Introduction
Pancreatic ductal adenocarcinoma (PDAC) is
considered a poor prognostic disease with low resection and high
recurrence rates (1,2). To overcome such difficulties,
multidisciplinary therapeutic approaches, including surgical
treatment, chemotherapy, and radiotherapy, have recently been
reported, with improvements in prognosis (3-6).
Prompt and accurate diagnoses of patients suitable for the
aforementioned therapeutic approaches are important (2,7).
Recently, PDAC arising from genetic abnormalities, such as familial
pancreatic cancer, hereditary breast and ovarian cancer syndrome,
Lynch syndrome, and Peutz-Jeghers syndrome, has been analyzed and
reported as a representative example of such backgrounds (8-10).
Approximately 10% of PDAC cases are reported to have one of the
aforementioned genetic backgrounds (11). Thus, further investigation regarding
such oncological viewpoints in patients with PDAC is needed.
Moreover, recent developments in cancer treatment
have led to prognostic improvements in malignant diseases,
including PDAC. Such improvements extend the opportunity to treat
patients with malignant disease and a history of other primary
malignancies (11-14).
In the clinical setting, once malignant disease has been treated,
periodic medical check-ups, including imaging studies, are
performed for follow-up of previously treated malignancies. We
hypothesized that such routine studies may beneficially affect the
early diagnosis of other malignant diseases, including PDAC.
However, few studies have reported the clinicopathological
characteristics of patients with PDAC and a history of other
primary malignancies (11-13).
Therefore, further studies are needed to better understand the
clinicopathological characteristics of patients with PDAC.
This study aimed to analyze the clinicopathological
characteristics of patients with PDAC, with a focus on the history
of other primary malignancies.
Materials and methods
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The study was approved by the Institutional Review Board
of Toyama Prefectural Central Hospital (approved number: 57-52).
Written informed consent was obtained from all individual
participants included in the study.
We enrolled 102 consecutive patients with surgically
resected and pathologically proven PDAC who were intended for
curative resection between April 2013 and March 2018. PDAC patients
with intraductal papillary mucinous neoplasm and those surgically
treated with non-curative intent were excluded. The mean age of the
enrolled patients was 70.1 years (range, 34-90 years), and the
majority of patients were men (men:women ratio, 61:41). The mean
follow-up period of the patients was 27.6 months (range, 1.2-80.1
months). Data from patients' medical records, including blood
examinations, imaging studies, medical histories, pathological
findings, and postoperative therapies, were retrospectively
analyzed. Patients with a history of other primary malignancies
(including concomitant diseases) were included in the HoM(+) group,
and patients with no history of other primary malignancies were
included in the HoM(-) group.
Staging and resectability classification were
performed according to the Union for International Cancer Control
Tumor-Node-Metastasis (TNM) classification (eighth edition)
(15).
In this study, data are presented as mean ± standard
deviation. Student's t-test was used to compare quantitative data.
The Chi-square test, Fisher's exact test, or likelihood ratio test
were used to compare qualitative data, as appropriate. Disease-free
survival and overall survival were calculated from the date of
initial surgery for PDAC to the date of relapse or death from any
cause. Disease-free survival was censored at the last date on which
the absence of recurrence was confirmed. Overall survival was
censored at the date of last follow-up. Survival curves were
estimated using the Kaplan-Meier method and compared using the
log-rank test. Multivariate analysis was performed using a Cox
proportional hazards model. All statistical analyses were conducted
using SPSS version 22.0 (IBM Corp.). A P-value <0.05 was
considered statistically significant.
Results
The patients' clinical characteristics are
summarized in Table I. The mean age
of the patients with PDAC who underwent pancreatoduodenectomy
(n=71; 69.6%) and distal pancreatectomy (n=31; 30.4%) was 70 and 71
years, respectively. The median follow-up period was ~25
months.
 | Table IPatient baseline characteristics. |
Table I
Patient baseline characteristics.
| Characteristic | Patients (n=102) |
|---|
| Mean ± SD age, years
(range) | 70.1±9.0 (34-90) |
| Sex (n) | |
|
Male | 61 |
|
Female | 41 |
| Tumor location
(n) | |
|
Ph | 70 |
|
Pb | 19 |
|
Pt | 13 |
| Preoperative therapy
(n) | |
|
Yes | 11 |
|
No | 91 |
| Surgical procedure
(n) | |
|
PD | 71 |
|
DP | 31 |
Of the 102 patients included in the study, 25
(24.5%) had a history of other primary malignancies (including
concomitant diseases). The details of the malignancies are shown in
Table II. There was no significant
difference in patient characteristics between the two groups,
except for age [74.2 vs. 68.9 years for patients in the with
[HoM(+)] and without [HoM(-)] a history of other primary
malignancies groups, respectively; P=0.005] (Table III).
 | Table IIHistory of malignant diseases. |
Table II
History of malignant diseases.
| Characteristic | Patients |
|---|
| Total number of
patients | 102 |
| History of malignant
disease (including synchronous disease) | |
|
Yes | 25 |
|
No | 77 |
| Interval to diagnosis
of PDAC (years)a | |
|
Mean ±
SD | 10.5±11.8 |
|
Range | (0-48) |
| Details of malignant
disease (n)a,b | |
|
Colorectal
cancer | 7 |
|
Bladder
cancer | 4 |
|
Breast
cancer | 3 |
|
Gastric
cancer | 3 |
|
Lung
cancer | 3 |
|
Biliary
cancer | 1 |
|
Esophageal
cancer | 1 |
|
GIST | 1 |
|
HCC | 1 |
|
Lymphoma | 1 |
|
Prostate
cancer | 1 |
|
Renal
cancer | 1 |
|
Thyroid
cancer | 1 |
|
Uterine
sarcoma | 1 |
 | Table IIIComparison of patient baseline
characteristics. |
Table III
Comparison of patient baseline
characteristics.
| | HoM | |
|---|
|
Characteristics | n (%) | + (n=25) | - (n=77) | P-value |
|---|
| Age (years) | | | | 0.005 |
|
Mean ±
SD | - | 74.2±7.4 | 68.9±9.2 | |
|
Range | - | (57-85) | (34-90) | |
|
≤59 | 10(10) | 1 | 9 | |
|
60-74 | 59(58) | 10 | 49 | |
|
≥75 | 33(32) | 14 | 19 | |
| Sex | | | | 0.982 |
|
Male | 61(60) | 15 | 46 | |
|
Female | 41(40) | 10 | 31 | |
| Tumor location | | | | 0.380 |
|
Ph | 70(69) | 15 | 55 | |
|
Pb/Pt | 32(31) | 10 | 22 | |
| Preoperative
chemotherapy | | | | 0.208 |
|
Yes | 11(11) | 1 | 10 | |
|
No | 91(89) | 24 | 67 | |
| Surgery | | | | 0.483 |
|
PD | 71(70) | 16 | 55 | |
|
DP | 31(30) | 9 | 22 | |
Table IV compares
the clinical characteristics of patients with PDAC and with or
without a history of other primary malignancies. In the HoM(+)
group, the most common reason for consultation was the follow-up of
previous malignancies (n=8; 32.0%), followed by jaundice (n=6;
24.0%), the progression of diabetes mellitus (n=4; 16.0%), and
health check abnormalities (n=4; 16.0%). In the HoM(-) group, the
most common reason for consultation was jaundice (n=20; 26.0%),
followed by the progression of diabetes mellitus (n=16; 20.8%) and
health check abnormalities (n=14; 18.2%). The difference between
the two groups was significant (P<0.001).
 | Table IVComparison of patient clinical
characteristics. |
Table IV
Comparison of patient clinical
characteristics.
| | HoM | |
|---|
|
Characteristics | n (%) | (+) (n=25) | - (n=77) | P-value |
|---|
| Reason for
consultation | | | | <0.001 |
|
F/u of
HoM | 8(8) | 8 | - | |
|
F/u of other
diseases | 9(9) | 1 | 8 | |
|
Health
check | 18(18) | 4 | 14 | |
|
Jaundice | 26(25) | 6 | 20 | |
|
Progression
of DM | 8(8) | 4 | 4 | |
|
Pain | 13(13) | 2 | 11 | |
|
Others | 8(8) | 0 | 8 | |
| Resectability | | | | 0.645 |
|
R | 81(79) | 22 | 59 | |
|
BR | 15(15) | 2 | 13 | |
|
UR | 6(6) | 1 | 5 | |
| Margin status | | | | 0.774 |
|
R0 | 66(65) | 16 | 50 | |
|
R1 | 33(32) | 9 | 24 | |
|
R2 | 2(2) | 0 | 2 | |
|
RX | 1(1) | 0 | 1 | |
| UICC final
stage | | | | 0.474 |
|
0 | 1(1) | 0 | 1 | |
|
IA | 5(5) | 0 | 5 | |
|
IB | 3(3) | 1 | 2 | |
|
IIA | 18(18) | 5 | 13 | |
|
IIB | 37(36) | 9 | 28 | |
|
III | 27(26) | 9 | 18 | |
|
IV | 11(11) | 1 | 10 | |
| Adjuvant
therapy | | | | 0.106 |
|
No | 28(27) | 10 | 18 | |
|
Yes | 74(73) | 15 | 59 | |
| Recurrence | | | | 0.399 |
|
No | 27(26) | 5 | 22 | |
|
Yes | 75(74) | 20 | 55 | |
Most cases were classified as ‘resectable’ (88.0% in
the HoM(+) group and 76.6% in the HoM(-) group). The proportion of
patients classified as ‘resectable’ did not differ significantly
between the two groups (P=0.645).
Complete (R0) resection was achieved in 64.0% of
patients in the HoM(+) group (n=16) and 64.9% of patients in the
HoM(-) group (n=50). There was no significant difference between
the two groups (P=0.774).
The final stage of the largest proportion of
patients (36.3%) in both groups was stage 2B, with no difference
between the groups (P=0.474).
Adjuvant chemotherapy was administered to 60.0% of
patients in the HoM(+) group (n=15) and 76.6% of patients in the
HoM(-) group (n=59). There was no significant difference between
the two groups (P=0.106). The recurrence rate also did not differ
significantly between the two groups [80.0% in the HoM(+) group
(n=20) vs. 71.4% in the HoM(-) group (n=55); P=0.399].
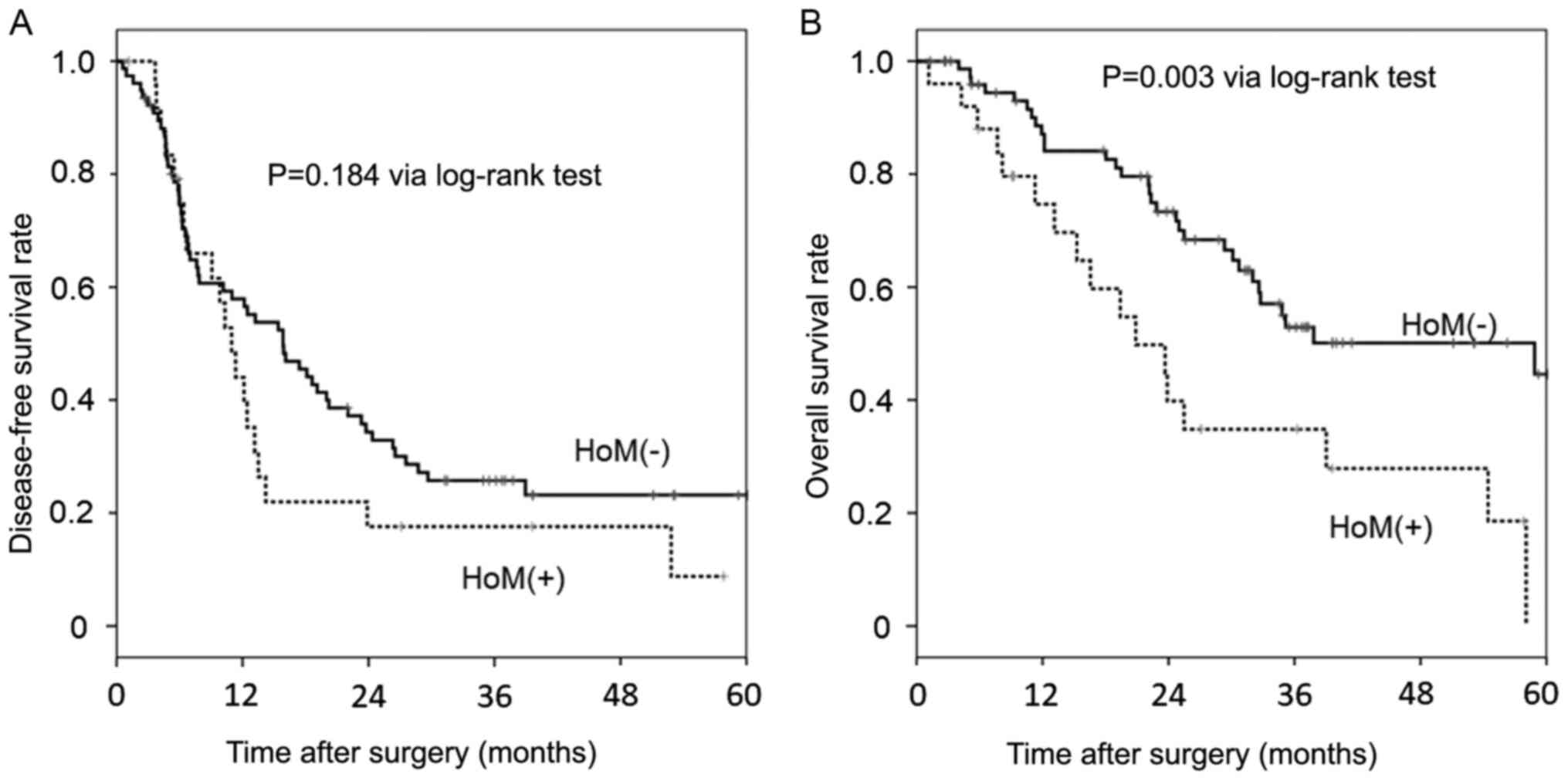
Postoperative disease-free survival and overall
survival rates are shown in Fig. 1.
Disease-free survival did not differ significantly between the two
groups (P=0.184). Conversely, overall survival was significantly
poorer in the HoM(+) group than in the HoM(-) group (P=0.003). In
the univariate analysis, a history of other primary malignancies
was associated with poorer overall survival [hazard ratio (HR):
2.424, 95% confidence interval (CI): 1.332-4.411; P=0.004].
Advanced TNM stage (stage III-IV) (HR: 1.719, 95% CI: 0.975-3.031;
P=0.061) and the initiation of adjuvant chemotherapy (HR: 0.533,
95% CI: 0.274-1.036; P=0.063) were also factors associated with
overall survival. In the multivariate analysis, a history of other
primary malignancies was identified as an independent predictor of
poor overall survival (HR: 2.416, 95% CI: 1.324-4.406; P=0.004)
(Table V).
 | Table VUnivariate and multivariate analyses
of prognostic factors for the overall survival of patients with
PDAC. |
Table V
Univariate and multivariate analyses
of prognostic factors for the overall survival of patients with
PDAC.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<75
vs. ≥75 years) | 1.139 | 0.626-2.072 | 0.670 | - | - | - |
| Sex (male
vs. female) | 1.100 | 0.618-1.957 | 0.746 | - | - | - |
| HoM (+ vs.
-) | 2.424 | 1.332-4.411 | 0.004 | 2.416 | 1.324-4.406 | 0.004 |
| NAC (+ vs.
-) | 0.666 | 0.238-1.862 | 0.438 | - | - | - |
| Tumor location (Ph
vs. Pb/Pt) | 1.478 | 0.824-2.650 | 0.190 | - | - | - |
| Resectability (R
vs. BR/UR) | 1.712 | 0.411-7.135 | 0.460 | - | - | - |
| Margin status (R0
vs. R1/R2/RX) | 0.856 | 0.478-1.534 | 0.602 | - | - | - |
| UICC final stage
(I-II vs. III-IV) | 1.719 | 0.975-3.031 | 0.061 | 1.644 | 0.929-2.908 | 0.088 |
| Adjuvant
chemotherapy (yes vs. no) | 0.533 | 0.274-1.036 | 0.063 | 0.575 | 0.292-1.131 | 0.109 |
Discussion
PDAC is still considered a poor prognostic disease,
despite developments in diagnosis and treatment (1,2). To
overcome such difficulties, early diagnosis, especially for
identifying suitable patients for therapeutic intervention, is
important (2,7). Moreover, recent advances in cancer
medicine, especially in the genetic aspect, have provided new
therapeutic and diagnostic modalities for specific populations of
patients with PDAC (8-10).
Thus, analyzing PDAC from the viewpoint of early diagnosis and
different oncogenic backgrounds has been an important recent
clinical issue. Therefore, we studied PDAC, focusing on patients
with a history of other primary malignancies.
In this study, the HoM(+) group had significantly
poorer overall survival than the HoM(-) group, and a history of
other primary malignancies was a significant prognostic factor for
overall survival in patients with PDAC. The results differed from
those of previous reports, as the survival rate of patients with
other primary malignancies was the same or better than that of
patients without other primary malignancies (11-13,16).
This difference may be explained as follows. Most previous reports
examined patients diagnosed and treated for PDAC before being
diagnosed and treated for other primary malignancies. Thus, other
primary malignancies occurred in patients who survived treatment
for PDAC. Therefore, the meaning and impact of ‘other primary
malignancies’ differed substantially from the meaning in our study.
In contrast, a study by He et al (16) differed significantly from the above
reports. The authors analyzed 67,555 PDAC cases included in the
Surveillance, Epidemiology, and End Results database to determine
whether these patients had a prior history of cancer. Our study
differed from the He et al (16) study in that our study group only
included patients who intended to undergo curative resection for
PDAC. The characteristics of patients in the Japanese local
regional hospital also differed from those in the Surveillance,
Epidemiology, and End Results database. We think that the clinical
information of patients with PDAC, especially their medical history
prior to diagnosis and treatment, is important for early diagnosis
of surgically treatable PDAC, as HoM(+) can impact the
prognosis.
We hypothesized that regular imaging screening for
previous diseases, which is required for follow-up, would be useful
for the early detection of PDAC, as reported by Hoshimoto et
al (17). However, our results
revealed no significant beneficial effect in the HoM(+) group, both
pre- and postoperatively, including that of survival. We believe
that the reasons for such discouraging results may be as follows.
First, the majority of patients in the HoM(+) group did not
continue regular medical follow-ups for previous malignant diseases
at the time of diagnosis of PDAC because half of the patients in
the HoM(+) group relapsed over 5 years after treatment for other
primary malignancies without recurrence. In addition, regular
follow-up of patients in the HoM(+) group did not always provide
useful imaging information for early diagnosis of PDAC (e.g.,
imaging that did not cover the upper abdomen or computed tomography
without contrast enhancement).
In this study, overall survival was significantly
poorer in the HoM(+) group than in the HoM(-) group, despite no
significant difference in disease-free survival between the two
groups. Factors according to PDAC stage, including resectability
and R0 resection rate, were not significantly different between the
two groups. We did not suppose that the preoperative PDAC status
would differ between the two groups. Therefore, we speculate that
such differences in overall survival may be due to the clinical
course after recurrence of PDAC.
Patients in the HoM(+) group were significantly
older than those in the HoM(-) group. Univariate and multivariate
analyses showed that age was not a significant prognostic factor
for patients with PDAC. The majority of deceased patients in our
study died of recurrence of PDAC. Therefore, other diseases did not
affect the postoperative course of PDAC. However, this fact did
affect the clinical course of PDAC, as age is an important factor
in selecting the therapeutic strategy. Recent studies have reported
the effectiveness of adjuvant chemotherapy for improving the
prognosis of PDAC after surgery (4-6).
Thus, postoperative multidisciplinary therapeutic approaches may be
restricted by age in the HoM(+) group.
Treatment of previous malignant diseases may affect
some aspects of patients' condition, including the immunological
and oncological status. In continuing therapeutic intervention for
recurrent PDAC after resection, various factors concerning
patients' condition have been shown to influence continuing
anticancer therapy (18). We
analyzed the neutrophil-to-lymphocyte ratio, a reliable indicator
of immunological status and inflammation (19). However, no significant difference
was observed between the two groups (Table SI). Therefore, we postulated that
patients in the HoM(+) group may not tolerate treatment for
recurrent disease owing to unknown causes.
Next, we considered the chemosensitivity of patients
in the HoM(+) group, which is induced by previous treatment for
other malignant diseases. The therapeutic strategy for solid organ
malignant disease is usually combined with local and systemic
therapy, such as surgical resection and chemotherapy. Furthermore,
hematopoietic malignancies are usually treated with chemotherapy.
Such anticancer drugs may affect the chemosensitivity of subsequent
PDAC. However, this study lacked detailed information on the
history of previous treatments for other malignant diseases.
Therefore, we were unable to test this hypothesis. Further
information is required.
Various genetic abnormalities associated with PDAC
have recently been reported owing to advancements in genomic
medicine (8-10).
Genetic mutations in the oncogene KRAS and tumor suppressor
genes TP53, p16/CDKN2A, and SMAD4 are
representative mutations for PDAC (20). Several studies have discussed the
role of each gene mutation in the development and progression of
PDAC (21,22). Epigenetic alterations have also been
reported to be responsible for the dysregulation of
tumor-associated genes (23,24).
Some of these genes have already become therapeutic targets for
selected patients (e.g., BRCA2 and PALB2) (25,26).
In our study, previous treatment for other primary malignancies was
similar to those reported previously (11,13,17).
Thus, these malignancies may have included some of the
aforementioned genetic and epigenetic abnormalities. Future
developments in genomic medicine will expand the indications for
such therapeutic interventions. Therefore, clinicians should pay
more attention to patients and patients' family history to gather
potential information on PDAC with genetic abnormalities, including
a history of other primary malignancies.
This study has some limitations owing to its small
sample size and retrospective design. First, our study group was
obtained from patients admitted to a local regional hospital in
Japan. Thus, there is a bias in race and ethnicity. Second, our
investigation may be subject to selection bias, as the patients
selected in this study were those suitable for surgical resection.
Unresectable patients were not included, including a certain
proportion of patients with PDAC. Moreover, most patients included
in this study were treated under the upfront surgery policy. The
beneficial effect of neoadjuvant chemotherapy has recently been
reported for patients with resectable PDAC (3,4). The
initiation of a multidisciplinary therapeutic approach that
includes neoadjuvant chemotherapy may radically change the
prognosis and treatment of PDAC. The aim of this study was to
examine the characteristics of patients with PDAC from a different
viewpoint, including early diagnosis for better prognosis, focusing
on a history of other primary malignancies. We emphasize the
importance of paying attention to the medical history of patients
with PDAC, which has a significant impact on prognosis. Finally,
there is ambiguity concerning the review of medical history,
especially for detailed information regarding previous treatments,
such as chemotherapy regimen or family history. Such information is
generally dependent on patients' and family members' anamnesis.
Therefore, the risk of incomplete and inaccurate information could
not be avoided. Thus, the construction of a system for sharing
correct information about patients' and family members' medical
history is important for the future treatment of malignant
diseases.
A history of other primary malignancies had a
negative impact on overall survival in patients with surgically
treated PDAC. Further studies, especially detailed analyses of the
differences in overall survival, recurrence-free survival, previous
treatments for other primary malignancies, and family history, are
needed to better understand the clinical features of PDAC.
Supplementary Material
Comparison of patient laboratory
data.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH, KA and KS designed the current study and wrote
the manuscript. TTo, KMo, ST, YS and YK collected the clinical
data, which included postoperative course data. HH, TK, SK, AH and
TTs performed statistical analysis. HH, MK and KMa interpreted
patient data. HH and MK confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The study was approved by the Institutional Review Board
of Toyama Prefectural Central Hospital (approved no. 57-52).
Written informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buscail L, Bournet B and Cordelier P: Role
of oncogenic KRAS in the diagnosis, prognosis and treatment of
pancreatic cancer. Nat Rev Gastroenterol Hepatol. 17:153–168.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanno A, Masamune A, Hanada K, Kikuyama M
and Kitano M: Advances in early detection of pancreatic cancer.
Diagnostics (Basel). 9(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Unno M, Motoi F, Matsuyama Y, Satoi S,
Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, Yoshitomi H,
et al: Randomized phase II/III trial of neoadjuvant chemotherapy
with gemcitabine and S-1 versus upfront surgery for resectable
pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 37 (Suppl
4)(S189)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reni M, Balzano G, Zanon S, Zerbi A,
Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C,
Chiaravalli M, et al: Safety and efficacy of preoperative or
postoperative chemotherapy for resectable pancreatic adenocarcinoma
(PACT-15): A randomised, open-label, phase 2-3 trial. Lancet
Gastroenterol Hepatol. 3:413–423. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ocuin LM, Miller-Ocuin JL, Zenati MS,
Vargo JA, Singhi AD, Burton SA, Bahary N, Hogg ME, Zeh HJ III and
Zureikat AH: A margin distance analysis of the impact of adjuvant
chemoradiation on survival after pancreatoduodenectomy for
pancreatic adenocarcinoma. J Gastrointest Oncol. 8:696–704.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Raff JP, Noyer C, Boxer N, Sadan S, Costin
D, Roayaie S, Gordon M, Kaumaya M, Hopkins U, Cortese M, et al:
Early detection for pancreatic cancer in individuals at
elevated-risk, using endoscopic ultrasound (EUS) and magnetic
resonance imaging (MRI) of the abdomen: Feasibility and preliminary
outcomes. J Clin Oncol. 38 (Suppl 15)(e16798)2020.
|
|
8
|
Matsubayashi H, Takaori K, Morizane C,
Maguchi H, Mizuma M, Takahashi H, Wada K, Hosoi H, Yachida S,
Suzuki M, et al: Familial pancreatic cancer: Concept, management
and issues. World J Gastroenterol. 23:935–948. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohmoto A, Yachida S and Morizane C:
Genomic features and clinical management of patients with
hereditary pancreatic cancer syndromes and familial pancreatic
cancer. Int J Mol Sci. 20(561)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Llach J, Carballal S and Moreira L:
Familial pancreatic cancer: Current perspectives. Cancer Manag Res.
12:743–758. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shin SJ, Park H, Sung YN, Yoo C, Hwang DW,
Park JH, Kim KP, Lee SS, Ryoo BY, Seo DW, et al: Prognosis of
pancreatic cancer patients with synchronous or metachronous
malignancies from other organs is better than those with pancreatic
cancer only. Cancer Res Treat. 50:1175–1185. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eriguchi N, Aoyagi S, Hara M, Okuda K,
Tamae T, Fukuda S, Hashino K, Sato S, Fujiki K, Furukawa S and Jimi
A: Synchronous or metachronous double cancers of the pancreas and
other organs: Report on 12 cases. Surg Today. 30:718–721.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gerdes B, Ziegler A, Ramaswamy A, Wild A,
Langer P and Bartsch DK: Multiple primaries in pancreatic cancer
patients: Indicator of a genetic predisposition? Int J Epidemiol.
29:999–1003. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Z, Zhou Y, Guan C, Ding Y, Tao S,
Huang X, Chen L, Zhang F and Zhang R: The impact of previous cancer
on overall survival of bladder cancer patients and the
establishment of nomogram for overall survival prediction. Medicine
(Baltimore). 99(e22191)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours. 8th
edition. John Wiley & Sons, Toyama, Toyama, pp930-8550,
2017.
|
|
16
|
He X, Li Y, Su T, Lai S, Wu W, Chen L, Si
J and Sun L: The impact of a history of cancer on pancreatic ductal
adenocarcinoma survival. United European Gastroenterol J.
6:888–894. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hoshimoto S, Hishinuma S, Shirakawa H,
Tomikawa M, Ozawa I and Ogata Y: Outcomes in patients with
pancreatic cancer as a secondary malignancy: A retrospective
single-institution study. Langenbecks Arch Surg. 404:975–983.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adamska A, Domenichini A and Falasca M:
Pancreatic ductal adenocarcinoma: Current and evolving therapies.
Int J Mol Sci. 18(1338)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Asari S, Matsumoto I, Toyama H, Shinzeki
M, Goto T, Ishida J, Ajiki T, Fukumoto T and Ku Y: Preoperative
independent prognostic factors in patients with borderline
resectable pancreatic ductal adenocarcinoma following curative
resection: The neutrophil-lymphocyte and platelet-lymphocyte
ratios. Surg Today. 46:583–592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iacobuzio-Donahue CA, Velculescu VE,
Wolfgang CL and Hruban RH: Genetic basis of pancreas cancer
development and progression: Insights from whole-exome and
whole-genome sequencing. Clin Cancer Res. 18:4257–4265.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Le A, Rajeshkumar NV, Maitra A and Dang
CV: Conceptual framework for cutting the pancreatic cancer fuel
supply. Clin Cancer Res. 18:4285–4290. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Neureiter D, Jäger T, Ocker M and
Kiesslich T: Epigenetics and pancreatic cancer: Pathophysiology and
novel treatment aspects. World J Gastroenterol. 20:7830–7848.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo M, Jia Y, Yu Z, House MG, Esteller M,
Brock MV and Herman JG: Epigenetic changes associated with
neoplasms of the exocrine and endocrine pancreas. Discov Med.
17:67–73. 2014.PubMed/NCBI
|
|
25
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Villarroel MC, Rajeshkumar NV,
Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH,
Eshleman JR, Klein A, Laheru D, et al: Personalizing cancer
treatment in the age of global genomic analyses: PALB2 gene
mutations and the response to DNA damaging agents in pancreatic
cancer. Mol Cancer Ther. 10:3–8. 2011.PubMed/NCBI View Article : Google Scholar
|