Introduction
Infantile hemangiomas (IHs) are a common type of
vascular tumor (1), with an
incidence rate ranging from 0.2-10.0% (2). IHs often occur in the face and neck,
and grow rapidly in the first 3-12 months of life, and
spontaneously subside at the age of 3-7 years (3). However, in the course of its
development, 15% of children will experience complications that
affect their appearance and physiological functions, which can be
life-threatening, such as permanent deformities, visual and
auditory impairment, local tissue ulcers, bleeding, infection,
airway damage (4-6).
For untreated hemangiomas, the color and texture of the tumor
location is often different from the normal skin after regression,
and the majority of children still require surgical repair in
adulthood (7). Therefore, active
treatment of IHs is necessary, and the sooner the treatment, the
better the effect, which can minimize the incidence of
complications (8), and also
decrease the physical and psychological damage to children and
their parents.
In recent years, as the treatment continues to
optimize (9), a variety of methods
including drug, laser (10) and
surgical excision (11) have
emerged. However, laser and surgical excision have ulcers, scars,
permanent pigmentation and anesthesia risks (12,13),
so drug therapy becomes particularly important due to it being less
invasive, convenient and economical. Such drugs mainly include
vincristine (14), bleomycin
(15), interferon (16), steroids (17), propranolol (18). Vincristine is primarily used in
patients with life-threatening diseases, or when they have received
treatment with other drugs that have proved ineffective (19). Bleomycin was first used in the
treatment of cystic lymphangioma in 1977(20); interferon causes lower limb
disability in infants (21).
Aforementioned drugs cannot be used as a routine treatment method
for IHs. Steroids have been recognized as a first-line drug used
for IHs in the past, but there are adverse reactions such as
Cushing's syndrome and developmental retardation, which cause
irreversible damage to the growth and development of children
(22). Therefore, it is
particularly important to find a new drug with low side effects and
high efficiency.
In 2008, propranolol was first discovered in
infantile hemangioma (IH) treatment by Léauté-Labrèze et al
(23) when they treated a child
with nasal hemangioma and hypertrophic cardiomyopathy, they
accidentally found that propranolol could relieve the symptoms of
hemangioma. They then switched to propranolol for children who did
not respond to steroids and achieved good clinical results. Since
then, some clinical studies have shown that propranolol also has a
certain level of efficacy and safety (24,25).
The purpose of the present meta-analysis was to compare the
clinical efficacy of propranolol and steroids in the treatment of
IHs.
Materials and methods
Data sources and searches
PubMed, Cochrane Library, Embase and Web of Science
databases were searched before date March 31, 2020. The key words
used as search terms included ‘infantile hemangiomas’,
‘propranolol’, ‘steroids’, ‘steroids’ and ‘prednisolone’.
Selection criteria
The selection criteria included: i) Published
English literature on randomized controlled trials, case-control
trials and retrospective studies of propranolol vs. steroids in the
treatment of IHs; ii) infants diagnosed with IHs, where hemangioma
were located on the body surface, they were <6 years of age
(26), had no other underlying
diseases, had received no other treatment or medication history,
and had no limit on race, sex, single or multiple tumors; iii) the
following outcome indicators included the number of effective
cases, recurrent cases, adverse reactions and surgical resections
following drug treatment. Effectiveness is based on the Visual
Analogue Scale (VAS) to evaluate color and size: Grade I, poor
response (0-25% regression); Grade II, fair response (26-50%
regression); Grade III, good response (51-75% regression); and
Grade IV, excellent response (76-100% regression), and 75% was
considered effective (25). The
recurrence rate refers to the growth rebound at the time of dose
reduction or treatment termination during follow-up.
Literature screening and data
extraction
There were two researchers that independently
conducted literature screening and data extraction, screened out
the literature that did not meet the selection criteria, obtained
the full text of the included literature, and after cross-checking,
handed over the divergent documents to the third researcher for
assistance and decision. Data extraction included author, year of
publication, type of study, average age, sex, location of
hemangiomas, treatment time, and dose.
Quality assessment
The quality evaluation was conducted by means of
methodological index for non-randomized studies (MINORS), with a
total of 12 criteria: i) A clearly stated aim; ii) Inclusion of
consecutive patients; iiii) Prospective collection of data; iv)
Endpoints appropriate to the aim of the study; v) Unbiased
assessment of the study endpoint; vi) Follow-up period appropriate
to the aim of the study; vii) Loss to follow up less than 5%; viii)
Prospective calculation of the study size; ix) An adequate control
group; x) Contemporary groups; xi) Baseline equivalence of groups;
xii) Adequate statistical analyses. Each of which received a score
of 0-2. A score of 0 meant that no report had been made; a score of
1 meant that information was reported but insufficient; and a score
of 2 meant that sufficient information was reported and provided
(27).
Statistical analysis
A meta-analysis was performed on the extracted data
with Review Manager 5.3. Chi2 and df are qualitative
tests for heterogeneity. When the P-value of df ≤0.05, it means
that the test for heterogeneity is meaningful. I2 is a
quantitative test for heterogeneity, if I2<50%, it
meant that the heterogeneity was acceptable, then the fixed effects
model was used for meta-analysis, if I2≥50%, the factors
leading to heterogeneity were analyzed in subgroups. If
heterogeneity existed but had no obvious clinical significance,
then the random effects model was selected. The odds ratio (OR) and
95% confidence interval (CI) were used to analyze and count the
curative effect indexes. Z represents statistical results, and the
P-value of Z is ≤0.05, indicating that the combined results are
statistically significant.
Results
Literature search and data
extraction
A total of 641 articles were obtained after the
preliminary retrieval, including the Cochrane Library (n=8), Embase
(n=116), PubMed (n=321) and Web of Science (n=196), and two
researchers included 28 articles after reading the titles and
abstracts. A total of nine of studies were finally included after
full-text evaluations of all, including 221 cases of propranolol
and 201 cases of steroids (Fig. 1).
The full details were extracted (Tables
I and II).
 | Table ICharacteristics of the patients
included in the present study. |
Table I
Characteristics of the patients
included in the present study.
| Authors (year) | Study design | Sex (M:F) | Age | Location | Duration | Dose
(mg/kg/day) | Complications | Refs. |
|---|
| Price et al
(2011) | RS | NR | 4.5 months | H&N, 53; torso,
5; | A: 7.9 months | A: 2 | A: Hypoglycemia1;
rash 2 | (49) |
| | | | | extremity, 7;
perineum, 3 | B: 5.2 months | B: 4 | B: Cushing
syndrome, 42; hypertensions, 2; gastroesophageal reflux, 4; other,
5 | |
| Bertrand et
al (2011) | RS | NR | NR | H&N, 24 | A: 10.6 months | A: 2.7 | A: Sleep
disturbances, 6; hypotension, 1; vomiting, 1 | (51) |
| | | | | | B: 12.7 months | B: 2.8 | B: Oral thrush, 2;
insomnia, 1; hypertension, 1 | |
| Rössler et
al (2012) | RS | A: 10:20 | A: 135.1 days | NR | A: 198 days | A: 2 | A: Hypoglycemia, 3;
sleep disturbances, 3 | (52) |
| | | B: 9:21 | B: 85.8 days | | B: 120 days | B: 2 | B: Cushingoid
effects, 30; Hypertensions, 2; insomnia, 2; others, 4 | |
| Malik et al
(2013) | RCT | A: 7:3 | A: 4.6 months | NR | A: 9.9 months | A: 1-3 | A: Hypoglycemia, 1;
Somnolence, 1 | (53) |
| | | B: 2:3 | B: 5.5 months | | B: 13.5 months | B: 1-4 | B: Cushing
syndrome, 5; gastroesophageal reflux, 3 | |
| Bauman et al
(2014) | RCT | A: 2:6 | 2 weeks to | A: H&N, 10;
Torso, 1 | A: 323 days | NR | A: Dehydration, 1;
others, 31 | (54) |
| | | B: 3:8 | 6 months | B: H&N, 8 | B: 300 days | | B: Growth
retardation, 9; others, 34 | |
| Hoornweg et
al (2014) | RS | A: 2:5 | NR | H&N, 41 | A: 6.5 months | NR | NR | (55) |
| | | B: 4:25 | | | B: 15.9 months | | | |
| Kim et al
(2017) | RCT | 15:19 | 3.3 months | A: H&N, 13;
torso, 2; extremity, 3 | NR | A: 2 | A: Hypotension, 5;
Hypertension, 7; others, 4 | (56) |
| | | | | B: H&N, 15;
extremity, 3 | | B: 2 | B: Hypotension, 1;
Hypertension, 7; growth disability, 2; others, 5 | |
| Polites et
al (2018) | RS | 11:41 | 1-5 months | H&N, 25;
extremity, 9; perineum, 8; | NR | A: 2 | A: Ulceration, 5;
reflux, 1; loose stools, 1; Shaking spells, 1 | (57) |
| | | | | multiple, 10 | | B: 2-3 | B: Ulceration, 5;
cushingoid effects, 9; hyper tension, 3 | |
| Ali et al
(2018) | RCT | 2:03 | NR | H&N, 42; | NR | A: 2 | A: Mild flue like
symptoms, 1 | (58) |
| | | | | multiple, 18 | | B: 2 | B: Cushingoid
effects, 3 | |
 | Table IIStatistical results of propranolol
vs. steroid therapy for His. |
Table II
Statistical results of propranolol
vs. steroid therapy for His.
| Authors (year) | Total number | Effective
number | Recurrence (n) | Adverse reaction
(n) | Surgical excision
(n) | Refs. |
|---|
| Price et al
(2011) | A: 68 | A: 56 | A: 2 | A: 3 | A: 8 | (49) |
| | B: 42 | B: 12 | B: 0 | B: 14 | B: 12 | |
| Bertrand et
al (2011) | A: 12 | A: 12 | NR | A: 2 | NR | (51) |
| | B: 12 | B: 9 | | B: 7 | | |
| Rossler et
al (2012) | A: 39 | A: 25 | A: 5 | A: 6 | NR | (52) |
| | B: 38 | B: 23 | B: 3 | B: 9 | | |
| Malik et al
(2013) | A: 10 | A: 10 | NR | A: 2 | NR | (53) |
| | B: 10 | B: 9 | | B: 9 | | |
| Bauman et al
(2014) | A: 11 | A: 7 | A: 2 | A: 9 | NR | (54) |
| | B: 8 | B: 23 | B: 1 | B: 7 | | |
| Hoornweg et
al (2014) | A: 14 | A: 14 | NR | A: 0 | A: 0 | (55) |
| | B: 29 | B: 21 | | B: 16 | B: 10 | |
| Kim et al
(2017) | A: 17 | A: 17 | NR | NR | NR | (56) |
| | B: 17 | B: 15 | | | | |
| Polites et
al (2018) | A: 29 | A: 27 | A: 1 | A: 7 | NR | (57) |
| | B: 23 | B: 17 | B: 0 | B: 10 | | |
| Ali et al
(2018) | A: 30 | A: 25 | NR | A: 1 | NR | (58) |
| | B: 30 | B: 20 | | B: 3 | | |
Study quality assessment and
publication bias evaluation
According to the scoring criteria, 3 studies with
scores >20 were classified as high quality, 5 studies between 17
and 20 were classified as medium quality, and 1 study <17 was
classified as low quality (Table
III).
 | Table IIIQuality assessment of the included
studies using the methodological index for non-randomized
studies. |
Table III
Quality assessment of the included
studies using the methodological index for non-randomized
studies.
| Authors (year) | A clearly stated
aim | Inclusion of
consecutive patients | Prospective
collection of data | Endpoints
appropriate to the aim of the study | Unbiased assessment
of the study endpoint | Follow-up period
appropriate to the aim of the study | Loss to follow up
less than 5% | Prospective
calculation of the study size | An adequate control
group | Contem- porary
groups | Baseline
equivalence of groups | Adequate
statistical analyses | Total | Refs. |
|---|
| Price et al
(2011) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 19 | (49) |
| Bertrand et
al (2011) | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 19 | (51) |
| Rossler (2012) | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 16 | (52) |
| Malik et al
(2013) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 | (53) |
| Bauman et al
(2014) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 21 | (54) |
| Hoornweg et
al (2014) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 0 | 2 | 17 | (55) |
| Kim et al
(2017) | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 18 | (56) |
| Polites et
al (2018) | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 1 | 2 | 19 | (57) |
| Ali et al
(2018) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 | (58) |
In terms of publication bias, the OR value of
effectiveness was used as the abscissa and the reciprocal of OR
value as the ordinate to draw a funnel diagram. The funnel chart
had a symmetrical inverted funnel shape, indicating that the risk
of publication bias of the included studies in the present study
was small (Fig. 2).
Results of effectiveness
A total of 9 studies were included the present
study, including 230 cases of propranolol and 209 cases of steroid.
There was no statistically significant heterogeneity test between
studies (P=0.09; I2=41%). The results revealed that the
effective rate of propranolol was better than that of steroids, and
the difference was statistically significant (OR, 3.96; 95% CI,
2.47-6.37; P<0.00001; Fig.
3).
Results of recurrence rate
A total of four articles were included, including
138 cases of propranolol and 85 cases of steroids. There was no
statistically significant heterogeneity test between studies
(P=0.30; I2=0%). The results revealed that there was no
significant difference in the recurrence rate between propranolol
and steroid (OR, 1.83; 95% CI, 0.59-5.70; P=0.3; Fig. 4).
Results of adverse reactions
A total of 8 articles were included, including 204
cases of propranolol and 184 cases of steroids. There was no
statistically significant heterogeneity test between the studies
(P=0.15; I2=35%). The results revealed that the
incidence of adverse reactions of propranolol was lower than that
of the steroid group, which was statistically significant (OR,
0.21; 95% CI, 0.12-0.36; P<0.00001; Fig. 5).
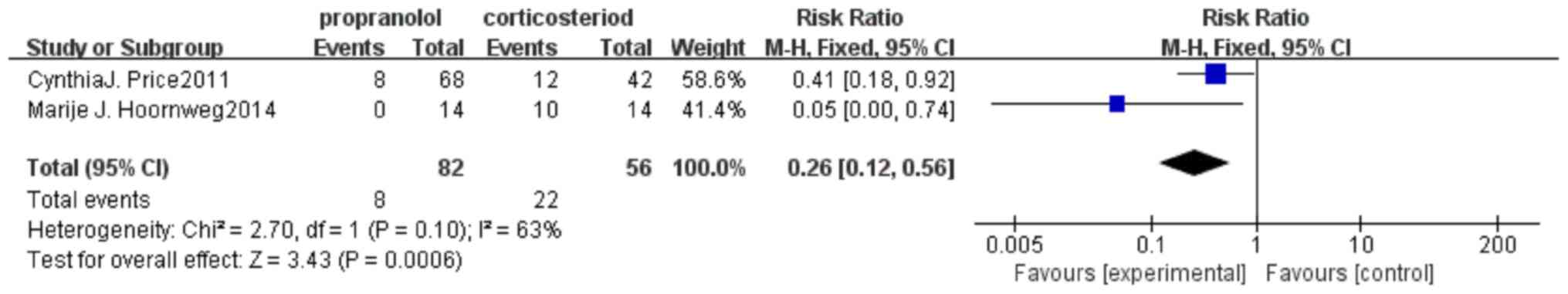
Results of surgical resection
rate
A total of two articles were included, including 82
cases of propranolol and 56 cases of steroids. There was no
statistically significant heterogeneity test between studies
(P=0.05; I2=74%). The results revealed that the surgical
resection rate of propranolol was lower than that of steroid, and
the difference was statistically significant (OR, 0.19; 95% CI,
0.08-0.46; P=0.0002; Fig. 6).
Discussion
IHs are a complex mixture of clonal endothelial
cells associated with epidermal, dendritic and mast cells (28). Of IHs, >10% can cause upper
airway obstruction (29), ulcers,
bleeding, soft tissue malformations and high-output heart failure
(30). Vascular endothelial growth
factor (VEGF) and basic fibroblast growth factor (bFGF) can
regulate the growth of IHs (31).
Steroids mainly act on the proliferation stage of IHs by inhibiting
the expression levels of immature VEGF and bFGF, which blocks
angiogenesis and prevents the growth of the tumor body (32-34).
In addition, estrogen serves a role in the growth of IHs (35), and thus, steroids may be able to
bind to estrogen receptors, thereby reducing the effects of
estrogen and ultimately inhibiting hemangioma growth. The
effectiveness of steroid therapy is ~78% (36); however, long-term use often causes
serious side effects. The majority of children will have obvious
cushings-like changes (37), and
certain patients will have behavior changes (such as irritability
or insomnia), high blood pressure, gastrointestinal irritation,
fungal infections and may even influence height development
(38). The effectiveness of
propranolol is ~88% for the treatment of IHs (36). In the short term, propranolol mainly
acts on beta receptors on capillary endothelial cells, resulting in
the receptor being unable to bind with adrenaline, and thus
inhibits vasodilation (39).
Moreover, propranolol can increase the constriction of hemangioma
pericytes and further contracts the blood vessels (40). The role of propranolol in the medium
term is major mainly inhibits cell proliferation and angiogenesis
by blocking the relevant regulatory factors and signaling pathways
of angiogenesis, including blocking the mitogen that activates the
protein kinase pathway (Rac/MAPK) (41), blocking the PI3K signaling pathway
(42), reducing hypoxia inducible
factor 1(43) and blocking
DLL4/Notch1Akt signaling (44). At
the same time, propranolol can decrease the synthesis and release
of NO, inhibit vascular smooth muscle relaxation, and cause
hemangioma vasoconstriction (45).
The long-term effect of propranolol is to induce endothelial cell
apoptosis, mainly by reducing the expression levels of STAT3 and
the anti-apoptotic protein Bcl2(44), whilst promoting the expression
levels of the apoptotic proteins caspase-3, -8 and -9(46) and the tumor suppressor gene
p53(47). Studies have shown that
when the therapeutic dose of propranolol is ≥2 mg/kg/day, a better
therapeutic effect can be obtained (48).
A total of 9 controlled experiments were included in
the present study, among which 221 patients received propranolol
and 201 patients received steroids. The results of the
meta-analysis of the 9 groups showed that propranolol had a higher
efficacy and less adverse reactions than steroids in the treatment
of IHs. Adverse reactions to propranolol occurred in 30 patients,
including asymptomatic hypotension, vomiting and non-specific rash,
but in some children the symptoms were completely reversible after
drug withdrawal. Adverse reactions to steroids occurred in 75
patients, including Cushings-like changes, oral sores,
irritability, insomnia, arterial hypertension, arterial hemorrhage,
gastroesophageal reflux, hypertrichosis, dysplasia,
hypercholesterolemia. There was no significant difference in the
recurrence rate between the two; propranolol was also lower than
steroids in the rate of surgical resection due to poor therapeutic
effect or unsatisfactory aesthetic recovery. Meanwhile, Price et
al (49) found that propranolol
costs about half as much as steroids. As propranolol has higher
efficacy and safety levels than steroids, it may replace steroids
as a new first-line therapeutic drug (50).
There were three limitations to the present study.
First, there is a small number of literatures included in the
present study, and there are not enough randomized controlled
trials, so there is a certain selectivity bias. Secondly, due to
the lack of analysis of different sites of IHs in the included
literature, the occurrence sites of hemangioma could not be studied
separately, and the sensitivity of different sites of IHs to the
two drugs could not be proved to be different. Thirdly, for
children with complications, there is a lack of long-term follow-up
to observe whether the complications have completely disappeared,
as well as the recovery time after drug withdrawal, so it is
impossible to more fully evaluate the long-term effects and harms
of propranolol on children.
In summary, the present study proves that
propranolol has better clinical efficacy and lower complications
than steroids for the treatment of IHs, and provides a certain
theoretical basis for the selection of treatment schemes for IHs.
However, more high-quality randomized controlled trials are needed
to strengthen the evidence. At the same time, the mechanism
underlying propranolol on IHs still needs further study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and YY designed the study and drafted the
manuscript. YY, YL and YX participated in study screening, data
extraction and statistics. YL and JZ were involved in result
validation and quality assessment. JZ and YY confirm the
authenticity of all the raw data. All authors contributed to
revising the manuscript and all authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aizman L, Van Den Anker J, Tender J,
Krishnan A and Kirkorian AY: Special management considerations for
propranolol use in breastfed infants of mothers taking
antihypertensives. Pediatr Dermatol. 37:537–540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Munden A, Butschek R, Tom WL, Marshall JS,
Poeltler DM, Krohne SE, Alió AB, Ritter M, Friedlander DF,
Catanzarite V, et al: Prospective study of infantile haemangiomas:
Incidence, clinical characteristics and association with placental
anomalies. Br J Dermatol. 170:907–913. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maguiness SM: Vascular tumors and
malformations in children, introduction. Semin Cutan Med Surg.
35(107)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Howard MA, Olitsky SE, Rychwalski P and
Mungan N: Management of periocular infantile hemangioma. J Pediatr
Ophthalmol Strabismus. 56:344–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Léauté-Labrèze C, Harper JI and Hoeger PH:
Infantile haemangioma. Lancet. 390:85–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuzuriha S, Nagai F and Noguchi M: How to
manage disfiguring scars in involuted infantile hemangioma. Adv
Wound Care (New Rochelle). 8:221–229. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hochman M: The role of surgery in the
management of infantile hemangiomas: What is the best timing?
Otolaryngol Clin North Am. 51:119–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Wu HW, Yuan W and Zheng JW:
Propranolol therapy for infantile hemangioma: Our experience. Drug
Des Devel Ther. 11:1401–1408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen ZY, Wang QN, Zhu YH, Zhou LY, Xu T,
He ZY and Yang Y: Progress in the treatment of infantile
hemangioma. Ann Transl Med. 7(692)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chinnadurai S, Sathe NA and Surawicz T:
Laser treatment of infantile hemangioma: A systematic review.
Lasers Surg Med. 48:221–233. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leone F, Benanti E, Marchesi A, Marcelli
S, Gazzola R and Vaienti L: Surgical excision of infantile
hemangiomas: A technical refinement to prevent bleeding
complications. J Pediatr Med Chir. 36(7)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kagami S, Kuwano Y, Shibata S, Uwajima Y,
Yamada D, Miyamoto A, Miyagawa T, Araki M, Takahashi K, Isomura S,
et al: Propranolol is more effective than pulsed dye laser and
cryosurgery for infantile hemangiomas. Eur J Pediatr.
172:1521–1526. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Satterfield KR and Chambers CB: Current
treatment and management of infantile hemangiomas. Surv Ophthalmol.
64:608–618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Techasatian L and Phukam N: Treatment
modalities and outcomes of infantile hemangiomas at srinagarind
hospital. J Med Assoc Thai. 99 (Suppl 5):S74–S80. 2016.PubMed/NCBI
|
|
15
|
Qiu Y, Lin X, Ma G, Chang L, Jin Y, Chen H
and Hu X: Eighteen cases of soft tissue atrophy after intralesional
bleomycin a5 injections for the treatment of infantile hemangiomas:
A long-term follow-up. Pediatr Dermatol. 32:188–191.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
White CW, Sondheimer HM, Crouch EC, Wilson
H and Fan LL: Treatment of pulmonary hemangiomatosis with
recombinant interferon alfa-2a. N Engl J Med. 320:1197–1200.
1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chai Y, Zhou Z, Song J, Lv R, Xu G, Bi J,
Li X, Li Z and Huo R: Safety of ntralesional injection of
lauromacrogol combined with triamcinolone for infantile
hemangiomas. J Dermatol. 46:770–776. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sinha S and Lloyd MS: Propranolol for
surgeons in the treatment of infantile hemangiomas. J Craniofac
Surg. 31:134–137. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wasserman JD, Mahant S, Carcao M, Perlman
K and Pope E: Vincristine for successful treatment of
steroid-dependent infantile hemangiomas. Pediatrics.
135:e1501–e1505. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Düzenli Kar Y, Özdemir ZC, Acu B and Bör
Ö: Infantile hemangioma: Efficacy of low-dose propranolol and of
intralesional bleomycin injection for propranolol non-response.
Pediatr Int. 61:459–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Zheng JW and Yuan WE: Treatment
of alarming head and neck infantile hemangiomas with
interferon-α2a: A clinical study in eleven consecutive patients.
Drug Des Devel Ther. 9:723–727. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Soliman YS and Khachemoune A: Infantile
hemangiomas: Our current understanding and treatment options.
Dermatol Online J. 24(13030/qt5jt8q9km)2018.PubMed/NCBI
|
|
23
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kado M, Shimizu A, Matsumura T, Mochizuki
M, Mizuno H and Hayashi A: Successful treatment of infantile
hemangiomas with propranolol in low-birth-weight infants. J
Craniofac Surg. 28:789–793. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ainipully AM, Narayanan SK, Vazhiyodan AP
and Somnath P: Oral propranolol in infantile hemangiomas: Analysis
of factors that affect the outcome. J Indian Assoc Pediatr Surg.
24:170–175. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Polites SF, Watanabe M, Crafton T, Jenkins
TM, Alvarez-Allende CR, Hammill AM and Dasgupta R: Surgical
resection of infantile hemangiomas following medical treatment with
propranolol vs. corticosteroids. J Pediatr Surg. 54:740–743.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Frieden IJ, Haggstrom AN, Drolet BA,
Mancini AJ, Friedlander SF, Boon L, Chamlin SL, Baselga E, Garzon
MC, Nopper AJ, et al: Infantile hemangiomas: Current knowledge,
future directions. Proceedings of a research workshop on infantile
hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr
Dermatol. 22:383–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Darrow DH: Management of infantile
hemangiomas of the airway. Otolaryngol Clin North Am. 51:133–146.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blei F and Guarini A: Current workup and
therapy of infantile hemangiomas. Clin Dermatol. 32:459–470.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ozeki M, Nozawa A, Hori T, Kanda K, Kimura
T, Kawamoto N and Fukao T: Propranolol for infantile hemangioma:
Effect on plasma vascular endothelial growth factor. Pediatr Int.
58:1130–1135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khan ZA, Boscolo E, Picard A, Psutka S,
Melero-Martin JM, Bartch TC, Mulliken JB and Bischoff J:
Multipotential stem cells recapitulate human infantile hemangioma
in immunodeficient mice. J Clin Invest. 118:2592–2599.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Greenberger S, Boscolo E, Adini I,
Mulliken JB and Bischoff J: Corticosteroid suppression of VEGF-A in
infantile hemangioma-derived stem cells. N Engl J Med.
362:1005–1013. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matsuda S, Gomi F, Oshima Y, Tohyama M and
Tano Y: Vascular endothelial growth factor reduced and connective
tissue growth factor induced by triamcinolone in ARPE19 cells under
oxidative stress. Invest Ophthalmol Vis Sci. 46:1062–1068.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang L, Wu HW, Yuan W and Zheng JW:
Estrogen-mediated hemangioma-derived stem cells through estrogen
receptor-α for infantile hemangioma. Cancer Manag Res. 9:279–286.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu X, Qu X, Zheng J and Zhang L:
Effectiveness and safety of oral propranolol vs. other treatments
for infantile hemangiomas: A meta-analysis. PLoS One.
10(e0138100)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bota M, Popa G, Blag C and Tataru A:
Infantile hemangioma: A brief review. Clujul Med. 88:23–27.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sethuraman G, Yenamandra VK and Gupta V:
Management of infantile hemangiomas: Current trends. J Cutan
Aesthet Surg. 7:75–85. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cavalli R, Buffon RB, de Souza M, Colli AM
and Gelmetti C: Tumor lysis syndrome after propranolol therapy in
ulcerative infantile hemangioma: Rare complication or incidental
finding? Dermatology. 224:106–109. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee D, Boscolo E, Durham JT, Mulliken JB,
Herman IM and Bischoff J: Propranolol targets the contractility of
infantile haemangioma-derived pericytes. Br J Dermatol.
171:1129–1137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pan WK, Li P, Guo ZT, Huang Q and Gao Y:
Propranolol induces regression of hemangioma cells via the
down-regulation of the PI3K/Akt/ eNOS/VEGF pathway. Pediatr Blood
Cancer. 62:1414–1420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
de Jong S, Itinteang T, Withers AH, Davis
PF and Tan ST: Does hypoxia play a role in infantile hemangioma?
Arch Dermatol Res. 308:219–227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun B, Dong C, Lei H, Gong Y, Li M, Zhang
Y, Zhang H and Sun L: Propranolol inhibits proliferation and
invasion of hemangioma-derived endothelial cells by suppressing the
DLL4/Notch1/Akt pathway. Chem Biol Interact. 294:28–33.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sharifpanah F, Saliu F, Bekhite MM,
Wartenberg M and Sauer H: β-Adrenergic receptor antagonists inhibit
vasculogenesis of embryonic stem cells by downregulation of nitric
oxide generation and interference with VEGF signalling. Cell Tissue
Res. 358:443–452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
England RW, Hardy KL, Kitajewski AM, Wong
A, Kitajewski JK, Shawber CJ and Wu JK: Propranolol promotes
accelerated and dysregulated adipogenesis in hemangioma stem cells.
Ann Plast Surg. 73 (Suppl 1):S119–S124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yao TH, Pataer P, Regmi KP, Gu XW, Li QY,
Du JT, Ge SM and Tu JB: Propranolol induces hemangioma endothelial
cell apoptosis via a p53-BAX mediated pathway. Mol Med Rep.
18:684–694. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang H, Hu DL, Shu Q and Guo XD: Efficacy
and adverse effects of oral propranolol in infantile hemangioma: A
meta-analysis of comparative studies. World J Pediatr. 15:546–558.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Price CJ, Lattouf C, Baum B, McLeod M,
Schachner LA, Duarte AM and Connelly EA: Propranolol vs.
corticosteroids for infantile hemangiomas: A multicenter
retrospective analysis. Arch Dermatol. 147:1371–1376.
2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lie E and Püttgen KB: Corticosteroids as
an adjunct to propranolol for infantile haemangiomas complicated by
recalcitrant ulceration. Br J Dermatol. 176:1064–1067.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bertrand J, McCuaig C, Dubois J, Hatami A,
Ondrejchak S and Powell J: Propranolol versus prednisone in the
treatment of infantile hemangiomas: a retrospective comparative
study. Pediatr Dermatol. 28:649–654. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rössler J, Schill T, Bähr A, Truckenmüller
W, Noellke P and Niemeyer C: Propranolol for proliferating
infantile haemangioma is superior to corticosteroid therapy-A
retrospective, single centre study. J Eur Acad Dermatol Venereol.
26:1173–1175. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Malik MA, Menon P, Rao KLN and Samujh R:
Effect of propranolol vs prednisolone vs propranolol with
prednisolone in the management of infantile hemangioma: a
randomized controlled study. J Pediatr Surg. 48:2453–2459.
2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bauman NM, McCarter RJ, Guzzetta PC, Shin
JJ, Oh AK, Preciado DA, He J, Greene EA and Puttgen KB: Propranolol
vs prednisolone for symptomatic proliferating infantile
hemangiomas: a randomized clinical trial. JAMA Otolaryngol Head
Neck Surg. 140:323–330. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hoornweg MJ, Saeed P, Tanck MW, Hage JJ,
Coumou AD and Van Der Horst CMAM: Comparison of intralesional
corticosteroid and propranolol treatment of periorbital infantile
hemangiomas: an outcome study of 61 cases. Eur J Ophthalmol.
24:940–947. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kim KH, Choi TH, Choi Y, Park YW, Hong KY,
Kim DY, Choe YS, Lee H, Cheon J-E, Park J, et al: Comparison of
efficacy and safety between propranolol and steroid for infantile
hemangioma: A randomized clinical trial. JAMA Dermatol.
153:529–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Polites SF, Rodrigue BB, Chute C, Hammill
A and Dasgupta R: Propranolol versus steroids for the treatment of
ulcerated infantile hemangiomas. Pediatr Blood Cancer.
65(e27280)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ali A, Aiman U, Haseen MA, Mir MA, Imran
G, Bharadwaj R and Yaseen M: The effect of oral propranolol versus
oral corticosteroids in management of pediatric hemangiomas. World
J Plast Surg. 7:16–24. 2018.PubMed/NCBI
|