Introduction
Endometriosis is a pathology of the female
reproductive tract (1), with a
morbidity rate of ~10% in women of reproductive age (2). Ovarian endometriomas (OE) are clinical
manifestations of endometriosis and comprise a risk factor for the
development of ovarian clear cell adenocarcinoma (CCC) and
endometrioid carcinoma, regarded as endometriosis-associated
ovarian cancers (EAOC) (3,4). The primary histological type of EAOC
in Asia is CCC, accounting for 20% of all ovarian cancers (5). The prevalence of EAOC will likely
increase along with that of endometriosis; however, the underlying
mechanisms associated with carcinogenesis are controversial. Hence,
it is important to elucidate the mechanisms underlying malignant
transformation and pathogenesis of EAOC.
Endometriosis is caused due to the reflux of
menstrual blood containing endometrial tissues (6), and OE form when menstrual blood flows
into the ovary (6), carrying
endometrial epithelium and endometrial stroma into the ovary
(7). Moreover, iron within the
menstrual blood serves as an etiological factor for the development
of ovarian cancer from OE (8-10).
However, the precise mechanism through which iron ions flow in
endometrial epithelial cells remains unknown.
Iron ions are primarily absorbed by the epithelium
of the intestinal tract (11),
after which iron is transported by divalent metal transporter1
(DMT1) that imports and ferroportin (FPN) that exports iron ions
(12,13). Furthermore, transferrin receptors
(TfR) uptake iron ions in other organs (14). These transporters regulate
intracellular iron concentrations (11). Meanwhile, iron transporters in OE
and CCC have rarely been investigated, and the mechanism of iron
flow through endometrial epithelial cells of OE or CCC tumor cells
remains unclear.
Macrophages within the endometrial stroma of OE
accumulate around the epithelium to englobe iron (7). The M1 macrophage phenotype induces
inflammatory cytokines and bactericidal activity (15), and M2 macrophages polarize toward
tumor-associated macrophages (TAMs) after T cells and tumor cells
release cytokines such as IL-4, IL-13, and macrophage
colony-stimulating factor (M-CSF) (15). Thereafter, TAMs migrate to hypoxic
regions, where they release growth factors and angiogenetic factors
that promote proliferation and metastasis (16). Although M2 macrophages have been
detected in OE and CCC (17,18),
their effect on OE carcinogenesis has not been investigated.
The present study aimed to immunohistochemically
determine the expression of iron transporters in the endometrial
epithelium of OE and tumor cells of CCC. We also evaluated the
relation of macrophages to endometrial epithelial carcinogenesis in
OE.
Materials and methods
Samples
The study was approved by the Institutional Review
Board of Tokyo Women's Medical University Hospital (Approval number
5371). We retrospectively analyzed 20 OE tissues without malignant
lesions and 18 ovarian CCC that were surgically excised at Tokyo
Women's Medical University Hospital between January 2014 and
December 2019. Opt-out informed consent was obtained from all
patients.
Perls Prussian blue staining
Tissues were stained with Perls Prussian blue using
potassium hexacyanoferrate (II) trihydrate (Wako Pure Chemical
Industries, Osaka, Japan) and enhanced with Nuclear Fast Red (Cosmo
Bio Co, Tokyo, Japan).
Hematoxylin and eosin (H&E)
staining and immunohistochemistry
Paraffin-embedded tissue sections (4 µm) were
deparaffinized for 10 min. Then the sections were stained with
H&E according to routine hospital procedures. For
immunohistochemistry, antigen was retrieved by autoclaving for 20
min or microwave heating for 15 min in 10 mM sodium citrate buffer,
pH 6.0 or 9.0. Nonspecific protein binding in tissue sections was
blocked using Protein Block Serum-Free (Dako A/S, Glostrup,
Denmark) at 25˚C, for 20 min. The sections were incubated overnight
at 4˚C with primary antibodies, followed by secondary antibodies
(Dako) for 30 min at 25˚C, and finally with 3,3'-diaminobenzidine
(Nichirei Biosciences, Tokyo, Japan) to visualize antigen binding.
Nuclei were stained with hematoxylin (Wako Pure Chemical
Industries). Primary antibodies against the following were used:
DMT1 (1:800), TfR (1:500), CD68 (1:400), CD11c (1:500), CD163
(1:500), and CD206 (1:5,000) (all from Abcam, Cambridge, United
Kingdom), FPN (1:400; Novus Biologicals, Centennial, CO, USA), Ki67
(1:100) and cytokeratin (1:200) (both from Dako), and CD10 (1: 50;
Leica Biosystems, Wetzlar, Germany). Samples prepared in the same
manner but without incubation with the primary antibodies were used
as negative controls (data not shown).
Immunohistochemistry analysis
The intensity of DMT1, TfR, and FPN staining was
scored using an arbitrary scale as 0, absent; 1, light; 2,
moderate; 3, intense. We used the score that was the most prevalent
in 10 fields. Due to endometrial epithelial detachment, 10 fields
could not be evaluated in three OE samples, and hence, these
samples were excluded from the analysis.
We evaluated CD11c, CD163, and CD206 expression as
the average number of antibody-positive cells counted in 10 fields
at x400 magnification. We evaluated ratios (%) of Ki67-positive
nuclei and averaged the numbers of cells counted at x400
magnification in 10 fields. Two pathologists assessed the
immunohistochemical scores and numbers of positive cells in all
sections.
Statistical analysis
Data were statistically analyzed using
JMP® 14 (SAS Institute Inc., Cary, NC, USA). Continuous
variables are presented as mean ± standard deviation. Categorical
variables (Nulliparous and Premenopause) are presented as numbers
(percentage) and compared with χ2 test or Fisher's exact
test. Shapiro-Wilk test was performed to determine if the
continuous variables were normally distributed. The levels of CA125
are expressed as the median with interquartile range (IQR) as they
were not normally distributed. Comparison of two groups with
normally distributed data (mean age and maximal cyst/tumor
diameter) was performed using Welch's t-test; otherwise, comparison
of continuous variables (median CA125) was performed using the
non-parametric Wilcoxon rank sum test. Two groups and more than two
groups were compared using the non-parametric Wilcoxon rank sum
test and Steel-Dwass test (used after Kruskal-Wallis test),
respectively, to confirm the significance of iron transport
proteins and the number of positively immunostained cells.
Statistical significance was set at P<0.05.
Results
Clinical features
Table I lists the
clinical features of the 18 CCC patients and 20 OE patients. The
average age of the CCC (56 years) and OE groups (47 years) differed
significantly (P=0.0105). Most CCC patients had a stage I disease,
according to the International Federation of Gynecology and
Obstetrics (FIGO) staging guidelines. Of the 18 CCC patients, seven
had a medical history of OE, two had a histopathological diagnosis
of OE, one had both medical history and histopathological diagnosis
of OE, and the medical history of OE in eight patients was unknown.
The maximal tumor diameter was significantly larger in CCC than
that in OE (P<0.001).
 | Table ICharacteristics of patients with CCC
(n=18) or OE (n=20). |
Table I
Characteristics of patients with CCC
(n=18) or OE (n=20).
| Variable | CCC | OE | P-value |
|---|
| Mean age (SD),
years | 56(11) | 47(3) | 0.0105 |
| Nulliparous, n
(%) | 9(50) | 13(65) | 0.5118 |
| Premenopause, n
(%) | 5(27) | 19(95) | <0.001 |
| Maximal cyst/tumor
diameter, mean (SD), mm | 123(39) | 46(21) | <0.001 |
| Median CA125 (IQR),
U/ml | 45 (21-184) | 58 (28-172) | 0.6742 |
| FIGO stage, n
(%) | | | |
|
I | 14(77) | | |
|
II | 1(5) | | |
|
III | 3(16) | | |
|
IV | 0 (0) | | |
| OE positive, n
(%) | 10(55) | | |
| Iron deposition
positive, n (%) | 13(72) (in cancer
cells and stroma) | 4(20) (in
endometrial epithelium) and 20(100) (throughout stroma) | |
Iron deposition in OE and CCC
We initially investigated iron localization in OE
and CCC by staining tissue sections with Prussian blue (Fig. 1). Iron was deposited in the
endometrial epithelium (Fig. 1A
arrow) of four OE samples and englobed macrophages in the stroma
just below the epithelium (Fig. 1A)
of all OE samples. Iron was deposited throughout the stroma in all
OE specimens (Table I). Stroma
located beneath the aggregated englobed macrophages (referred to
herein as ‘deep layer’; Fig. 1B,
region within the dotted line) also contained iron. Iron was
deposited in CCC cancer cells and stroma in 13 specimens (Fig. 1C and Table I).
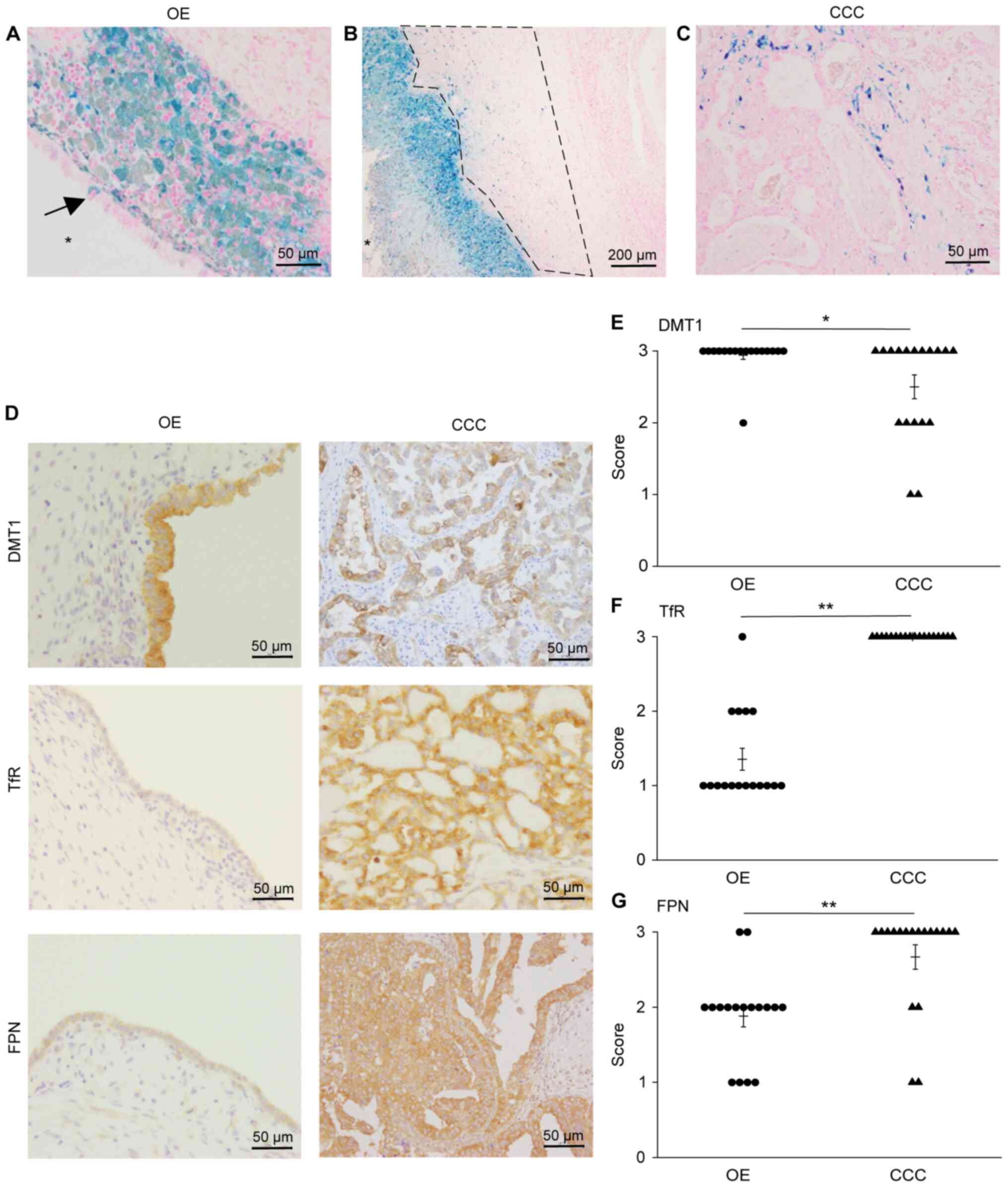 | Figure 1Iron deposits detected by Prussian
blue staining and expression of iron transport proteins in the
endometrial epithelium of OE and ovarian CCC tumor cells. (A) Iron
deposits in the endometrial epithelium of OE (arrow). Macrophages
englobed with iron are seen immediately below the epithelium.
Magnification, x400. Scale bar, 50 µm. The star (*) indicates the
lumen of OE. (B) Deep layer represents stroma beneath aggregated
englobed macrophages (region within the dotted line). Iron deposits
observed in englobed macrophages and deep layer. Magnification,
x100. Scale bar, 200 µm. The star (*) indicates the lumen of OE.
(C) Iron deposits in tumor cells and stroma of CCC. Magnification,
x400. Scale bar, 50 µm. (D) Immunolocalization of DMT1, TfR and
FPN. Magnification, x400. Scale bar, 50 µm. Comparison of (E) DMT1,
(F) TfR and (G) FPN expression. *P<0.05 and
**P<0.001 via Wilcoxon rank sum test. CCC, clear cell
adenocarcinoma; OE, ovarian endometrioma; DMT1, divalent metal
transporter 1; TfR, transferrin receptor; FPN, ferroportin. |
Immunostaining for iron transport
proteins
Considering that iron deposition was observed in
both OE and CCC, we investigated the expression of iron transport
proteins DMT1, TfR, and FPN by immunostaining (Fig. 1D). All specimens of OE epithelium
stained positive for these proteins. Specifically, DMT1 was scored
3 (intense staining) in 16 (Fig.
1E), TfR was scored 1 (light staining) in 12 (Fig. 1F), and FPN was scored 2 (moderate
staining) in 11 (Fig. 1G) OE
specimens. All three transporters were expressed at high levels in
CCC. Comparing the expression of the iron transport proteins in OE
and CCC specimens, we observed no remarkable difference in DMT1
expression (Fig. 1E), whereas
expression of TfR and FPN was significantly higher (P<0.001) in
CCC than in OE (Fig. 1F and
G).
Macrophages with englobed iron
infiltrate OE and CCC
Fig. 1 indicates
that iron ions flowed into and drained from cells. Iron was
deposited not only in epithelial cells but also in the stroma of
OE. Although Prussian blue staining revealed iron deposition in
macrophages located immediately below the epithelium, it was
difficult to ascertain whether iron ions in the entire stroma were
englobed by macrophages. Hence, we examined the distribution of
macrophages in the stroma to determine if they englobed iron ions
as well.
We detected the pan-macrophage marker CD68 in the
deep stroma layer, and the iron englobed macrophages below the
epithelium (Fig. 2A, CD68).
Immunohistochemical staining for CD11c (M1 macrophage marker),
CD163, and CD206 (M2 macrophage markers) markers revealed the
presence of CD11c+, CD163+, and
CD206+ cells immediately just below the OE epithelium,
and only M2 macrophages in the deep layer of the stroma (Fig. 2A, region within the dotted line).
Double staining for iron and CD163 revealed the presence of M2
macrophages englobed iron ions in the deep layer of the OE stroma
(Fig. 2A arrow). We located
significantly more CD163+ and CD206+ cells,
than CD11c+ cells in the deep layer of OE (Fig. 2C, P<0.001). CD68+
cells, along with conspicuous staining of CD163+ and
CD206+ cells, were observed in the stroma of CCC tumors
(Fig. 2B). We also observed
macrophages with englobed iron (Fig.
2B arrow) and far more abundant M2 than M1 macrophages in CCC
specimens (Fig. 2D, P<0.001).
There were no statistically significant differences in the numbers
of cells positive for CD11c, CD163, and CD206 between OE and CCC
(data not shown).
 | Figure 2Immunolocalization of CD68, CD11c,
CD163 and CD206. (A) Stroma under macrophages englobed with iron in
OE specimens designated as deep layer (region within the dotted
line). Distribution of CD68, CD11c, CD163 and CD206 in the deep
layer. Double staining for iron and CD163 shows M2 macrophages
englobed with iron in the stroma (arrows). The star (*) indicates
the lumen of OE. Magnification, x400. Scale bar, 50 µm. (B)
Distribution of CD68, CD11c, CD163 and CD206 in ovarian CCC
specimens. Double staining for iron and CD163 shows M2 macrophages
englobed with iron in the stroma of CCC (arrows). Magnification,
x400. Scale bar, 50 µm. Comparison of numbers of antibody-positive
cells in (C) OE and (D) CCC. **P<0.001 via
Steel-Dwass test (used after Kruskal-Wallis test). NS, not
significant; CCC, clear cell adenocarcinoma; OE, ovarian
endometrioma. |
Endometrial epithelium infiltrates the
stroma of OE
We postulated that the macrophages with englobed
iron in the deep layer of the OE stroma resulted from endometrial
epithelium infiltration of the OE stroma. We further hypothesized
that M2 macrophages colonized the epithelium and invaded the
stroma.
The endometrial epithelium forms rows in typical OE
(Fig. 3A). Fig. 2 demonstrates more abundant M2 than
M1 macrophages in the deep layers of the stroma (Fig. 3A; CD68, CD11c, CD163, and CD206 are
shown in Fig. 2A). The positive
rate of Ki67 (tumor growth marker) was low in the endometrial
epithelium (4.6%±1.6, Fig. 4A).
 | Figure 3Keratin staining indicates
endometrial epithelium infiltration of the stroma. (A) Endometrial
epithelium of typical OE was used as a control. Endometrial
epithelium formed a row and was stained with keratin (region within
the black square in the H&E-stained figure; magnification,
x400). CD68+, CD11c+, CD163+ and
CD206+ cells below the epithelium and the deep stroma
layer (region within the dotted line). (B) Endometrial epithelium
of OE. Keratin staining in the area where endometrial epithelium
infiltrated stroma and formed lumens (region within the black
square in the H&E-stained figure; magnification, x400).
CD68+ cells around infiltrated epithelium. CD163 and
CD206 were stained more intensely than CD11c. (C and D) Endometrial
epithelium adjacent to ovarian CCC from two patients. Keratin
staining in the area where endometrial epithelium infiltrated the
stroma and formed lumens (region within the black square in
H&E-stained figure; magnification, x400). CD68+
cells surrounded infiltrated epithelium. CD163 and CD206 were
stained more intensely than CD11c. H&E staining magnification,
x100; scale bar, 200 µm. Other images magnification, x400; scale
bar, 50 µm. The star (*) indicates the lumen of OE. CCC, clear cell
adenocarcinoma; OE, ovarian endometrioma; H&E,
hematoxylin-eosin. |
Staining for the epithelial cell marker keratin
revealed the regions where the endometrium epithelium had
infiltrated the stroma and had formed lumens in one OE specimen
(Fig. 3B: Patient no. 1, region
within the black square in the H&E staining figure) and in two
CCC specimens with OE adjacent to CCC (Fig. 3C-D: Patient no.2-3, region within
the black square in the H&E staining figure). These regions
contained more abundant CD163+ and CD206+
cells than CD11c+ cells (Figs. 3B-D and S1, and Ki67 positive rate of 14.3-38.5%;
Patient no.1: 38.5±6.0%, Patient no. 2: 36.1±3.7%, Patient no. 3:
14.3±2.4% was observed in the endometrium epithelium infiltrating
the stroma (Fig. 4B-D).
Endometrium, as well as ovarian follicles, stained positive for
keratin. To avoid misinterpreting these findings as normal, we
distinguished endometrial epithelium from ovarian follicles using
CD10, a diagnostic marker of endometriosis (19). The endometrial stroma was positive,
while the follicular epithelium was negative for CD10 (Fig. S2).
Discussion
In the present study, we detected iron transporters
in OE and CCC using immunohistochemistry and identified M2
macrophages englobed with iron throughout the stroma of OE. The
endometrial epithelium infiltrating the stroma was highly
proliferative.
Although the precise mechanisms associated with the
carcinogenesis of EAOC remain under investigation, the
co-occurrence of the AT-rich interaction domain 1A (ARID1A) and the
phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit
alpha (PIK3CA) mutation promotes CCC growth (20-23).
Moreover, an iron-rich environment contributes to cell
proliferation in OE, with oxidative stress and reactive oxygen
species implicated in DNA damage (8,10,23,24).
Thus, these findings suggest a relationship between iron and OE
tumorigenesis. However, the mechanisms through which endometrial
epithelium and tumor cells transport iron ions have remained
unknown. Therefore, we aimed to demonstrate iron flow using
immunohistochemistry. The transmembrane protein for ferric metal
iron, DMT1, plays a major role in the intestinal uptake of iron
ions (11). This protein is located
not only on the cell surface but also in endosomes (12), with TfR also located on the cell
surface. Because transferrin-binding trivalent iron molecules bind
TfR, the complex becomes internalized in cells via endocytosis
(12). Alternatively, FPN is the
only transporter that exports iron ions in humans (25). The iron-responsive element (IRE) and
iron regulatory proteins regulate DMT1, TfR, and FPN expression
(26,27). Hence, iron metabolism is strictly
controlled by these mechanisms. This control system collapses in
cancer, and the iron overload associated with cancer is largely due
to iron dysregulation (28).
Specifically, DMT1 expression is upregulated in colorectal cancer
as compared to healthy colons (29). Furthermore, the expression of TfR
and FPN is high and low in high-grade serous ovarian carcinoma,
respectively, implying storage of excess iron ions in cells within
this carcinoma type (30). The
present findings indicate the upregulation of DMT1 and
downregulation of TfR and FPN expression in OE. Moreover, the
levels of the iron transporters differed in CCC tissues. We also
verified that iron ions flow in and out of the endometrial
epithelium, indicating that the differential abundance of
transporters is associated with intercellular iron concentrations,
which participate in carcinogenesis.
Excessive iron was detected not only in the
endometrial epithelium but also in the stroma of OE, and M2
macrophages englobed iron ions in the stroma. Macrophages polarized
toward the M2 phenotype generally produce anti-inflammatory
cytokines and participate in tissue repair and angiogenesis
(15), whereas M1 macrophages
induce inflammatory cytokines and bactericidal activity. Therefore,
M2 macrophages are regarded as TAMs (15). These TAMs have been detected within
ascites from patients with ovarian cancer, where interaction
between tumor cells and M2 macrophages induced tumor progression
via signal transducer and activator of transcription 3 (STAT3)
(31). In agreement with these
findings, we detected more M2 than M1 macrophages in the stroma of
OE and CCC. Furthermore, we identified M2 macrophages englobed with
iron in the deep layer of OE stroma, which became a central focus
of the study. Considering that ovarian cancer is an epithelial
cancer, these M2 macrophages englobed with iron likely influence
epithelial carcinogenesis by infiltrating endometrial epithelium in
the stroma. The endometrial epithelium OE typically forms smooth
rows of cells (7,32). However, we demonstrate that
endometrial epithelium infiltrates and forms lumens within the
stroma. Moreover, the infiltrating epithelium was highly positive
for Ki67 compared with normal endometrial epithelium. This
indicates that the endometrial epithelium has potent proliferative
capacity, which is a state closer to cancer.
The infiltrative endometrial epithelium adjacent to
CCC is likely to be a precancerous lesion. However, infiltrative
endometrial epithelium in OE also has potential carcinogenic
capacity, as benign OE has loss-of-function mutations in ARID1A
like in CCC (33,34). Considering that OE harbors ARID1A
loss-of-function mutations, M2 macrophages in these regions might
accelerate carcinogenesis of the endometrial epithelium
infiltrating the stroma. We suggest that OE carcinogenesis occurs
not only in endometrial cysts but also in epithelium infiltrating
the stroma. Studies in a larger patient cohort are needed to verify
these findings. In a preliminary study, we performed an experiment
using an ovarian clear cell adenocarcinoma cell line and identified
that TfR and DMT1 could be detected by western blotting. We thought
that we should conduct additional experiments using ovarian
endometrioma, ovarian clear cell adenocarcinoma, and normal ovary
tissues as the study's primary aim was to compare between ovarian
endometrioma and ovarian clear cell adenocarcinoma. Due to the lack
of ovarian endometrioma and normal ovary cell lines, we intend
further explore the role of iron transporters using an animal
model, where we will be able to access more tissue samples and
perform western blots. Nevertheless, our results provide insights
into a potential underlying mechanism of carcinogenesis from OE to
CCC while elucidating tumor growth features. Confirming these
findings using pathological specimens, we could recognize the
possibility of them being precancerous lesions. Moreover, careful
follow-up is needed for the prevention and early detection of CCC.
Further investigations of the mechanisms responsible for the
infiltration of the endometrial epithelium into the stroma are
warranted.
In summary, the present study discovered iron
transport proteins in the epithelium of OE and CCC tumor cells. We
also revealed M2 macrophages englobed with iron in the stroma of OE
and CCC. Epithelium invading the stroma of OE implies
carcinogenesis under the influence of M2 macrophages englobed with
iron. These findings offer a new perspective on the malignant
transformation of OE into EAOC, which may serve as a precancerous
index factor.
Supplementary Material
Number of cells positive for CD11c,
CD163 and CD206 in the endometrial epithelium-infiltrating stroma.
(A) Number of antibody-positive cells around the infiltrated
epithelium in (A) patient no. 1, (B) patient no. 2 and (C) patient
no. 3. The data are shown as the mean ± SD in 10 fields of
view.
Endometrial epithelium and ovarian
follicle infiltrated by OE stroma. CD10 staining of (A) ovarian
follicle and (B) endometrial stroma. (C) Keratin-stained epithelium
of ovarian follicle. Magnification, x400. Scale bar, 50 μm. OE,
ovarian endometrioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KA, YN and HO contributed to study design. KA, YN,
TT and HO developed the methodology and assessed the authenticity
of the data. KA, YN and TT validated the data and performed formal
analysis. YN and TT provided resources. KA wrote the original
draft. KA, YN, TT and HO reviewed and edited the manuscript. HO
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Tokyo Women's Medical University Hospital (approval no.
5371; Tokyo, Japan). Opt-out informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rogers PA, D'Hooghe TM, Fazleabas A,
Gargett CE, Giudice LC, Montgomery GW, Rombauts L, Salamonsen LA
and Zondervan KT: Priorities for endometriosis research:
Recommendations from an international consensus workshop. Reprod
Sci. 16:335–346. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fukunaga M, Nomura K, Ishikawa E and
Ushigome S: Ovarian atypical endometriosis: Its close association
with malignant epithelial tumours. Histopathology. 30:249–255.
1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hooper G: The diagnosis and treatment of
endometriosis. Can Med Assoc J. 42:243–246. 1940.PubMed/NCBI
|
|
7
|
Clement PB: The pathology of
endometriosis: A survey of the many faces of a common disease
emphasizing diagnostic pitfalls and unusual and newly appreciated
aspects. Adv Anat Pathol. 14:241–260. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamaguchi K, Mandai M, Toyokuni S,
Hamanishi J, Higuchi T, Takakura K and Fujii S: Contents of
endometriotic cysts, especially the high concentration of free
iron, are a possible cause of carcinogenesis in the cysts through
the iron-induced persistent oxidative stress. Clin Cancer Res.
14:32–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fujimoto Y, Imanaka S, Yamada Y, Ogawa K,
Ito F, Kawahara N, Yoshimoto C and Kobayashi H: Comparison of redox
parameters in ovarian endometrioma and its malignant
transformation. Oncol Lett. 16:5257–5264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Greenshields AL, Shepherd TG and Hoskin
DW: Contribution of reactive oxygen species to ovarian cancer cell
growth arrest and killing by the anti-malarial drug artesunate. Mol
Carcinog. 56:75–93. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Gunshin H, Mackenzie B, Berger UV, Gunshin
Y, Romero MF, Boron WF, Nussberger S, Gollan JL and Hediger MA:
Cloning and characterization of a mammalian proton-coupled
metal-ion transporter. Nature. 388:482–488. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Tabuchi M, Yoshimori T, Yamaguchi K,
Yoshida T and Kishi F: Human NRAMP2/DMT1, which mediates iron
transport across endosomal membranes, is localized to late
endosomes and lysosomes in HEp-2 cells. J Biol Chem.
275:22220–22228. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ross SL, Tran L, Winters A, Lee KJ, Plewa
C, Foltz I, King C, Miranda LP, Allen J, Beckman H, et al:
Molecular mechanism of hepcidin-mediated ferroportin
internalization requires ferroportin lysines, not tyrosines or
JAK-STAT. Cell Metab. 15:905–917. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gammella E, Buratti P, Cairo G and
Recalcati S: The transferrin receptor: The cellular iron gate.
Metallomics. 9:1367–1375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Yamada Y, Uchiyama T, Ito F, Kawahara N,
Ogawa K, Obayashi C and Kobayashi H: Clinical significance of M2
macrophages expressing heme oxygenase-1 in malignant transformation
of ovarian endometrioma. Pathol Res Pract. 215:639–643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Canet B, Pons C, Espinosa I and Prat J:
CDC42-positive macrophages may prevent malignant transformation of
ovarian endometriosis. Hum Pathol. 43:720–725. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Toki T, Shimizu M, Takagi Y, Ashida T and
Konishi I: CD10 is a marker for normal and neoplastic endometrial
stromal cells. Int J Gynecol Pathol. 21:41–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chandler RL, Damrauer JS, Raab JR,
Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D,
Xiong J, et al: Coexistent ARID1A-PIK3CA mutations promote ovarian
clear-cell tumorigenesis through pro-tumorigenic inflammatory
cytokine signalling. Nat Commun. 6(6118)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamamoto S, Tsuda H, Takano M, Tamai S and
Matsubara O: Loss of ARID1A protein expression occurs as an early
event in ovarian clear-cell carcinoma development and frequently
coexists with PIK3CA mutations. Mod Pathol. 25:615–624.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Samartzis EP, Noske A, Dedes KJ, Fink D
and Imesch P: ARID1A mutations and PI3K/AKT pathway alterations in
endometriosis and endometriosis-associated ovarian carcinomas. Int
J Mol Sci. 14:18824–18849. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang HN, Lin MC, Huang WC, Chiang YC and
Kuo KT: Loss of ARID1A expression and its relationship with
PI3K-Akt pathway alterations and ZNF217 amplification in ovarian
clear cell carcinoma. Mod Pathol. 27:983–990. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Donovan A, Lima CA, Pinkus JL, Pinkus GS,
Zon LI, Robine S and Andrews NC: The iron exporter
ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab.
1:191–200. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schümann K, Moret R, Künzle H and Kühn LC:
Iron regulatory protein as an endogenous sensor of iron in rat
intestinal mucosa. Possible implications for the regulation of iron
absorption. Eur J Biochem. 260:362–372. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miyazawa M, Bogdan AR, Hashimoto K and
Tsuji Y: Regulation of transferrin receptor-1 mRNA by the interplay
between IRE-binding proteins and miR-7/miR-141 in the 3'-IRE
stem-loops. RNA. 24:468–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Miller LD, Coffman LG, Chou JW, Black MA,
Bergh J, D'Agostino R Jr, Torti SV and Torti FM: An iron regulatory
gene signature predicts outcome in breast cancer. Cancer Res.
71:6728–6737. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xue X, Ramakrishnan SK, Weisz K, Triner D,
Xie L, Attili D, Pant A, Győrffy B, Zhan M, Carter-Su C, et al:
Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling
to promote colorectal tumorigenesis. Cell Metab. 24:447–461.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Basuli D, Tesfay L, Deng Z, Paul B,
Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, et al: Iron
addiction: A novel therapeutic target in ovarian cancer. Oncogene.
36:4089–4099. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takaishi K, Komohara Y, Tashiro H, Ohtake
H, Nakagawa T, Katabuchi H and Takeya M: Involvement of
M2-polarized macrophages in the ascites from advanced epithelial
ovarian carcinoma in tumor progression via Stat3 activation. Cancer
Sci. 101:2128–2136. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tran-Harding K, Nair RT, Dawkins A, Ayoob
A, Owen J, Deraney S, Lee JT, Stevens S and Ganesh H: Endometriosis
revisited: An imaging review of the usual and unusual
manifestations with pathological correlation. Clin Imaging.
52:163–171. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yachida N, Yoshihara K, Suda K, Nakaoka H,
Ueda H, Sugino K, Yamaguchi M, Mori Y, Yamawaki K, Tamura R, et al:
ARID1A protein expression is retained in ovarian endometriosis with
ARID1A loss-of-function mutations: Implication for the two-hit
hypothesis. Sci Rep. 10(14260)2020.PubMed/NCBI View Article : Google Scholar
|


















