Introduction
According to the GLOBOCAN 2018 data, colorectal
cancer (CRC) is the third leading cause of cancer-related mortality
worldwide (1). Despite the high
mortality rate of CRC, the substantial progress in
multidisciplinary approaches, including chemotherapy, has improved
the survival of patients with advanced disease, with a median
overall survival of 30 months achieved in clinical trials (2). Overall, the majority of the patients
are treated in the outpatient setting.
Pulmonary thromboembolism (PTE) is a serious
complication and constitutes one of the leading causes of death
among cancer outpatients (3). It is
known that tumor-derived tissue factor activates the clotting
cascade, and various factors, such as decreased physical activity
and mechanical compression of veins by the tumor, may promote
thrombus formation in patients with cancer (4). Moreover, bevacizumab, which is used in
the treatment of several malignancies, including CRC, is believed
to cause vascular endothelial disorders by inhibiting vascular
endothelial growth factor and promoting thrombosis (5). An increased risk of venous
thromboembolism (VTE), including PTE, has been reported in patients
with cancer receiving bevacizumab (6).
The D-dimer test is useful for the exclusion of
acute PTE in non-cancer patients; however, as it has high
sensitivity but low specificity, it is only useful if the clinical
probability is low. Alternatively, although the pre-test
probability of PTE is higher among patients with cancer compared
with non-cancer patients, the proper utilization of the D-dimer
test remains unclear (7).
In patients with unresectable, advanced or recurrent
CRC treated with bevacizumab, Mochizuki et al (8) reported that the cutoff value of the
D-dimer test for diagnosis of VTE, including PTE, is 3.0 µg/ml,
with a sensitivity of 75%, specificity of 72% and negative
predictive value (NPV) of 98%, and may thus be useful for exclusion
diagnosis. Conversely, as cancer progression, as well as
thrombosis, increase the D-dimer level, the significance of D-dimer
monitoring in the clinical course of patients with cancer remains
unclear. Moreover, the recurrence rate of lung metastasis as the
site of the first recurrence in patients with CRC is only 5.5%
(9), contrast-enhanced CT scan of
the chest is not routine practice in patients undergoing CT scans
for routine staging of malignancies, and even contrast-enhanced
chest CT scans are associated with high false-negative rates
(10). Therefore, the actual
association between PTE and D-dimer monitoring remains unknown.
The present study was undertaken to investigate the
reliability and validity of D-dimer monitoring in PTE diagnosis by
reassessment of CT images in selected patients with unresectable,
advanced or recurrent CRC who received bevacizumab as primary
treatment and were subjected to contrast-enhanced chest CT
examination.
Materials and methods
Patients and study design
The medical records of 63 patients with
histologically confirmed unresectable, advanced or recurrent CRC
who received bevacizumab combination chemotherapy as primary
treatment at the NTT Medical Center Tokyo (Tokyo, Japan) between
April 2015 and December 2018 were retrospectively reviewed. A total
of 25 patients who met the selection criteria [D-dimer tests were
performed repetitively, both at baseline and during the bevacizumab
treatment period, and abdominal contrast-enhanced chest CT scans
performed for any cause (Fig. 1)]
were included in the present study. None of the eligible patients
had PTE prior to bevacizumab combination chemotherapy or were
receiving anticoagulation therapy.
In our selected study population, the presence or
absence of PTE and the diagnostic accuracy of D-dimer testing for
PTE diagnosis were retrospectively examined, along with an
assessment of the patient background and Khorana score (11), which is a score for predicting
thrombosis in patients with cancer. The best overall response was
assessed according to the Response Evaluation Criteria in Solid
Tumors (version 1.1) (12). PTE was
retrospectively reassessed by the investigators and a radiologist
using chest and abdominal contrast-enhanced CT images.
This study was conducted after obtaining approval
from the Institutional Review Board of NTT Medical Center Tokyo
(approval no. 19-62).
D-dimer level measurement
A plasma D-dimer latex immunoassay (LIA) was
performed using the Nanopia® D-dimer assay kit (Sekisui
Medical Co., Ltd.). The measurable D-dimer LIA value ranges from
0.5 to 60 µg/ml, while the upper limit of normal is 1.0 mg/ml.
Statistical analysis
Statistical analyses were performed to calculate the
sensitivity, specificity and NPV. The cutoff value of the upper
limit of the normal D-dimer value was set to 3.0 µg/ml with
reference to the report by Mochizuki et al (8). All statistical analyses were performed
using EZR software (version 1.35; Saitama Medical Center, Jichi
Medical University, Saitama, Japan). P<0.05 was considered to
indicate statistically significant differences.
Results
Patient characteristics
A total of 25 patients who underwent a total of 250
D-dimer tests after receiving bevacizumab combination chemotherapy
were included in this investigation. The characteristics of the
included patients are summarized in Table I. The Khorana score was 0 points in
22 cases (88%), and there were no patients with scores of ≥2
points. The median baseline D-dimer value in our study population
was 1.3 µg/ml, and the D-dimer value was >1.0 µg/ml, which is
the upper limit among patients without cancer, in 15 cases (60%).
PTE was detected in 4 of the 25 cases (16%) on contrast-enhanced CT
images, with all cases being asymptomatic.
 | Table IPatient characteristics and incidence
rate of PTE. |
Table I
Patient characteristics and incidence
rate of PTE.
| Characteristics | No. (%) |
|---|
| Age, years
(range) | 66 (42-81) |
| Sex | |
|
Male | 12 (48.0) |
|
Female | 13 (52.0) |
| Concurrent
regimen | |
|
mFOLFOX6 | 19 (76.0) |
|
CapeOX | 3 (12.0) |
|
FOLFIRI | 2 (8.0) |
|
CPT-11 | 1 (4.0) |
| Best overall
responsea | |
|
Complete
response | 0 (0) |
|
Partial
response | 7 (28.0) |
|
Stable
disease | 14 (56.0) |
|
Progressive
disease | 4 (16.0) |
| Khorana score | |
|
0 | 22 (88.0) |
|
1 | 3 (12.0) |
|
≥2 | 0 (0) |
| Baseline D-dimer,
µg/ml (range) | 1.3 (0.4-16.9) |
| Baseline
carcinoembryonic antigen, ng/ml (range) | 22.9
(1.7-2,330.0) |
| Baseline carbohydrate
antigen 19-9, U/ml (range) | 120 (0-284,810) |
| Number of bevacizumab
cycles (range) | 10 (2-36) |
| Number of D-dimer
tests per patient (range) | 8 (1-33) |
| Number of enhanced CT
scans per patient (range) | 3 (1-7) |
| PTE cases | 4 (16.0) |
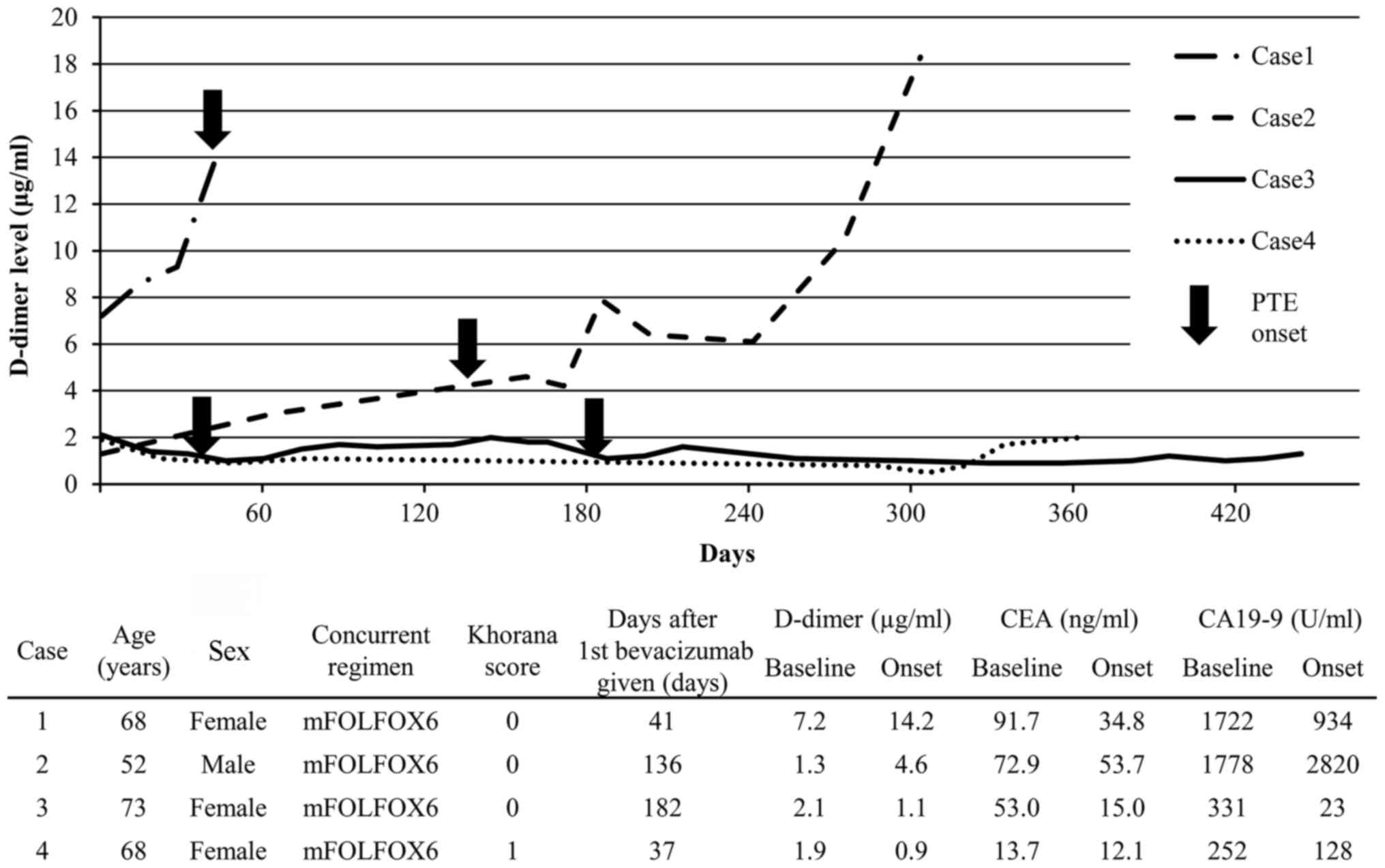
Clinical course of D-dimer values and
characteristics of PTE cases
The clinical course of the D-dimer values and the
characteristics of the 4 patients of interest are described in
Fig. 2. In Case 1, the baseline
D-dimer value was positive at 7.2 µg/ml, and at the onset of PTE it
further increased to 14.2 µg/ml. In Case 2, the baseline D-dimer
value was negative at 1.3 µg/ml, but at the onset of PTE it was
positive at 4.6 µg/ml, and the carbohydrate antigen (CA) 19-9 level
had also increased from 1,778 to 2,820 U/ml. In Cases 3 and 4, the
baseline D-dimer values were negative and were found to have
further decreased at the onset of PTE, whereas the CA19-9 and
carcinoembryonic antigen levels had also decreased. PTE in Case 1
was asymptomatic; however, increased D-dimer levels were detected
on blood tests. Therefore, contrast-enhanced CT scan was performed
for suspected PTE, and PTE was detected. PTE in Cases 2, 3 and 4
was detected by reassessment of contrast-enhanced CT images.
The sensitivity, specificity and NPV were 50.0, 90.5
and 90.5%, respectively, when 3.0 µg/ml was set as the D-dimer
cutoff value (Table II).
 | Table IIDiagnostic accuracy of D-dimer test
for PTE. |
Table II
Diagnostic accuracy of D-dimer test
for PTE.
| | Number of patients
(n=25) |
|---|
| Variables | PTE (+) | PTE (-) |
|---|
| D-dimer level,
µg/ml | | |
|
>3.0 | 2 | 2 |
|
≤3.0 | 2 | 19 |
| Measure of accuracy,
% | | |
|
Sensitivity | | 50.0 |
|
Specificity | | 90.5 |
|
Positive
predictive value | | 50.0 |
|
Negative
predictive value | | 90.5 |
Discussion
Despite its retrospective design and small sample
size, to the best of our knowledge, this is the first study to
evaluate the reliability and validity of D-dimer monitoring for PTE
limited to patients with unresectable, advanced or recurrent CRC
treated with bevacizumab as primary treatment. CRC is a type of
cancer not included in the Khorana score, and it is often treated
by bevacizumab in the primary to tertiary treatment setting.
Therefore, CRC was selected as the best target to investigate PTE
in the present study.
PTE in patients with cancer is associated with high
recurrence rate and poor prognosis, despite it being asymptomatic
or an incidental finding. Therefore, it is important to accurately
diagnose even asymptomatic PTE, and the same treatment is
recommended for symptomatic and asymptomatic patients, including
patients diagnosed incidentally (13). The PTE cases detected in the present
study included asymptomatic PTE that was incidentally diagnosed
upon reassessment of contrast-enhanced chest CT images for routine
staging of malignancy, indicating that it is difficult to detect
all cases in clinical practice. Therefore, the primary purpose was
to investigate whether blood tests, such as the D-dimer testing,
would be useful for complementing PTE diagnosis.
However, the high NPV, which is a characteristic of
the D-dimer test in non-cancer patients, may be less reliable in
patients with cancer due to the higher incidence of PTE compared
with non-cancer patients. Furthermore, despite the fact that the
D-dimer test is a sensitive method for the diagnosis of acute PTE
in non-cancer patients, the sensitivity of D-dimer test was only
50% in patients with cancer, with a cutoff value of 3.0 µg/ml.
These results suggest that periodic chest CT scans are necessary,
even if the D-dimer test is negative. In other words, the D-dimer
test has low sensitivity in patients with bevacizumab-treated
unresectable, advanced or recurrent CRC, and may only be useful in
the detection and diagnosis of PTE, rather than the exclusion of
PTE. In addition, patients with cancer have additional factors that
may also affect D-dimer levels, such as age, inflammation and tumor
progression, and the D-dimer levels may decrease with disease
control by treatment; therefore, D-dimer level routine monitoring
may be important, in addition to its measurement at baseline and at
the timing of appearance of suspicious symptoms. The cutoff value
of the D-dimer test in PTE diagnosis requires further verification,
but this could not be performed in the present study owing to the
insufficient sample size. However, if D-dimer levels rapidly
increase during routine monitoring, as in Cases 1 and 2 reported
herein, the possibility of PTE should be considered.
There were several major limitations to the present
study. First, this was a retrospective observational study with a
small sample size. Second, PTE was evaluated by contrast-enhanced
chest CT images, but without considering the possibility of deep
vein thrombosis (DVT) in the lower limbs or in the injection site
of the central venous catheter. There may be an association between
PTE and DVT, and the source of PTE may be thrombi formed in the
veins in the lower limbs or pelvis. In particular, the lower limbs
are rarely evaluated by CT scans for the purpose of disease
assessment in patients with CRC, and ultrasonography is generally
only performed when suspicious symptoms are observed. The PTE cases
in the present study may have developed DVT prior to the onset of
PTE, and the relevance between the onset of DVT and the variation
of D-dimer levels and NPV was not elucidated in this study.
Finally, on routine monitoring of D-dimer levels, the reasons for
the low sensitivity of D-dimer testing may include the negative
conversion of the D-dimer levels due to the organization of the
thrombus with the lapse of time after thrombus formation and the
detection of minute thromboembolism by reassessment of
contrast-enhanced CT scans. Future research should be aimed at
elucidating this issue.
Despite these limitations, it was herein
demonstrated that PTE, including asymptomatic and incidentally
diagnosed cases, appears to be a frequent occurrence among patients
with CRC, such as those included in the present study, and the
D-dimer test may be less useful for exclusion diagnosis, but may
prove useful for appropriate PTE detection and diagnosis in
addition to chest CT scans. In addition, D-dimer level monitoring
is hypothesized to be important not only at baseline, but also
during treatment. However, the optimal reference values and the
appropriate measurement and timing of D-dimer testing require
further study.
Acknowledgements
The authors would like to thank the staff of
Chemotherapy Center, NTT Medical Center Tokyo.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
DK and KU: Study conception and design. DK, MK and
KU: Data collection and analysis. DK, MK and KU have seen and can
confirm the authenticity of the raw data. DK and KU: Drafting of
the manuscript. SS, JS, JI, AH, RO, TK and MY: Critical review and
revision of the manuscript for important intellectual content. All
the authors contributed to the discussion and have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of NTT Medical Center Tokyo (approval no. 19-62). As this
was a retrospective observational study, consent was not obtained
from individual patients. An information disclosure document about
this study was created and published for the study patient,
guaranteeing the opportunity for the study patient to refuse.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khorana AA: Venous thromboembolism and
prognosis in cancer. Thromb Res. 125:490–493. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fernandes CJ, Morinaga LTK, Alves JL,
Castro MA, Calderaro D, Jardim CVP and Souza R: Cancer-associated
thrombosis: The when, how and why. Eur Respir Rev.
28(180119)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nalluri SR, Chu D, Keresztes R, Zhu X and
Wu S: Risk of venous thromboembolism with the angiogenesis
inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA.
300:2277–2285. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alahmari AK, Almalki ZS, Alahmari AK and
Guo JJ: Thromboembolic events associated with bevacizumab plus
chemotherapy for patients with colorectal cancer: A meta-analysis
of randomized controlled trials. Am Heal Drug Benefits. 9:221–231.
2016.PubMed/NCBI
|
|
7
|
Crawford F, Andras A, Welch K, Sheares K,
Keeling D and Chappell FM: D-dimer test for excluding the diagnosis
of pulmonary embolism. Cochrane Database Syst Rev.
2016(CD010864)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mochizuki S, Yoshino T, Kojima T, Fuse N,
Ikematsu H, Minashi K, Yano T, Tahara M, Kaneko K, Doi T, et al:
Therapeutic significance of a D-dimer cut-off level of >3 µg/ml
in colorectal cancer patients treated with standard chemotherapy
plus bevacizumab. Jpn J Clin Oncol. 40:933–937. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Es N, Bleker SM and Di Nisio M:
Cancer-associated unsuspected pulmonary embolism. Thromb Res. 133
(Suppl 1):S172–S178. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Key NS, Khorana AA, Kuderer NM, Bohlke K,
Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW,
et al: Venous thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J Clin Oncol.
38:496–520. 2020.PubMed/NCBI View Article : Google Scholar
|