Introduction
Carcinoma of the esophagus is one of the most lethal
neoplasms worldwide (1,2). In China, however, it ranks among the
top 3 most common malignancies, demonstrating an incidence of
nearly 5 million and claiming a cancer-related death of around 4
million per year, turning out a major health threat (3,4). Quite
different from the situation in western countries that most
esophageal cancer evolving from Barrett's esophagus and
demonstrating a major histoloty of adenocarcinoma, situation in
China is that squamous cell carcinoma predominates in more than 95%
EC patients. However, accounting for nearly 95% of all cases in
China, most esophageal squamous cell carcinoma (ESCC) locates in
the intrathoracic portion and surgical resection remains the
preferred modality of radical treatment, especially for the early-
or mid-staged lesions (5). After
esophagectomy, reconstruction using a gastric conduit is the most
common procedure (6), although
various other anastomotic techniques have been demonstrated
(7-10).
However, reconstruction surgery following resection of the
esophagus is frequently associated with occurrence of anastomotic
leakage. Once it occurs, patients suffered decreased quality of
life, protracted hospitalization or even death. This is why there
were many innovations and modifications in reconstructive surgery
including functional end-to-end stapling, triangulating stapling,
T-shaped linear stapling, pre-embedded stapling, and so forth
(11-15).
Although efficacy of mechanical anastomosis had been
reported previously (12,16,17),
much effort had still been tried to better off the clinical outcome
and simplify the procedure (18,19).
Chen et al (20) reported
that use of pleural flaps in the upper mediastinum would reduce the
incidence of cervical subcutaneous emphysema and anastomotic
leakage into pleural cavity. Sugimura et al (8) introduced a modified Collard
anastomosis which would be more effective in the reduction of
anastomotic stenosis. Sun et al (21) demonstrated an embedded three-layer
esophagogastric anastomotic maneuvre which would facilitating the
reduction of morbidity as well as improvement of short-term
outcomes. In the present study we introduced a novel method of
cervical esophagogastric anastomosis, so-called ‘modified one-piece
mechanical anastomosis (MOMA)’ in McKeown esophagogastrectomy and
compared its feasibility, efficacy and safety with conventionally
double-layer hand-sewn anastomosis (CDHA). We made a minor
modification based on the traditional mechanical anastomosis (TMA).
We hypothesized non-inferiority when comparing MOMA to CDHA, and in
our early practical experience MOMA had been proven feasible, and
would significantly speed up the surgical procedure in abdominal
phase and cervical anastomosis.
Patients and methods
Study design and patients
From March 2016 to March 2018, 96 consecutive
patients with thoracic esophageal squamous cell carcinoma in the
Department of Thoracic Surgery of Fujian Cancer Hospital and Fujian
Medical University Cancer Hospital were hospitalized and
preoperatively evaluated for the eligibility for surgical
resection. As a result, 80 of them met the criteria and were
enrolled. All patients were diagnosed by gastroscopy and
pathologically proven, no surgical contraindications had been
demonstrated and no patient had suffered from a double cancer. The
surgical criteria for thoracic esophageal cancer is cT1-4aN0-1M0.
Forty patients received a modified anastomotic (MOMA) and the other
40 conventionally hand-sewn maneuver (CDHA). Resections were
carried out by 2 different surgical teams (MOMA by X. Chen, J.
Zhang and G. Weng and CDHA by K. Zhu, S. Lin and Y. Cai), while
patients were treated with the same perioperative regimen in
process of hospitalization. The screening items included: Complete
blood count (CBC), comprehensive chemistry profile, esophageal
barium swallow, upper gastrointestinal (GI) endoscopic
ultrasonography (EUS) and biopsy and chest/upper abdomen computed
tomography (CT) with intravenous (IV) contrast. The histopathologic
features of cancerous specimens were classified in accordance with
the 8th AJCC (American Joint Committee on Cancer) criteria on
esophageal cancer (22,23), and the TNM staging system as well
(24). Patients receiving induction
chemotherapy, however, would not undergo surgery until down-staging
was achieved and surgical indication was met. Clinicopathologic
parameters including age, gender, smoking status, Brinkman index,
ECOG score, history of gastric surgery, cellular histology,
preoperative weight loss, body mass index (BMI), preoperative
albumin, preoperative BUN, tumor location, American Society of
Anesthesiology (ASA) classification, Charlson comorbidity index
(CCI) (25), pathologic TNM stage,
follow-up data and history of neoadjuvant therapy and postoperative
therapy were collected. Intraoperative characteristics like
thoracic duct ligation, pyloric emptying procedure, jejunostomy,
length of hospital stay, total operation time, time of anastomosis,
estimated blood loss, total chest/gastric tube retention time,
total chest/gastric tube drainage volume and number of
resected/metastasized lymph nodes (r/m LNs). Patients' surgical
outcome information included resection margin, blood transfusion,
postoperative pneumonitis, anastomotic leakage/stenosis,
postoperative arrhythmia, bleeding, gastric conduit palsy/tearing,
recurrent laryngeal nerve palsy, chylothorax, 30-day re-admission
and mortality. The final follow-up date was September 24, 2019. The
study protocol was approved by the Human Ethics Review Committee of
Fujian Cancer Hospital and Fujian Medical University Cancer
Hospital, and a signed informed consent was obtained from each
patient.
Surgical approaches
The operation began with a thoracic phase by open
right thoracotomy, in which resection of the tumor together with
lymphadenectomy was carried out. An open abdominal phase followed,
in which the stomach was prepared and then brought up through the
chest into the neck for a circular end-to-end stapled anastomosis,
with the proximal stomach conduit at the apex of the pleural
cavity.
Dissection of the esophagus was initiated from the
mediastinal visceral pleura at the inferior margin of arch of
azygos vein with ultrasonic shears, moving down from the posterior
and then to the anterior wall of the esophagus. After the azygos
vein was transected, the dissection was continued up into the upper
mediastinum, carefully preserving both sides of the bronchial
arteries and thoracic duct, and keeping from injuring both sides of
recurrent laryngeal nerves (RLNs) while dissecting the suspicious
metastatic lymph nodes nearby.
At the end of esophagectomy, the patient was
repositioned to supine position. Gastric mobilization as well as
preparation of gastric conduit was then carried out in an open
manner. For MOMA group, gastric mobilization was initiated from the
middle at the greater curvature of stomach on the greater omentum,
with a distance of ≥2 cm from the arch of gastroepiploic vessels
(Fig. 1A, arrow ①), firstly moving
clockwise to the starting point of the right gastroepiploic artery,
then anticlockwise to dissect the left gastroepiploic,
splenogastric, short gastric and retrogastric vessels. After
removal of No. 18 and 19 LNs, the left gastric vessels together
with No. 17 LN were then dissected. The omental bursa was opened,
with the lesser curvature of the stomach and the esophagogastric
conjunction well dissected and fully released. Then the right
gastric vessels was ligated at the level of 3rd or 4th branch from
the rightmost (Fig. 1A, arrow ② and
1B, arrow ①), the stomach was then
cut from the ligation/start point (Fig.
1A, arrow ② and 1B, arrow ②)
along with the lesser curvature (Fig.
1C and D) to the endpoint
(Fig. 1A, arrow ③ and 1E, arrow) at ≤3 cm (Fig. 1A, arrow ④, marked as yellow thick
line) to the cardia without full transection at the esophagogastric
junction with endocutter, making the stomach a thin gastric conduit
of around 3.5 cm in diameter (Fig.
1F, arrow) and ensuring the adequate length for the replacement
of resected esophagus. Then some stitches were placed to ensure the
security of the gastric conduit, and the uppermost stitch (Fig. 1E, arrow) was used as a landmark to
indicate the cutting margin of remnant gastric conduit later.
A straight incision was made in front of the
sternocleidomastoid muscle in the left neck, after removal of 1L
LNs, the cervical esophagus was freed. The gastric conduit,
together with the dissected esophagus and cut lesser curvature of
the stomach, was pulled up from the abdomen into the neck through
hiatus, esophageal bed in the retromediastinum and then inlet of
thoracic cage, carefully not to have it torn. After an appropriate
size of anvil (all Johnson & Johnson, and size of stapler used
was as followed: no. 21 in 23 patients and no. 25 in 17 patients)
was inserted and well placed (Fig.
2A, arrow ①), an incision was made at the lesser curvature site
on the esophagogastric junction for the entrance of stapler shaft
(Fig. 2A, site of ultrasonic shears
cut). Then a circular end-to-end stapled anastomosis was
accomplished (Fig. 2B, arrow
showing the anastomosis) with the anastomotic site on the posterior
wall of gastric conduit and close to the greater curvature to
ensure better blood flow. The remnant gastric conduit was
transected at least 3 cm afar off from the anastomotic line, i.e.,
along with the line of marked stitch (Fig. 2C, arrow showing the marking stitch),
ensuring the adequate blood supply (Fig. 2D, arrow ① for anastomosis and ② for
transecting line, distance within them should be ≥3 cm).
For the CDHA group, all the other procedures were
identical except that during the preparation of gastric conduit,
the lesser curvature of the stomach was fully transected without
preserving the remnant part of the lesser curvature (Fig. 1A, arrow ④, marked as yellow thick
line), then the gastric conduit was pulled up to the neck and a
conventional double-layer hand-sewn anastomosis was carried out
with 4-0 Mersilk in an interrupted manner in both layers.
In the patients without jejunostomy, a nasojejunal
feeding tube were inserted to ensure that enteral alimentation was
started in the early postoperative period.
Definition of postoperative
complications and follow-up
Patients routinely underwent postoperative
gastrointestinal endoscopy at 12 months if complaints of symptoms
such as dysphagia arise. In this study, anastomotic stricture is
defined as a condition that requires balloon dilation at the
stenotic anastomosis within 90 postoperative days (PODs), with
endoscopic proof of a stenosis through which a 9-mm endoscope
cannot be passed. Anastomotic leakage is defined as the presence of
extraluminal contrast by postoperative CT after swallowing contrast
medium, endoscopic visualization of dehiscence or fistula, or flow
of saliva or pus through the cervical wound within 30 PODs. If pus
was discovered from the cervical wound with uncertain anastomotic
leakage found, patients undergo a contrast medium swallow study and
a CT study after open drainage of the cervical wound to confirm the
existence of anastomotic leakage. Other overall postoperative
morbidities are redefined as greater than grade II by the
Clavien-Dindo classification. Follow-up appointments for all
patients took place at 1, 3, 6, 12 and then every 6 months
following surgery at Fujian Medical University Cancer Hospital. All
patients would be followed up to 5 years or until death.
Statistical analysis
All data were analyzed by SPSS 23.0 (SPSS, Inc.).
The quantitative data were expressed as the mean ± standard
deviation (SD) and compared using the unpaired Student's t-test.
The counting data were expressed by frequency or rate, and the
comparison between groups was carried out by Pearson's
χ2 or Fisher's exact test as appropriate. All patients
received a follow-up. The Kaplan-Meier method with log-rank test
was used for estimating and comparing probability of unadjusted
disease-free survival (DFS) and overall survival (OS) within
groups. A P-value <0.05 was considered statistically
significant.
Results
Basic characteristics of study
population
Ninety-six consecutive patients were screened and 80
patients with thoracic esophageal cancer were enrolled and received
surgery from April 2016 through March 2018 (Table I). The average age for CDHA and MOMA
groups was 63.53±1.14 and 61.58±0.85 years old, respectively
(P=0.173). Except for preoperative albumin (P=0.029), no
statistical difference had been demonstrated in the items of
gender, smoker, Brinkman index, ECOG score, preoperative BUN, BMI,
preoperative weight loss, tumor location, ASA classification,
Charlson comorbidity index, induction therapy, postoperative
radiotherapy, postoperative chemotherapy, pathologic TNM staging,
nerve involvement or vascular invasion (Table I, all P>0.05).
 | Table IBasic characteristics of study
population (n=80). |
Table I
Basic characteristics of study
population (n=80).
| Variables | CDHA (n=40) | MOMA (n=40) |
t/χ2 | P-value |
|---|
| Age, years (mean ±
SD) | 63.53±1.14 | 61.58±0.85 | 1.375 | 0.173 |
| Sex, n | | | 0.853 | 0.356 |
|
Male | 27 | 23 | | |
|
Female | 13 | 17 | | |
| Smoker, n | | | 2.452 | 0.117 |
|
Yes | 24 | 17 | | |
|
No | 16 | 23 | | |
| Brinkman index
(mean ± SD) | 435.00±60.29 | 305.00±64.05 | 1.478 | 0.143 |
| Average follow-up,
months | 24.70 | 18.58 | / | NA |
| ECOG, n | | | / | >0.999 |
|
≤1 | 40 | 40 | | |
|
>1 | 0 | 0 | | |
| BMI,
kg/m2 (mean ± SD) | 21.34±0.41 | 22.33±0.48 | 1.554 | 0.124 |
| Preoperative
albumin, g/l (mean ± SD) | 38.03±0.53 | 40.04±0.73 | 2.219 | 0.029 |
| Preoperative BUN,
g/l (mean ± SD) | 5.21±0.24 | 5.17±0.24 | -0.141 | 0.888 |
| Preoperative weight
loss, na | | | 0.734 | 0.392 |
|
>0, ≤5
kg | 36 | 38 | | |
|
>5, ≤10
kg | 4 | 2 | | |
| Tumor location,
n | | | 2.040 | 0.361 |
|
Upper | 6 | 4 | | |
|
Middle | 28 | 25 | | |
|
Lower | 6 | 11 | | |
| ASA classification,
n | | | 0.392 | 0.531 |
|
II | 35 | 33 | | |
|
III | 5 | 7 | | |
| CCI, n | | | 0.251 | 0.617 |
|
≤3 | 12 | 10 | | |
|
>3 | 28 | 30 | | |
| Induction therapy,
na | | | 3.127 | 0.077 |
|
Yes | 39 | 35 | | |
|
No | 1 | 5 | | |
| Postoperative RT,
na | | | 3.127 | 0.077 |
|
Yes | 5 | 1 | | |
|
No | 35 | 39 | | |
| Postoperative CT,
n | | | 1.867 | 0.172 |
|
Yes | 11 | 6 | | |
|
No | 29 | 34 | | |
| pTNM staging,
n | | | 0.487 | 0.485 |
|
0-II | 24 | 27 | | |
|
III | 16 | 13 | | |
| Nerve involvement,
n | | | 0.000 | >0.999 |
|
Yes | 7 | 7 | | |
|
No | 33 | 33 | | |
| Vascular invasion,
n | | | 2.990 | 0.084 |
|
Yes | 15 | 8 | | |
|
No | 25 | 32 | | |
Intraoperative characteristics
All patients received Mckeown procedure with
different anastomotic ways. As shown in Table II, all patients in both groups
received open thoracotomy and laparotomy. Although number of
patients receiving thoracic duct ligation (8 vs. 17, P=0.030) and
jejunotomy (14 vs. 31, P<0.001) in the CDHA and MOMA groups was
various, no significant difference had been demonstrated in the
following items: Pyloric emptying procedure, length of hospital
stay (25.35±1.29 vs. 24.40±1.16 days, P=0.586), chest tube
retention time (9.80±0.68 vs. 11.15±0.52 days, P=0.119), total
chest tube drainage (2517.90±469.05 vs. 2715.35±298.77 ml,
P=0.724), gastric tube retention time (10.35±0.39 days vs.
11.58±0.51 d, P=0.059), total gastric tube drainage (1568.55±182.01
vs. 1738.70±170.54 ml, P=0.497), average resected LNs (22.43±1.75
vs. 24.83±1.62, P=0.317) or metastasized LNs (0.93±0.28 vs.
0.95±0.25, P=0.946). It's of note that in comparison to the CDHA
group, total operation time (207.73±2.66 vs. 225.40±3.43 min,
P<0.001) and time of anastomosis (10.95±0.44 vs. 23.03±0.47 min,
P<0.001) were significantly shorter and the estimated blood loss
was obviously less (144.50±21.14 ml vs. 241.75±23.75 min, P=0.003).
The average follow-up time in CDHA and MOMA groups was 24.70 and
18.58 months, respectively, both longer than one year.
 | Table IIIntraoperative characteristics
(n=80). |
Table II
Intraoperative characteristics
(n=80).
| Parameters | CDHA (n=40) | MOMA (n=40) |
t/χ2 | P-value |
|---|
| TD ligation, n | | | 4.713 | 0.030 |
|
Yes | 8 | 17 | | |
|
No | 32 | 23 | | |
| Pyloric emptying
procedure, n | | | / | NA |
|
None | 40 | 40 | | |
|
Balloon
dilation | 0 | 0 | | |
| Jejunostomy, n | | | 14.679 | <0.001 |
|
Yes | 14 | 31 | | |
|
No | 26 | 9 | | |
| Length of hospital
stay, days (mean ± SD) | 25.35±1.29 | 24.40±1.16 | 0.547 | 0.586 |
| Total operation
time, min (mean ± SD) | 225.40±3.43 | 207.73±2.66 | 4.067 | <0.001 |
| Time of
anastomosis, min (mean ± SD) | 23.03±0.47 | 10.95±0.44 | 18.781 | <0.001 |
| Estimated blood
loss, ml (mean ± SD) | 241.75±23.75 | 144.50±21.14 | 3.059 | 0.003a |
| Chest tube
retention time, days (mean ± SD) | 9.80±0.68 | 11.15±0.52 | 1.575 | 0.119 |
| Total chest tube
drainage, ml (mean ± SD) | 2517.90±469.05 | 2715.35±298.77 | 0.355 | 0.724 |
| Gastric tube
retention time, days (mean ± SD) | 10.35±0.39 | 11.58±0.51 | 1.917 | 0.059 |
| Total gastric tube
drainage, ml (mean ± SD) | 1568.55±182.01 | 1738.70±170.54 | 0.682 | 0.497 |
| Average resected
LNs (mean ± SD) | 22.43±1.75 | 24.83±1.62 | 1.007 | 0.317 |
| Metastasized LNs
(mean ± SD) | 0.93±0.28 | 0.95±0.25 | 0.067 | 0.946 |
Patients' surgical outcome
The perioperative surgical outcomes of patients
within 30 PODs were indicated in Table III. Briefly, in the CDHA and MOMA
groups, 37 and 39 patients achieved R0 resection margin (P=0.294),
11 and 10 patients received blood transfusion (P=0.799), 9 and 8
patients had postoperative pneumonitis (P=0.785), 1 and 0 patient
suffered anastomotic leakage (P=1.000), 2 and 4 patients suffered
anastomotic stenosis (P=0.392), 5 and 3 had postoperative
arrhythmia (P=0.454), 0 and 1 patient suffered bleeding (P=1.000),
4 and 3 patients suffered gastric conduit palsy (P=0.692), 0 and 2
patients suffered gastric conduit tearing (P=0.494), 6 and 8
patients suffered recurrent laryngeal nerve palsy (P=0.556), 1 and
1 patient suffered chylothorax (P=1.000), 3 and 2 patients had
30-day re-admission (P=0.643) and none had 90-day mortality,
respectively. After comparing their DFS and OS, no statistical
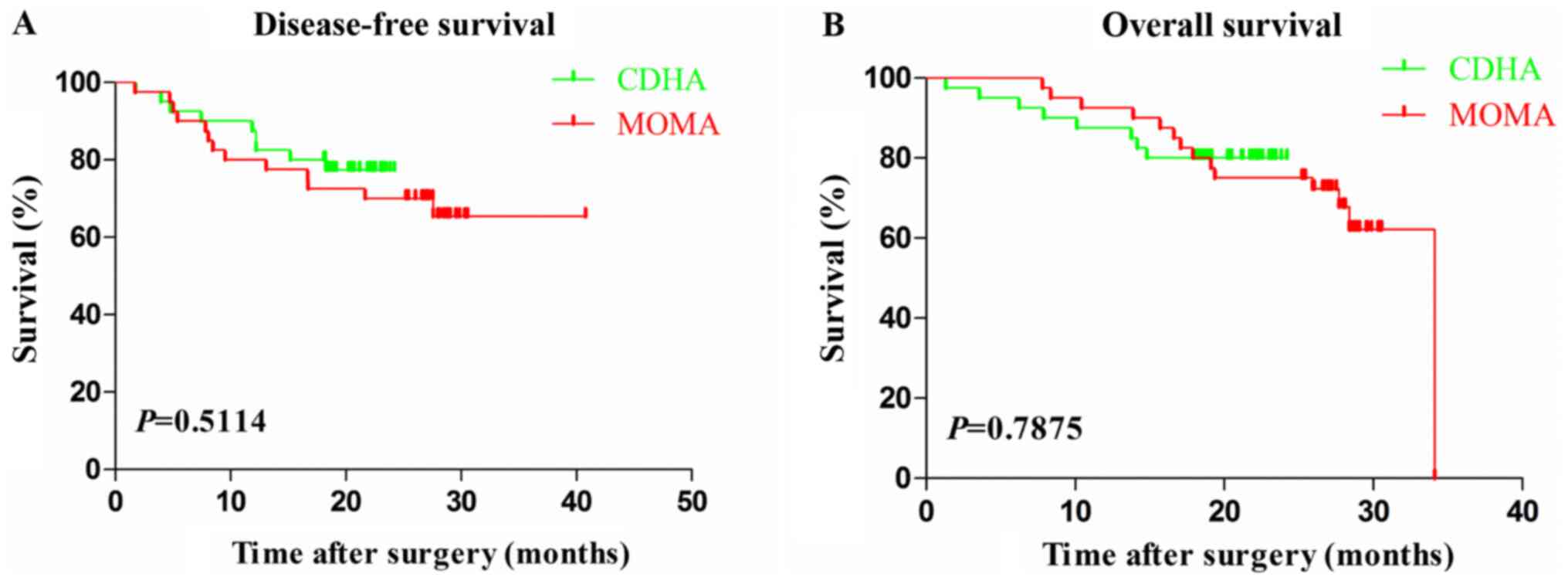
difference had been demonstrated within these two groups (Fig. 3A and B; P=0.5114 and 0.7875, respectively).
 | Table IIIPerioperative surgical outcome
(n=80). |
Table III
Perioperative surgical outcome
(n=80).
| Parameters | CDHA, n (n=40) | MOMA, n (n=40) | χ2 | P-value |
|---|
| Resection
margin | | | 1.099 | 0.294 |
|
R0 | 37 | 39 | | |
|
R1 | 3 | 1 | | |
| Blood
transfusion | | | 0.065 | 0.799 |
|
Yes | 11 | 10 | | |
|
No | 29 | 30 | | |
| Pneumonitis | | | 0.075 | 0.785 |
|
Yes | 9 | 8 | | |
|
No | 31 | 32 | | |
| Anastomotic
leakagea | | | / | >0.999 |
|
Yes | 1 | 0 | | |
|
No | 39 | 40 | | |
| Anastomotic
stenosis | | | 0.734 | 0.392 |
|
Yes | 2 | 4 | | |
|
No | 38 | 36 | | |
| Arrhythmia | | | 0.561 | 0.454 |
|
Yes | 5 | 3 | | |
|
No | 35 | 37 | | |
|
Bleedinga | | | / | >0.999 |
|
Yes | 0 | 1 | | |
|
No | 40 | 39 | | |
| GC palsy | | | 0.157 | 0.692 |
|
Yes | 4 | 3 | | |
|
No | 36 | 37 | | |
| GC
tearinga | | | / | 0.494 |
|
Yes | 0 | 2 | | |
|
No | 40 | 38 | | |
| RLN palsy | | | 0.346 | 0.556 |
|
Yes | 6 | 8 | | |
|
No | 34 | 32 | | |
|
Chylothoraxa | | | / | >0.999 |
|
Yes | 1 | 1 | | |
|
No | 39 | 39 | | |
| 30-day
re-admission | | | 0.215 | 0.643 |
|
Yes | 3 | 2 | | |
|
No | 37 | 38 | | |
| 90-day
mortality | 0 | 0 | / | NA |
Discussion
Esophagectomy remains the gold standard in the
treatment of esophageal cancer with curative intent. However, this
operation is complicated and associated with high morbidity and
mortality (26-29).
Anastomosis-related complications especially anasomotic leakage is
one of the most lethal comorbidies, usually resulting in pyothorax,
mediastinitis, tracheal fistula, arterial fistula or septicemia,
and ending up with multiple organ failure eventually. In order to
achieve satisfactory esophagogastric anastomosis, much effort had
been tried either to optimize the anasomotic procedure (18,19,30-37),
to better off the blood flow at the anastomotic site on the grafted
conduits (11,38,39),
or to manage prophylactic measurements to ensure the confinement of
inflammation and facilitate the healing in case of leakage
(20,21).
In the present study we evaluated the utility of
MOMA and compared it with CDHA in cervical esophagogastric
anastomosis after sub-total esophagectomy in TE-SCC patients. Major
modifications of MOMA lie in gastric conduit preparation and
anastomotic maneuver, without fully transecting the lesser
curvature while preserving it for no longer than 3 cm at the
conjunctional part and pulling the conduit up to the neck to
fulfill a circular end-to-end stapled anastomosis, quite different
from conventional way by transecting the gastroesophageal junction
with the continuation of extracorporeal gastroplasty by fully
cutting off the lesser curvature of stomach (40,41).
As could be expected and eventually testified in our study that
this modification would firstly simplify the procedure of gastric
conduit preparation and esophagogastric anastomosis by avoiding the
action of transecting lower esophagus and making pulling-up
stitches at the apex of gastric conduit, and secondly decrease the
amount of hemorrhage although it would probably be due only to the
shorter duration of the operation, especially hand-sewn cervical
anastomosis (40-43).
Major clinical findings in our study indicated that
in comparison to CDHA, time consumption in total operation and
anastomosis in MOMA group was statistically shortened, and
therefore estimated blood loss was reduced accordingly. However,
anastomosis-related complications like anastomotic leakage and
stricture bore no difference within these two maneuvers. Recently,
Li et al (19) reported a
T-shaped linear-stapled cervical esophagogastric anastomosis in a
sample size of 32 patients, demonstrating a time consumption in
anastomosis at 17.6 min, which was much longer than ours.
Furthermore, their anastomotic method was similar with the
triangulating anastomosis, which was reported to have higher rate
of leakage at the site of staple overlapping (17).
Besides the beneficiary aspects mentioned above,
analyses demonstrated no different incidence of postoperative
complications like pneumonitis, arrhythmia, bleeding, gastric
conduit palsy, RLN palsy, chylothorax, 30-day re-admission and
mortality (all P>0.05) in both groups. However, it should be
noticed that there were 2 patients suffering from the gastric
conduit tearing at the endpoint on the lesser curvature because of
the inadequate cutting. As a result, the gastric conduits had to be
returned to the abdomen to get the torn part fixed, re-cut and
pulled up to the neck again. So, we had to address the importance
that in the MOMA procedure the remnant part of the gastroesophageal
junction left should not be longer than 3 cm lest the conduit gets
torn in process of being pulled up into the neck. In addition,
before the gastric conduit was about to be pulled up, adequate
muscle relaxant should be administered and transient respiratory
cessation could be used to ensure the safety of pulling-up action.
As most causes of anastomotic leakage were likely due to gastric
conduit compression and congestion of the gastric conduit stump
caused by the sternoclavicular joint of the thoracic inlet,
therefore, when the width of the thoracic inlet was less than three
fingerbreadths, the left sternoclavicular joint was resected and
the thoracic inlet was dilated to ensure the adequate space for the
passover of the gastric conduit. After taking these factors into
account, our early experience confirmed the feasibility and safety
of this procedure.
Some limitation of this study should be noted. With
a retrospective study at a sample-size of 40 in each group,
although the results supported the feasibility of MOMA maneuver,
further study is necessary to validate the efficacy and safety of
this procedure. In addition, in order to facilitate proving the
feasibility, open procedure was used in both groups to compare MOMA
and CDHA, however, with the global acceptance of minimally invasive
procedure and traditional mechanical anastomosis (TMA) (44), further study would be designated to
compare MOMA and TMA, and even the effectiveness of MOMA in both
minimally invasive settings.
In conclusion, MOMA suggests a feasible, effective
and reproducible alternative in McKeown esophagogastrectomy for the
treatment of TE-SCC, providing significantly shorter operation and
anastomosis time, and less estimated intraoperative blood loss as
well.
Acknowledgements
The authors would like to thank Professor Junqiang
Chen and Professor Jiancheng Li from the Department of
Radiotherapic Oncology (Fujian Cancer Hospital, Fuzhou, China) for
their kind help in the process of the study and paper writing.
Funding
This work was supported by the Science and Technology Program of
Fujian Province, China (grant no. 2018Y2003; to KZ and XC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KZ and XC conceived and designed the study. KZ
provided administrative support. KZ, JZ, XC, YD, SL, YC and GW
provided study materials or patients. JZ, XC, YD, SL and YC
collected and assembled the data. KZ, JZ, XC, YD, SL, YC and GW
analyzed and interpreted the data. KZ and XC were responsible for
confirming the authenticity of the raw data and the paper itself.
All authors wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Human Ethics
Review Committee of Fujian Cancer Hospital and Fujian Medical
University Cancer Hospital (Fuzhou, China), and written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: . Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tu CC and Hsu PK: The frontline of
esophageal cancer treatment: Questions to be asked and answered.
Ann Transl Med. 6(83)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Okamura A, Watanabe M, Imamura Y, Hayami
M, Yuda M, Yamashita K, Shoji Y and Mine S: Cervicothoracoscopic
approach in esophagectomy. Ann Surg Oncol. 25(333)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Constantinoiu S, Achim F and Constantin A:
Use of the stomach in esophageal reconstructive surgery in era of
minimally invasive approach. Chirurgia (Bucur). 113:809–825.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsuji T, Ojima T, Nakamori M, Nakamura M,
Katsuda M, Hayata K, Kitadani J, Maruoka S, Shimokawa T and Yamaue
H: Triangulating stapling vs functional end-to-end stapling for
cervical esophagogastric anastomosis after esophagectomy for
thoracic esophageal cancer: Study protocol for a randomized
controlled trial. Trials. 20(83)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sugimura K, Miyata H, Matsunaga T, Asukai
K, Yanagimoto Y, Takahashi Y, Tomokuni A, Yamamoto K, Hirofumi A,
Nishimura J, et al: Comparison of the modified Collard and
hand-sewn anastomosis for cervical esophagogastric anastomosis
after esophagectomy in esophageal cancer patients: A propensity
score-matched analysis. Ann Gastroenterol Surg. 3:104–113.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Song YN, Qi Y, Zhang CY, Sheng YL, Wu K,
Zhu SL, Han L, Shan TT, Ye GC, Zhang QY, et al: A new technology
for reducing anastomotic fistula in the neck after esophageal
cancer surgery. J Thorac Dis. 11:3084–3092. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yasuda T, Shiraishi O, Iwama M, Makino T,
Kato H and Kimura Y: Novel esophageal reconstruction technique via
transmediastinal route from posterior to anterior mediastinum after
esophagectomy. J Thorac Cardiovasc Surg. 156:859–866.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shimakawa T, Naritaka Y, Asaka S, Miyazawa
M, Murayama M, Yamaguchi K, Usui T, Yokomizo H, Yoshimatsu K,
Shiozawa S and Katsube T: Innovations for cervical
esophagogastrostomy in thoracic esophageal cancer operations.
Anticancer Res. 38:2323–2327. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang ZQ, Jiang YQ, Xu W, Cai HR, Zhang Z,
Yin Z and Zhang Q: A novel technique for cervical
gastro-oesophageal anastomosis during minimally invasive
oesophagectomy. Int J Surg. 53:221–229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin TH and Huang PM: Early postoperative
endoscopy for evaluation of the anastomosis after esophageal
reconstruction. Thorac Cardiovasc Surg. 66:376–383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yuan Y, Zeng XX, Zhao YF and Chen LQ:
Modified double-layer anastomosis for minimally invasive
esophagectomy: An effective way to prevent leakage and stricture.
World J Surg. 41:3164–3170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Markar SR, Arya S, Karthikesalingam A and
Hanna GB: Technical factors that affect anastomotic integrity
following esophagectomy: Systematic review and meta-analysis. Ann
Surg Oncol. 20:4274–4281. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Worrell S, Mumtaz S, Tsuboi K, Lee TH and
Mittal SK: Anastomotic complications associated with stapled versus
hand-sewn anastomosis. J Surg Res. 161:9–12. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li J, Shen Y, Tan L, Feng M, Wang H, Xi Y,
Leng Y and Wang Q: Cervical triangulating stapled anastomosis:
Technique and initial experience. J Thorac Dis. 6 (Suppl
3):S350–S354. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Wang B, Liang T, Guo NN and Zhao M:
Pre-embedded cervical circular stapled anastomosis in
esophagectomy. Thorac Cancer. 11:723–727. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Wang Z, Zhang G, Fu J and Wu Q:
T-shaped linear-stapled cervical esophagogastric anastomosis for
minimally invasive esophagectomy: A pilot study. Tumori.
106:506–509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen X, Liu S, Chen P, He H and Wang F:
Application of pleural flaps in laparoscopic-thoracoscopic
esophagectomy for esophageal cancer. J Thorac Dis. 12:973–979.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun HB, Li Y, Liu XB, Zhang RX, Wang ZF,
Zheng Y, Qin JJ, Li HM, Chen XK and Wu Z: Embedded three-layer
esophagogastric anastomosis reduces morbidity and improves
short-term outcomes after esophagectomy for cancer. Ann Thorac
Surg. 101:1131–1138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Konda P, Ai D, Guerra CE,
Rodriguez-Restrepo A, Mehran RJ, Rice D, Hofstetter W, Heir J,
Kwater P, Gottumukkala V, et al: Identification of risk factors
associated with postoperative acute kidney injury after
esophagectomy for esophageal cancer. J Cardiothorac Vasc Anesth.
31:474–481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oweira H, Schmidt J, Mehrabi A, Kulaksiz
H, Schneider P, Schöb O, Giryes A and Abdel-Rahman O: Validation of
the eighth clinical American joint committee on cancer stage
grouping for esophageal cancer. Future Oncol. 14:65–75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rice TW: Esophageal cancer staging. Korean
J Thorac Cardiovasc Surg. 48:157–163. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamashita K, Watanabe M, Mine S, Fukudome
I, Okamura A, Yuda M, Hayami M and Imamura Y: The impact of the
Charlson comorbidity index on the prognosis of esophageal cancer
patients who underwent esophagectomy with curative intent. Surg
Today. 48:632–639. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yen YC, Chang JH, Lin WC, Chiou JF, Chang
YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH and Wu SY:
Effectiveness of esophagectomy in patients with thoracic esophageal
squamous cell carcinoma receiving definitive radiotherapy or
concurrent chemoradiotherapy through intensity-modulated radiation
therapy techniques. Cancer. 123:2043–2053. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yamashita K, Makino T, Yamasaki M, Tanaka
K, Hara T, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K,
Takiguchi S, et al: Comparison of short-term outcomes between 2-
and 3-field lymph node dissection for esophageal cancer. Dis
Esophagus. 30:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamashita H, Seto Y, Takenaka R, Okuma K,
Kiritooshi T, Mori K, Yamada K, Fukuda T, Kaminishi M, Abe O and
Nakagawa K: Survival comparison between radical surgery and
definitive chemoradiation in 267 esophageal squamous cell
carcinomas in a single institution: A propensity-matched study.
PLoS One. 12(e0177133)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jamieson GG, Mathew G, Ludemann R, Wayman
J, Myers JC and Devitt PG: Postoperative mortality following
oesophagectomy and problems in reporting its rate. Br J Surg.
91:943–947. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Kamiya K, Unno N, Miyazaki S, Sano M,
Kikuchi H, Hiramatsu Y, Ohta M, Yamatodani T, Mineta H and Konno H:
Quantitative assessment of the free jejunal graft perfusion. J Surg
Res. 194:394–399. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deng XF, Liu QX, Zhou D, Min JX and Dai
JG: Hand-sewn vs linearly stapled esophagogastric anastomosis for
esophageal cancer: A meta-analysis. World J Gastroenterol.
21:4757–4764. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang J, Yao F, Yao J, Xu L, Qian JL and
Shan LM: 21-versus 25-mm circular staplers for cervical
anastomosis: A propensity-matched study. J Surg Res. 246:427–434.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gao HJ, Mu JW, Pan WM, Brock M, Wang ML,
Han B and Ma K: Totally mechanical linear stapled anastomosis for
minimally invasive Ivor Lewis esophagectomy: Operative technique
and short-term outcomes. Thorac Cancer. 11:769–776. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhan B, Chen J, Du S, Xiong Y and Liu J:
Using the hand-sewn purse-string stapled anastomotic technique for
minimally invasive Ivor Lewis esophagectomy. Thorac Cardiovasc
Surg. 67:578–584. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang F, Zhang H, Zheng Y, Wang Z, Geng Y
and Wang Y: Intrathoracic side-to-side esophagogastrostomy with a
linear stapler and barbed suture in robot-assisted Ivor Lewis
esophagectomy. J Surg Oncol. 120:1142–1147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Valmasoni M, Capovilla G, Pierobon ES,
Moletta L, Provenzano L, Costantini M, Salvador R and Merigliano S:
A technical modification to the circular stapling anastomosis
technique during minimally invasive Ivor Lewis procedure. J
Laparoendosc Adv Surg Tech A. 29:1585–1591. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Schroder W, Raptis DA, Schmidt HM,
Gisbertz SS, Moons J, Asti E, Luyer MDP, Hölscher AH, Schneider PM,
van Berge Henegouwen MI, et al: Anastomotic techniques and
associated morbidity in total minimally invasive transthoracic
esophagectomy: Results from the EsoBenchmark database. Ann Surg.
270:820–826. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Goense L, Meziani J, Bülbül M, Braithwaite
SA, van Hillegersberg R and Ruurda JP: Pulmonary diffusion capacity
predicts major complications after esophagectomy for patients with
esophageal cancer. Dis Esophagus. 32(doy082)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zheng X, Yang YS, Hu WP, Xiao X, Luan SY,
Chen LQ and Yuan Y: Coniform gastric tube for end-to-end
anastomosis during minimally invasive McKeown esophagectomy. Ann
Thorac Surg. 109:e297–e300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang F, Liu S, Wang J, Chen X, Zheng Q,
Wang Z, Xu J and Chen S: Comparison of the stapled suture with the
manual suture in the application of minimally invasive
esophagectomy. Zhonghua Wei Chang Wai Ke Za Zhi. 17:881–883.
2014.PubMed/NCBI(In Chinese).
|
|
41
|
Chen X, Chen J, Zheng X, Chen Y, Lin Y,
Zheng Q, Zhu K and Pan J: Prognostic factors in patients with
thoracic esophageal carcinoma staged pT1-4aN0M0 undergone
esophagectomy with three-field lymphadenectomy. Ann Transl Med.
3(282)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu YJ, Fan J, He HH, Zhu SS, Chen QL and
Cao RH: Anastomotic leakage after intrathoracic versus cervical
oesophagogastric anastomosis for oesophageal carcinoma in Chinese
population: A retrospective cohort study. BMJ Open.
8(e021025)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shang QX, Chen LQ, Hu WP, Deng HY, Yuan Y
and Cai J: Three-field lymph node dissection in treating the
esophageal cancer. J Thorac Dis. 8:E1136–E1149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mariette C, Markar SR, Dabakuyo-Yonli TS,
Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T,
Carrère N, et al: Hybrid minimally invasive esophagectomy for
esophageal cancer. N Engl J Med. 380:152–162. 2019.PubMed/NCBI View Article : Google Scholar
|