic changes (such as smooth
muscle metaplasia) in the intervening years. Hence, DICER1 mutation
status does not absolutely distinguish adult and pediatric Cystic
Nephroma in all cases. In fact, also considering that ropy collagen
and inhibin immunoreactivity are far more common in adult Cystic
Nephroma/MEST than in pediatric lesion (being cellular stroma and
estrogen receptor immunoreactivity commonly present in both cases),
we argue that the marked differences in age, sex predominance,
morphology and immunohistochemical profile support the current WHO
Classification's separation of adult and pediatric cystic nephromas
as distinct entities. The patient in the current study was a
31-year-old female, affected by intermittent right-flank pain and
presenting with microscopic hematuria.
In 1956 Boggs and Kimmelstiel proposed these
diagnostic criteria for a multilocular cyst (18): i) a multilocular growth; ii) the
absence of communication not only between cysts but also between
cysts and pelvis; iii) cysts lined by epithelium; iv) residual
kidney essentially normal; and v) the absence of normal nephrons in
the septa of cysts. In 1989 Joshi and Beckwith modified these
criteria, specifying that: i) the lesion is composed entirely of
cysts and their septa; ii) Cystic Nephroma is a lesion with
separate and well-demarcated growth; iii) septa are the only solid
components which conform to the outlines of the cyst without
expansive nodules; iv) cysts are lined by flattened, cuboidal or
hobnail epithelium; and v) septa contain fibrous tissue in which
well-differentiated tubules may be present (19). The differential diagnosis of a
cystic renal growth varies from adults to children, including
several lesions such as polycystic kidney, nephroblastomas, Wilms'
tumour, hydronephrotic kidney, mesoblastic nephroma and cystic
renal cell carcinoma. Furthermore, due to the presence of
Echinococcus granulosus in some countries, the Cystic Nephroma must
be distinguished from the hydatid cyst (for which the treatment is
medical). In some studies, the authors highlighted the coexistence
of renal cell carcinoma or focal renal cell carcinoma with the
Cystic Nephroma (20,21). The reason for this coexistence may
be the potential malignant transformation of cystic epithelium or
keratin positive stromal cells. Osathanondh and Potter, considering
the lesion as a cyst, included Cystic Nephroma under ‘type 2
polycystic kidneys’ (22). However,
we consider that Cystic Nephroma is a neoplasm; in effect, some
studies showed that the tumor cells have multipotentiality in
cellular differentiations and others reported the possibility of
malignant transformation such as Raj et al who described a
malignant Cystic Nephroma in an asymptomatic man (23). In addition, according to the Bosniak
classification, for a type III cystic lesion (such as Cystic
Nephroma) the probability of a malignant transformation is ~55%,
justification for a resection of the cystic mass itself (24). For these reasons, the total excision
of cystic nephroma is to be recommended. On the other hand,
reliably distinguishing the Cystic Nephroma from a malignant lesion
of the kidney by preoperative imaging or gross examination is very
difficult. In effect, although the literature reports distinct
radiographic features, these are not universally present in all
cases. Imaging studies, such as US and CT, usually show the
multilocular nature of the Cystic Nephroma; however, the
differential diagnosis between a class II and III cyst, based on
the Bosniak classification, can be problematic. Therefore, surgical
intervention is necessary for both diagnosis and treatment.
According to the literature, nephrectomy is an adequate treatment
and it does not need any chemotherapy and radiotherapy (25,26).
In addition, the nephron-sparing technique can be an adapted choice
to treat the lesion (27). In fact,
our inability to classify the renal mass as benign using common
pre-operative imaging techniques has led us to adopt the same
therapeutic strategy as for renal cell carcinoma. To date, partial
nephrectomy, when surgically possible, is the first treatment
option for T1 tumors. In effect, the EAU guidelines strongly
recommend that consideration be given to performing a partial
nephrectomy in patients with T1 cancer (28). According to the current Tumor Node
Metastasis (TNM) staging system, stage T1 is defined as a tumor
limited to the kidney, with dimensions equal to or <7 cm
(29). The renal mass reported in
our clinical case, by virtue of its size, was classified as a stage
T1a, justifying the choice of a conservative intervention. In
addition, it should be noted that the nephron-sparing surgery for a
type III Bosniak cyst was also warranted in view of the young age
of the patient, the result of the extemporaneous histological
examination during the operation and the associated symptoms
(hematuria but also recurrent episodes of renal colic), which would
hardly regress without surgery. Rather, due to the tendency of the
cystic mass to increase in size with the passage of time, an
exacerbation of the symptoms itself would certainly have occurred.
In addition, regarding the comparison between surgery and
preoperative histological correlation using renal biopsy in the
case of an indeterminate cystic mass (such as Cystic Nephroma),
some authors recommend biopsy. However, in the case of biopsy, the
possibility of false negatives due to the small number of malignant
cells in the cystic mass, the risk of seeding along the needle path
and the risk of rupture of the cyst with spread of malignant cells
must be considered. Therefore, the renal biopsy should only be
performed when there are clinical grounds for suspecting that the
mass is inflammatory (in case of pyuria, not present in our case)
or when there are radiological signs suggestive of inflammation
(hyperdensity of the perirenal adipose tissue, a sign not present
in the images CT scan of the patient). To support this approach,
the EAU guidelines offer only a weak recommendation to perform a
preoperative kidney biopsy in patients with unclear kidney lesions
(28). Therefore, although
published reports consider nephrectomy as the classical surgical
technique to treat the MCN (30,31),
we suggest that nephron-sparing surgery, when surgically possible,
may be the best choice of treatment when the diagnosis of Cystic
Nephroma is suspected preoperatively and verified intraoperatively.
A definitive diagnosis can typically be made from the result of the
pathological examination when the operation is over. If the removed
lesion is of benign nature, only surveillance is necessary after
surgery.
In conclusion, considering the young age of the
patient, the case described above has been singular for the choice
of partial nephrectomy as a treatment modality. In addition, the
study case presented another unique characteristic: The direct
tumor extension into the renal pelvis through a calyx. This growth
pattern may be a singular feature of MCN. In this case, the lesion
was localized within the renal parenchyma but, as described in only
two other cases (32,33), herniation into the renal pelvis had
occurred. Although rare, Cystic Nephroma must be kept in mind in
the differential diagnosis of a renal mass. Because the age of
presentation ranges from infancy to adulthood, both pediatric and
adult surgeons may be called on to diagnose and treat Cystic
Nephroma. In summary, the present study reports a case of a MCN
with an unusual localization and for which the combination of
clinical and radiological findings may help in lesion
characterization, but only histology can provide the definitive
diagnosis. We advocate the use of nephron-sparing technique as the
most appropriate surgical treatment method for MCN when the
diagnosis is suspected pre-operatively and verified
intra-operatively on frozen section analysis; in the present case,
performing a conservative surgical treatment, the kidney function
was kept intact (a fundamental consideration when patients have a
long life expectancy).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MS and NS analyzed and interpreted the patient data
regarding the urological disease. RB was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Signed informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuzgunbay B, Turunc T, Bolat F and Kilinc
F: Adult cystic nephroma: A case report and a review of the
literature. Urol Oncol. 27:407–409. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Falidas E, Ntasi A, Mathioulakis S,
Vlachos K, Anyfantakis G, Boutzouvis S and Villias C: Multicystic
nephroma in an elderly man. Case report. G Chir. 32:483–486.
2011.PubMed/NCBI
|
|
3
|
Wilkinson C, Palit V, Bardapure M, Thomas
J, Browning AJ, Gill K and Biyani CS: Adult multilocular cystic
nephroma: Report of six cases with clinical, radio-pathologic
correlation and review of literature. Urol Ann. 5:13–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Edmunds W: Cystic adenoma of the kidney.
Trans Pathol Soc London. 43:89–90. 1892.
|
|
5
|
Castillo OA, Boyle ET and Kramer SA:
Multilocular cysts of kidney. A study of 29 patients and review of
literature. Urology. 37:156–162. 1991.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sacher P, Willi UV, Niggli F and Stallmach
T: Cystic nephroma: A rare benign renal tumor. Pediatr Surg Int.
13:197–199. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eble J and Bonsib SM: Extensively cystic
renal neoplasms: Cystic nephroma, cystic partially differentiated
nephroblastoma, multilocular cystic renal cell carcinoma and cystic
hamartoma of the renal pelvis. Semin Diagn Pathol. 15:2–20.
1998.PubMed/NCBI
|
|
8
|
Kajani N, Rosenberg BF and Bernstein J:
Multilocular cystic nephroma. J Urol Pathol. 1:33–42. 1993.
|
|
9
|
Eble JN: Cystic nephroma and cystic
partially differentiated nephroblastoma: Two entities or one? Adv
Anat Pathol. 1:99–102. 1994.
|
|
10
|
Adsay NV, Eble JN, Srigley JR, Jones EC
and Grignon DJ: Mixed epithelial stromal tumor of the kidney. Am J
Surg Pathol. 24:958–970. 2000.
|
|
11
|
Eble J: Cystic partially differentiated
nephroblastoma. In: World Health Organization Classification of
Tumours Pathology and Genetics of Tumours of the Urinary System and
Male Genital Organs. Eble JN, Sauter G, Epstein JI and Sesterhenn
IA (eds). IARC Press, Lyon, p55, 2004.
|
|
12
|
Bonsib SM: Cystic nephroma. In: World
Health Organization Classification of Tumours Pathology and
Genetics of Tumours of the Urinary System and Male Genital Organs.
Eble JN, Sauter G, Epstein JI and Sesterhenn IA (eds). IARC Press,
Lyon, p76, 2004.
|
|
13
|
Argani P, Bruder E and Dehner L:
Paediatric cystic nephroma. In: World Health Organization
Classification of Tumors: Pathology and Genetics of Tumors of the
Urinary System and Male Genital Organs. 4th edition. Moch H,
Humphrey PA, Ulbright TM and Reuter VE (eds). IARC Press, Lyon,
p52, 2016.
|
|
14
|
Bahubeshi A, Bal N, Rio Frio T, Hamel N,
Pouchet C, Yilmaz A, Soglio DB, Williams GM, Tischkowitz M, Priest
JR and Foulkes WD: Germline DICER1 mutations and familial cystic
nephroma. J Med Genet. 47:863–866. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Caliò A, Eble JN, Grignon DJ and Delahunt
B: Cystic nephroma in adults: A clinicopathologic study of 46
cases. Am J Surg Pathol. 40:1591–1600. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Srigley JR, Delahunt B, Eble JN, Egevad L,
Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et
al: ISUP renal tumor panel. The international society of urological
pathology (ISUP) vancouver classification of renal neoplasia. Am J
Surg Pathol. 37:1469–1489. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Michal M, Amin MB and Delahunt B: Mixed
epithelial stromal tumor family. In: World Health Organization
Classification of Tumors: Pathology and Genetics of Tumors of the
Urinary System and Male Genital Organs. 4th edition. Moch H,
Humphrey PA, Ulbright TM and Reuter VE (eds). IARC Press, Lyon,
pp70-71, 2016.
|
|
18
|
Boggs LK and Kimmelstiel P: Benign
multilocular cystic nephroma: Report of two cases of so-called
multilocular cyst of the kidney. J Urol. 76:530–541.
1956.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Joshi VV and Beckwith JB: Multilocular
cyst of the kidney (cystic nephroma) and cystic, partially
differentiated nephroblastoma. Terminology and criteria for
diagnosis. Cancer. 64:466–479. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Wall JG, Schroder FH and Scholtmeijer
RJ: Diagnostic workup and treatment of multilocular cystic kidney.
Difficulties in differentialdiagnosis. Urology. 28:73–77.
1986.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Posso M, Safadi D and Van Dyk OJ:
Unilateral polycystic or multicystic kidney associated with focal
mural renal cell carcinoma: Presentation of a case. J Urol.
109:559–563. 1973.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Osathanondh V and Potter EL: Pathogenesis
of polycystic kidneys: Type 2 due to inhibition of ampullary
activity. Arch Pathol. 77:474–484. 1964.PubMed/NCBI
|
|
23
|
Raj GV, Yowell C, Madden JF, Nosnik I,
Mouraviev V and Polascik TJ: Malignant cystic nephroma. Can J Urol.
13:3348–3350. 2006.PubMed/NCBI
|
|
24
|
Silverman SG, Pedrosa I, Ellis JH, Hindman
NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman
SS, et al: Bosniak classification of cystic renal masses, version
2019: An update proposal and needs assessment. Radiology.
292:475–488. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sharma S, Nagar R, Singh K and Chrungoo
RK: Cystic nephroma: An unusual renal lesion. J Urol.
163(1860)2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bastian PJ, Kuhlmann R, Vogel J and
Bastian HP: Local recurrence of a unilateral cystic nephroma. Int J
Urol. 11:329–331. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng EY, Cohn RA, Palmer LS, Fernbach S
and Firlit CF: A rare case of bilateral multilocular renal cysts. J
Urol. 157:1861–1862. 1997.PubMed/NCBI
|
|
28
|
Ljungberg B, Albiges L, Abu-Ghanem Y,
Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F,
Hora M, Kuczyk MA, et al: European association of urology
guidelines on renal cell carcinoma: The 2019 update. Eur Urol.
75:799–810. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the eighth edition of the tumor-
node-metastasis staging classification for urologic cancers. Eur
Urol. 73:560–569. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujita K, Ueki T and Matsushima H: An
atypical multilocular cystic nephroma presenting recurrent lumbago.
Nihon Hinyokika Gakkai Zasshi. 84:1883–1886. 1993.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
31
|
Egea JP, Compiano LO, Martínez F, García
FG, Gutierrez AS, Galiano JL and Ros MT: Multilocular cystic
nephroma. A diagnostic and therapeutic challenge. Report of two
cases. Arch Esp Urol. 57:431–434. 2000.PubMed/NCBI(In Spanish).
|
|
32
|
Kural AR, Öbek C, Özbay G and Önder AU:
Multilocular cystic nephroma: An unusual localization. Urology.
52:897–899. 1998.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gettman MT and Segura JW: An unusual case
of multilocular cystic nephroma with prominent renal pelvis
involvement treated with nephron sparing techniques. J Urol.
162(482)1999.PubMed/NCBI
|