Introduction
Head and neck cancer represents the seventh most
common cancer in Slovenia and is more common in men than women.
Squamous cell head and neck carcinoma (SCHNC) represents 90% of all
head and neck cancer. The annual incidence is around 470 cases
(1).
Despite multimodal treatment, 50 to 60% of stage III
and IV cancers will relapse locoregionally and/or at distant sites.
Surgical procedure and/or re-irradiation are therapeutical options,
but rarely feasible. Patients could be treated with systemic
therapy or best supportive care only. The decision depends on the
patient's platinum-free interval, performance status (PS) and
comorbidity (2).
In 2008, Vermorken et al reported the results
of the phase III EXTREME trial, in which the addition of cetuximab
to first-line platinum/5-fluorouracil chemotherapy (PFE regimen)
significantly improved progression-free survival (PFS) and overall
survival (OS) compared to platinum/5-fluorouracil (PE) only. The
EXTREME regimen become the new standard of care for patients having
very good PS (0-1) (3). Despite
that, the prognosis of patients with recurrent and/or metastatic
(R/M) SCHNC remains poor with a median OS of 10 months (4). In Slovenia, SCHNC survival rates are
comparable to those in Western Europe (5).
In our country, the decision about the most suitable
treatment for R/M SCHNC is made at the multidisciplinary tumour
board at the Maxillofacial Department or the Head and Neck
Surgical Clinic of two university clinical centres, Ljubljana,
and Maribor, and at the Institute of Oncology Ljubljana. All
patients referred for systemic therapy are treated at a single
centre, at the Medical Oncology Department of the Institute of
Oncology, Ljubljana.
We followed the international treatment guidelines
(6), but the treatment with
cetuximab was not fully reimbursed in the first years after
European Medicine Agency (EMA) approval, and our patient population
is also specific regarding the high percentage (20-25%) of grade 3
or 4 infusion reactions to cetuximab, which prevented our patients
from receiving cetuximab (7). The
primary aim of this retrospective study was to compare the outcomes
of our patients with R/M SCHNC treated with the PF and PFE regimens
in the routine clinical setting with outcome in a randomized trial
and to identify possible prognostic factors for PFS and OS. The
secondary aim was to assess the tolerability of the treatment.
Patients and methods
Study design
Patients with R/M SCHNC treated between April 2008
and May 2018 at the Institute of Oncology Ljubljana were included
in this retrospective study. Data on patients and tumour
characteristics and past treatments were retrieved from patients'
charts. Patients gave written consent prior to treatment. The study
protocol was reviewed and approved by the Institutional Ethics
Committee (approval ID: ERIDNPVO: 0023-2020).
Patient selection
The selection of patients for this aggressive
systemic treatment was performed at the multidisciplinary tumour
board. Inclusion criteria for the PF and PFE protocols: First-line
therapy for R/M SCHNC, PS 0-2, adequate haematologic, renal and
liver function, approved reimbursement for cetuximab (for PFE
only). Exclusion criteria for the PE and PFE protocols: Patients
with nasopharyngeal carcinoma, PS >2. Exclusion criteria for PFE
only: Infusion reaction to cetuximab grade >2 during the first
cycle of cetuximab, prior treatment with cetuximab (patients who
took part in the clinical study of concomitant radiation therapy
plus cetuximab plus cisplatin), known allergy to bee or wasp venom
grade >2, patients with bulky tumour in the oropharynx or larynx
which would prevent urgent intubation in grade 4 cetuximab allergy.
All patients who developed an allergy to cetuximab of grade >2
were treated with the PF protocol. Human papillomavirus (HPV)
status in oropharyngeal tumours was not assessed in all patients
and was not included in the analysis.
Treatment protocol
The PF regimen in both groups consisted of
5-fluorouracil (1000 mg/m2 daily, 24-h continuous
infusion) for 4 days and platinum-based chemotherapy [preferably
cisplatin (100 mg/m2, 3-h intravenous infusion), in case
of neurological or kidney disfunction carboplatin AUC 5, -1-h
infusion)] on day 2, every three weeks. In the PFE group, cetuximab
was administered on the third day of the cycle at an initial dose
of 400 mg/m2 in a 2-h intravenous infusion (preceded by
a test dose of 20 mg of cetuximab intravenously to test for an
allergy), followed by weekly doses of 250 mg/m2 in a 1-h
intravenous infusion. Dose modifications of chemotherapy and
cetuximab were permitted according to the drug-specified criteria.
Granulocyte-stimulating growth factors were used based on clinician
decision and standard recommendations.
Patients in both groups who achieved partial
remission or at least stable disease received up to six cycles of
chemotherapy. Patients in the PFE group who had at least stable
disease after a maximum of six cycles of chemotherapy continued
thereafter with cetuximab monotherapy every two weeks at a dose of
500 mg/m2 until disease progression or unacceptable
toxicity effects.
Patients in the PF group received no further active
treatment and were followed-up regularly for disease
progression.
Evaluation of therapy response
Response to the systemic therapy was evaluated
clinically at every clinical visit before continuing with scheduled
therapy. After the third or fourth cycle of therapy, computer
tomography (CT) imaging of the involved regions was planned and
obtained. Depending on the clinical situation, CT was obtained
earlier. Response evaluation criteria in solid tumours (RECIST 1.1)
were used (8). Assessment of
adverse effects during therapy was according to the Common
Terminology Criteria for Adverse Events (CTCAE) version
5.0(9). Infections, febrile
neutropenia, skin rash, diarrhoea, and hypomagnesaemia were
presented. Deaths during treatment were analyzed.
Statistical analysis
The characteristics of patients were categorically
presented as frequencies and proportions. Age was presented as mean
and range. Pearson chi-square test was used for statistical
comparisons for categorical data and unpaired Student's t-test was
used for comparing age between groups. In case of expected
parameter values of <5 in >20% of cells, Fisher's exact test
was used, which facilitates the analysis of smaller population
sizes. A P-value ≤0.05 was considered statistically
significant.
Treatment-free interval (TFI) was calculated from
the date of finishing primary treatment [surgery or
(chemo)radiotherapy] to the date of beginning of systemic treatment
of relapsed disease. Platinum-resistant patients were those who
progressed during the first 6 months after platinum-based
treatment.
PFS was defined as the time from the date of the
beginning of chemotherapy to the date of disease progression or
death from any cause. OS was calculated from the date of the
beginning of chemotherapy to the date of death from any cause.
Estimated survival rates and survival curves were
generated by the Kaplan-Meier method and compared using the
log-rank test. Univariate and multivariate regression analysis was
performed in all patients (PF and PFE together) to assess the
prognostic value of body mass index (BMI), type of systemic therapy
(PF vs. PFE therapy regimen), response rate, and subsequent lines
of chemotherapy. The prognostic significance was measured by hazard
ratio (HR), which was calculated using the Cox regression model.
All statistical analysis was performed using SPSS v.24.0 (IBM
Corp.).
Results
Patients characteristics
Sixty-seven patients (61 male, 6 female) were
included in the study. Exclusion criteria for cetuximab: 6 of 39
exposed to cetuximab (15.4%) had an allergy to cetuximab of grade
>2, one had an allergy to bee or wasp venom, 2 had received
prior cetuximab treatment, 2 refused cetuximab and 4 had an
extensive oropharyngeal tumour with a difficult urgent intubation
procedure. Finally, 34 patients were treated in the PF and 33 in
the PFE group (Table I). One
patient was African and all the others were Caucasian. The mean age
was 54.6 years. Most patients were in PS 1 (82.4% of the PF group
and 66.7% of the PFE group). The primary tumour was most often in
the oropharynx (47.1% in the PE and 39.4% in the PFE group). In the
PF group, half of patients relapsed locally and regionally, and 44%
had distant metastases. In the PFE group, one third of patients
relapsed locally and regionally, and half had distant metastases.
Of all patients, 7 patients were primary metastatic. The groups
were balanced according to age, gender, body mass index (BMI), PS,
tumour grade and primary site of progression (Table I). Median treatment free interval
(TFI) in 60 patients (primary metastatic excluded) was 18 months
(95% CI: 4.0-52.0). Of them, 4 patients were platinum resistant
(one in PS 0, others in PS 1). All 4 were treated with the PF
protocol.
 | Table ICharacteristics of patients treated
with PF (n=34) and PFE (n=33). |
Table I
Characteristics of patients treated
with PF (n=34) and PFE (n=33).
| Characteristics | PF | PFE | P-value |
|---|
| Mean age (range),
years | 54.7 (35-74) | 54.4 (32-70) | 0.88 |
| Sex, n (%) | | | 0.11a |
|
Female | 1 (2.9) | 5 (15.2) | |
|
Male | 33 (97.1) | 28 (84.8) | |
| Grade, n (%) | | | 0.26 |
|
2 | 13 (38.2) | 19 (57.6) | |
|
3 | 6 (17.6) | 5 (15.2) | |
|
Unknown | 15 (44.1) | 9 (27.3) | |
| Location of primary
tumour, n (%) | | | 0.17a |
|
Mouth | 0 (0.0) | 4 (12.1) | |
|
Oropharynx | 16 (47.1) | 13 (39.4) | |
|
Hypopharynx | 10 (29.4) | 9 (27.3) | |
|
Larynx | 5 (14.7) | 7 (21.2) | |
|
Paranasal
sinus | 1 (2.9) | 0 (0.0) | |
|
Other | 2 (5.9) | 0 (0.0) | |
| Primary stage, n
(%) | | | 0.05a |
|
I | 2 (5.9) | 0 (0.0) | |
|
II | 3 (8.8) | 0 (0.0) | |
|
III | 6 (17.6) | 12 (36.4) | |
|
IV | 23 (67.6) | 21 (63.6) | |
| Body mass index, n
(%) | | | 0.78a |
|
<18.5 | 5 (14.7) | 2 (6.1) | |
|
18-24.9 | 23 (67.6) | 24 (72.7) | |
|
25-30 | 5 (14.7) | 6 (18.2) | |
|
>30 | 1 (2.9) | 1 (3.0) | |
| Performance status,
n (%) | | | 0.15a |
|
0 | 5 (14.7) | 10 (30.3) | |
|
1 | 28 (82.4) | 22 (66.7) | |
|
2 | 1 (2.9) | 0 (0.0) | |
|
3 | 0 (0.0) | 1 (3.0) | |
| Primary treatment,
n (%) | | | 0.67a |
|
Radiotherapy | 3 (8.8) | 2 (6.1) | |
|
Surgery | 4 (11.8) | 3 (9.1) | |
|
Chemoradiation | 13 (38.2) | 13 (39.4) | |
|
Surgery and
radiotherapy | 5 (14.7) | 4 (12.1) | |
|
Surgery and
chemoradiotherapy | 8 (23.5) | 6 (18.2) | |
|
Chemotherapy | 1 (2.9) | 5 (15.2) | |
| Site of lesion at
relapse, n (%) | | | 0.44a |
|
Local | 19 (55.9) | 12 (36.4) | |
|
Regional | 18 (52.9) | 12 (36.4) | |
|
Distant | 15 (44.1) | 17 (51.5) | |
|
Primary
metastatic | 4 (11.8) | 3 (9.1) | |
Treatment characteristics
The treatment characteristics of both groups are
presented in Table II. The median
number of chemotherapy cycles for both groups was 4. Regarding the
platinum component, only 11.8% of the PF and 6.1% of the PFE group
were treated with carboplatin; all others received cisplatin at
least in one cycle. Cisplatin was used in all cycles in 67.7% of
the PF and 75.8% of the PFE group. Disease control was achieved in
58.8% of the PF and 72.7% of the PFE group. There were numerically
more partial responses in the PFE group and more stable disease in
the PF group, but the difference did not reach statistical
significance (P=0.17). Post-progression systemic treatment was
performed in 44% of patients in the PF and in 58% in the PFE
group.
 | Table IITreatment characteristics of patients
according to treatment with PF (n=34) and PFE (n=33). |
Table II
Treatment characteristics of patients
according to treatment with PF (n=34) and PFE (n=33).
|
Characteristics | PF, n (%) | PFE, n (%) | P-value |
|---|
| Number of
chemotherapy cycles | | | 0.93 |
|
1 | 4 (11.8) | 2 (6.1) | |
|
2 | 8 (23.5) | 1 (3.0) | |
|
3 | 6 (17.6) | 8 (24.2) | |
|
4 | 10 (29.4) | 16 (48.5) | |
|
5 | 3 (8.8) | 5 (15.2) | |
|
6 | 3 (8.8) | 1 (3.0) | |
| Cetuximab
cycles | | | NA |
|
1-3 | NA | 2 (6.1) | |
|
4-6 | NA | 4 (12.1) | |
|
7-9 | NA | 3 (9.1) | |
|
10-12 | NA | 4 (12.1) | |
|
13-15 | NA | 7 (21.2) | |
|
>15 | NA | 14 (42.4) | |
| Platinum
component | | | 0.79a |
|
Cisplatin
only | 23 (67.6) | 25 (75.8) | |
|
Carboplatin
only | 4 (11.8) | 2 (6.1) | |
|
Cisplatin
and carboplatin | 7 (20.6) | 6 (18.2) | |
| Response | | | 0.17 |
|
CR | 1 (2.9) | 0 (0.0) | |
|
PR | 10 (29.4) | 19 (57.5) | |
|
SD | 9 (26.5) | 5 (15.2) | |
|
Disease
control (CR+PR+SD) | 20 (58.8) | 24 (72.7) | |
|
PD | 9 (26.5) | 7 (21.2) | |
|
Not
evaluated | 5 (14.7) | 2 (6.1) | |
| Post-progression
therapy | | | 0.71 |
|
2nd-line
treatment | 8 (23.5) | 6 (18.2) | |
|
3rd-line
treatment | 7 (20.6) | 13 (39.4) | |
PFS and OS
Median follow-up time was 30.7 months. Median PFS
for all patients was 6.6 months (95% CI: 5.0-8.3); median PFS was
7.1 months (95% CI: 4.6-9.6) and 6.6 (95% CI: 4.2-9.1) for the PFE
vs. the PF group, respectively. There was no statistical difference
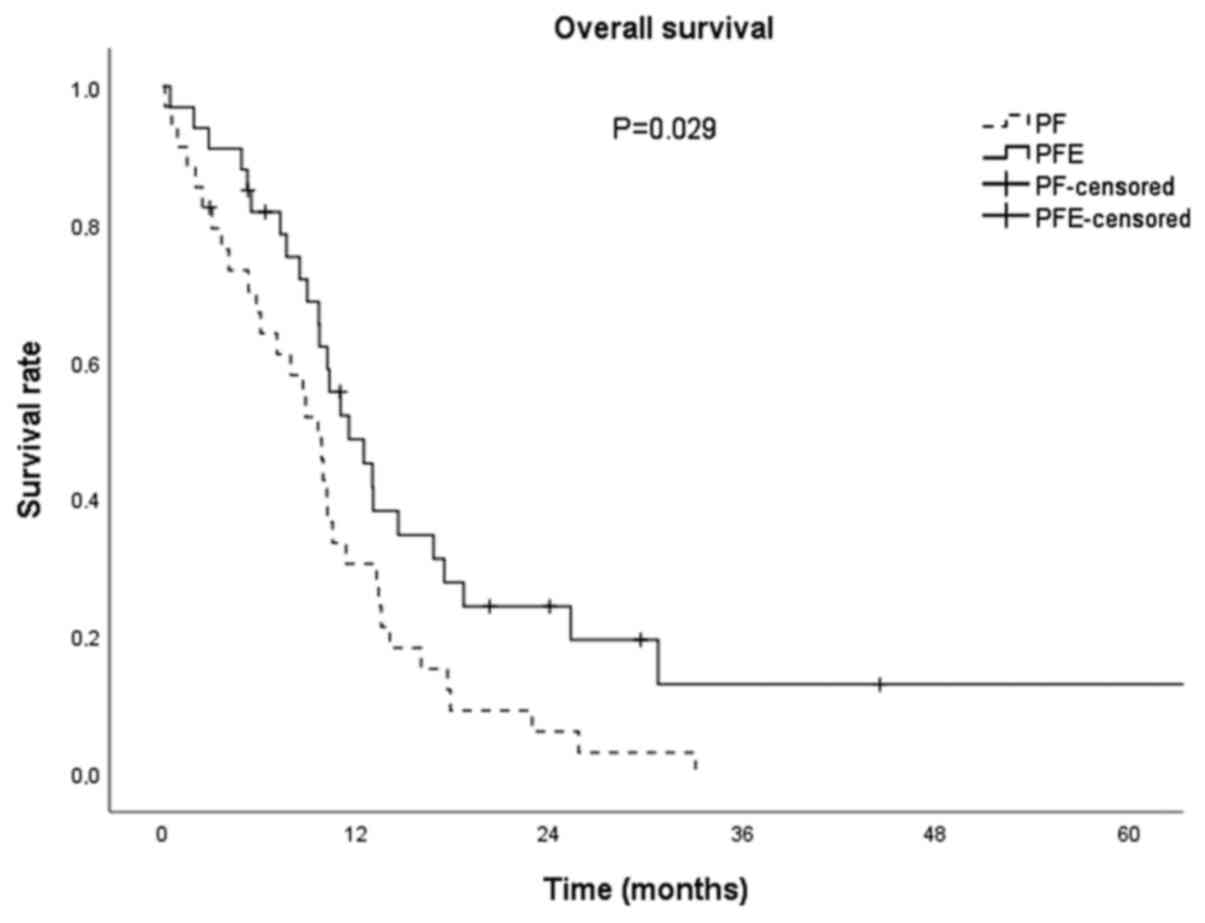
in median PFS between groups (P=0.852; Fig. 1). Median OS for all patients was
10.2 months (95% CI: 9.3-11.1). In the PFE group, OS was 11.5
months (95% CI: 8.1-14.9), and in the PF group 9.6 months (95% CI:
7.4-11.8) and was clinically importantly longer (for 1.9 months)
and statistically significant (P=0.029; Fig. 2).
Prognostic factors
Possible prognostic factors for PFS and OS are
presented in Tables III and
IV, respectively. For PFS, the
only independent prognostic factor was partial response to
treatment. For OS, in addition to the response rate, the number of
treatment lines (more than 1) was statistically significant. BMI
>25 was also nearly statistically significant.
 | Table IIIUnivariate and multivariate analyses
for prognostic factors regarding progression-free survival. |
Table III
Univariate and multivariate analyses
for prognostic factors regarding progression-free survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Body mass
index | | | | |
|
<25 | 1.00 | | | |
|
≥25 | 0.73
(0.37-1.44) | 0.73 | NA | NA |
| Cetuximab | | | | |
|
No | 1.00 | | | |
|
Yes | 0.95
(0.55-1.63) | 0.85 | NA | NA |
| Response rate | | | | |
|
Progressive
disease | 1.00 | | 1.00 | |
|
Complete
response | 0.34
(0.04-2.60) | 0.30 | 0.43
(0.05-3.50) | 0.43 |
|
Partial
response | 0.26
(0.13-0.52) | <0.01 | 0.32
(0.15-0.70) | <0.01 |
|
Stable
disease | 0.59
(0.27-1.28) | 0.18 | 0.57
(0.26-1.24) | 0.16 |
 | Table IVUnivariate and multivariate analyses
for prognostic factors regarding overall survival. |
Table IV
Univariate and multivariate analyses
for prognostic factors regarding overall survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Body mass
index | | | | |
|
<25 | 1.00 | | 1.00 | |
|
≥25 | 0.44
(0.21-0.94) | 0.035 | 0.46
(0.21-1.029 | 0.057 |
| Cetuximab | | | | |
|
No | 1.00 | | 1.00 | |
|
Yes | 0.56
(0.33-0.94) | 0.029 | 0.82
(0.46-1.45) | 0.494 |
| Response rate | | | | |
|
Progressive
disease | 1.00 | | 1.00 | |
|
Complete
response | 0.46
(0.06-3.53) | 0.462 | 0.26
(0.03-2.22) | 0.224 |
|
Partial
response | 0.13
(0.06-0.28) | <0.001 | 0.15
(0.06-0.38) | <0.001 |
|
Stable
disease | 0.30
(0.14-0.64) | 0.002 | 0.28
(0.13-0.64) | 0.002 |
| Subsequent
chemotherapy | | | | |
|
1 | 1.00 | | 1.00 | |
|
>1 | 0.39
(0.23-0.66) | 0.001 | 0.28
(0.15-0.52) | <0.001 |
Tolerability of treatment
The tolerability of treatment is presented in
Table V. During treatment with the
PF and PFE protocols, hypomagnesaemia occurred in 44.6 and 57.6% of
patients, respectively. Only one patient had grade 3
hypomagnesaemia (in the PFE group), all others were of grade 1 or
2. Skin rash of grade 1-3 was present in 85% of patients in the PFE
group. Of them, 27.3% had grade 3 skin rash. Diarrhoea was a rare
event; it was of grade 1 or 2 in 8.8% in the PF group and 6% in the
PFE group. Infections (skin, malignant wound infection, bladder
infection, oral mucositis, liver abscess, flu or pneumonia) were
present in 41.2% of patients in the PF group and 33.3% in the PFE
group. In both groups, one patient suffered febrile neutropenia.
During treatment, 5 (14.7%) patients in the PF and 4 (12.1%) in the
PFE group died due to adverse effects: Infection, bleeding, or
sudden cardiac event.
 | Table VAnalysis of adverse effects during
treatment with PF (n=34) and PFE (n=33). |
Table V
Analysis of adverse effects during
treatment with PF (n=34) and PFE (n=33).
| Adverse effect | PF, n (%) | PFE, n (%) | P-value |
|---|
|
Hypomagnesaemia | | | 0.60a |
|
Grade 1 | 12 (35.3) | 15 (45.5) | |
|
Grade 2 | 3 (8.8) | 3 (9.1) | |
|
Grade 3 | 0 (0) | 1 (3.0) | |
|
Grade 4 | 0 (0) | 0 (0) | |
| Skin rash | | |
<0.01a |
|
Grade 1 | NA | 10 (30.4) | |
|
Grade 2 | NA | 9 (27.3) | |
|
Grade 3 | NA | 9 (27.3) | |
|
Grade 4 | NA | 0 (0) | |
| Diarrhoea | | | 0.50a |
|
Grade 1 | 2 (5.9) | 0 (0) | |
|
Grade 2 | 1 (2.9) | 2(6) | |
|
Grade 3 | 0 (0) | 0 (0) | |
|
Grade 4 | 0 (0) | 0 (0) | |
| Infections | 14 (41.2) | 11 (33.3) |
>0.99a |
|
G1 | Unknown | Unknown | |
|
G2 | 4 (11.8) | 9 (27.3) | |
|
G3 | 7 (20.6) | 1 (3.0) | |
|
G4 | 0 (0) | 0 (0.0) | |
|
G5 | 3 (8.8) | 1 (3.0) | |
| Febrile
neutropenia | 1 (2.9) | 1 (3.0) | 1.00 |
| Death during
treatment | 5 (14.7) | 4 (12.1) | >0.99 |
| Causes of
death | | | |
|
Exsanguination | 2 (5.9) | 2(6) | |
|
Sudden
cardiac death | 1 (2.9) | 1(3) | |
|
Pneumonia | 1 (2.9) | 1(3) | |
|
Abscess | 1 (2.9) | 0 (0) | |
Discussion
In this retrospective study, we presented the
outcome and tolerability of the PFE and PE regimens in Slovenian
patients with R/M SCHNC treated in the routine clinical setting. In
a period of 10 years, half of the patients were treated in each
group. OS in the PFE group was 11.5 months and was 1.9 months
longer than in the PF group, which is statistically significant and
clinically important. PFS did not differ between the groups. A high
death rate due to disease progression and toxicity of treatment
were the main issues. Hypomagnesaemia and skin rash were manageable
with symptomatic measures.
In 2008, in the EXTREME study, OS of patients in the
PFE regimen arm significantly improved from 7.4 to 10.1 months and
PFS from 3.3 to 5.6 months, compared to those in the PF group. A
higher response rate (36 vs. 20%) and a significant reduction of
pain, and eating problems, and an improvement in speech were also
reported (3). Our retrospective
study showed a similar OS as the EXTREME study (11.5 months);
however, the benefit of median OS for PFE vs. PF regimen was
slightly smaller (1.9 months, compared to 2.7 months in the EXTREME
study). This clinically lower benefit could be due to
post-progression treatment in the PF group. Median PFS for PFE vs.
PF was not statistically significant in our report. Regarding
median PFS and OS with platinum-based chemotherapy and cetuximab,
similar results to ours were reported by other real-world studies
(10-14).
Due to poor prognosis of R/M HNSCC, data on possible
prognostic factors affecting OS and PFS are of great clinical value
(10-14).
In our study, favourable prognostic factors for OS were achieving
an objective response to therapy and receiving a subsequent line of
treatment after progression upon first-line treatment. OS tends to
be longer in slightly overweight patients (BMI >25). The only
prognostic factor for PFS was response rate (achieving partial
remission).
Depenni et al (10) found independent unfavourable
prognostic factors for OS and PFS in PFE regimen PS >0, presence
of residual tumour at the primary site, platinum resistance and
lack of objective response. Magnes et al (11) reported PS >1, leucocytosis and
increased C-reactive protein, treatment-free interval <12 months
and less intensive chemotherapy as prognostic factors for OS.
Similarly to our results, in a Japanese population, patients with
response to therapy (i.e. receiving ≥4 cycles of chemotherapy) had
a better prognosis for OS (12).
Response to systemic therapy is the major factor that affects
survival in R/M SCHNC (15,16). However, according to Anderson et
al, it is generally difficult to distinguish between cases
where response affects survival and cases where response identifies
patients with pre-treatment characteristics that favour longer
survival (17). Nevertheless, our
data and data in a Japanese population (12) show that patients able to receive
more lines of systemic therapy live longer.
Up to 57% of patients with SCHNC present with
malnutrition, with more than 10% weight loss from baseline body
mass. BMI has been found to be a useful tool to evaluate
nutritional status that should be routinely assessed before
treatment plans in SCHNC (18). The
association between BMI and survival is not consistent across
cancer types, stages and even sex, but in some cancer types BMI
>25 was associated with favourable OS (19). Having BMI >25 tends to carry a
beneficial prognosis for OS in our study. Similar to our results, a
Canadian observational study revealed that BMI >25 at diagnosis
is associated with improved survival; additionally, in their study,
BMI <19 was associated with decreased OS (20).
In our analysis, cetuximab treatment was not an
independent prognostic factor for OS. However, our study was not
randomized, but findings were similar to the randomized EXTREME
study, where patients with Karnofsky score <80 did not benefit
from the addition of cetuximab to chemotherapy (3). This indicates that PFE should be
preferred for patients in PS 0. Less intensive chemotherapies
combined with cetuximab also led to improved OS and represent
options for second-line treatment (21,22).
Fragile patients might be more susceptible to toxicity due to local
and systemic inflammatory responses triggered by cetuximab-induced
antibody-dependent cellular cytotoxicity (11).
The EXTREME regimen has considerable toxicity and
the logistics of managing three concomitant drugs. 5-fluorouracil
requires 24-h continuous infusion for 4 days and is associated with
an increased rate of mucositis, diarrhoea and cardiac events
(11). Cisplatin needs optimal
antiemetic treatment and close observation of renal function, high
hydration and magnesium supplementation. Cetuximab causes infusion
reaction, skin toxicity and hypomagnesaemia.
In our retrospective analysis, we found several
issues that should be discussed. Firstly, 15.4% of our patients
exposed to cetuximab had a grade 3 or 4 infusion reaction. This is
considerably higher than in the EXTREME study. We reported a
similar rate in our previous study (7). Secondly, the incidence of a grade 3
skin reaction (Table V) was also
high: 27.3% in comparison to 9% in the EXTREME study. These
patients needed intensive supportive care. Hypomagnesaemia was
predominantly of grade 1 or 2, but we performed very stringent
preventive measures for hypomagnesaemia. Diarrhoea (a side effect
of 5-fluorouracil) was not clinically important; <10% of
patients had grade 1 or 2 diarrhoea. A very important fact is that
41.2% in the PF group and 33.3% in the PFE group had infections. We
suppose that the high rate of infections in both groups could be
due to the high percentage of locoregional or regional relapse
(over 50% in PF and over 36% in PFE), which could cause tumour
wound infections and also aspiration pneumonia due to difficulties
in swallowing. The incidence of febrile neutropenia was low (2-3%).
A very important message from our study is reflected in the number
of deaths possibly related to treatment: 5 deaths (14.7% of
patients) in the PF group and 4 deaths (12.1% of patients) in the
PFE group. The causes are presented in Table V: Infections, bleeding and sudden
cardiac events.
In the EXTREME protocol, there were 10 (2.3%)
treatment-related deaths among 434 patients and an additional 4
(0.9%) cases of death due to sudden cardiac death. Despite these
complications and the small number of patients, OS of our patients
is better than real-world global OS, which is reported at 8.0
months (14). This is probably due
to the appropriate selection of patients for systemic therapy in
our daily practice. In a real-world population (14), one third of patients were platinum
resistant, which carries poor prognosis (10,11,14).
In our study, 4 (6%) patients were platinum resistant and died
within three months of therapy.
Because of the small number of patients, we cannot
conclude which regimen carries higher mortality and more adverse
effects. According to the ENCORE study, only 5% of serious adverse
events could be attributed to cetuximab (13).
Limitations of the study
The main limitations of our study are the small
number of patients and retrospective data collection. Many patients
were not treated with cetuximab due to limited access to the drug
(no reimbursement) or were unable to tolerate it (a substantial
rate of grade 3/4 infusion reactions). Finally, HPV status was not
assessed and its prognostic impact could not be evaluated.
The main advantage of this study is that all
patients were treated at a single comprehensive oncological centre
with specialist medical personnel (medical oncologists, specialist
nurses and nutritional therapists), which assured an optimal
treatment regimen and side effects management. In our real-world
practice, patients were treated with cisplatin as the preferred
agent at the same dose and in a comparable percentage as in the
EXTREME study. Our real-world treatment results will guide us to
further improve the selection of patients appropriate for this
aggressive treatment with short OS prolongation.
Recently, immunotherapy has become the new standard
of care, especially for patients with inflamed tumour (23,24).
The EMA has endorsed pembrolizumab as monotherapy or in combination
with PF chemotherapy in the first-line treatment of R/M SCHNC in
adults, based on programmed death-ligand 1 (PD-L1) expression for
those with combined positive score >1 and PS 0 or 1(25). It has been estimated that around
70-80% of patients with R/M SCHNC will be considered as eligible
(24). On the other side, the PFE
regimen still represents the optimal first-line therapy for the
remaining 20-30% of fit patients with PD-L1 negative R/M SCHNC or
patients contraindicated for anti-PD-L1 checkpoint inhibitors and
as second-line treatment after progression on PD-L1 checkpoint
inhibitors.
The analysis of patients with R/M HNSCC treated in
Slovenia in the 10-year period revealed that patients treated with
the PFE regimen have improved OS but not PFS when compared to the
PF regimen. Patients in either treatment group with objective
response to therapy, in good nutritional status and suitable for
further treatment at progression have a better prognosis. The
proportion of patients who died under treatment due to disease
progression and toxicity was high in both treatment arms. In
everyday clinical practice, the thorough selection of patients and
treatment at an experienced medical oncology centre is crucial.
Acknowledgements
The authors would like to thank Ms. Jelena Azarija,
Ms. Katja Leskovšek and Ms. Urška Rugelj from the Institute of
Oncology, Ljubljana (Ljubljana, Slovenia) for their help with data
collection.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ analyzed and interpreted the data and drafted the
manuscript. BZ conceived and designed the study, analyzed and
interpreted the data and critically revised the manuscript for
important intellectual content. CGK conceived and designed the
study, acquired the data, analyzed and interpreted the data,
drafted the manuscript and critically revised the manuscript for
important intellectual content. TZ and CGK are responsible for
confirming the authenticity of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Commission at the Institute of Oncology Ljubljana (Ljubljana,
Slovenia). All procedures followed in this study were in accordance
with the ethical standards of the responsible committee on human
experimentation (institutional and national) and the Declaration of
Helsinki of 1975, as revised in 2000. Individual patient consent
was not collected for the present study as this was a retrospective
database analysis, and the institutional informed consent form for
treatment included consent to use the patients data, materials
and/or test results for research purposes.
Patient consent for publication
Not applicable.
Competing interests
CGK presented these results at a meeting of
Slovenian and Croatian head and neck cancer specialists held in
Zagreb (Croatia) in December 2018 that was organised and sponsored
by Merck, although Merck had no influence on the presentation of
the results. TZ and BZ declare that they have no competing
interests.
References
|
1
|
Cancer in Slovenia 2016. Available from:
https://www.onko-i.si/fileadmin/onko/datoteke/dokumenti/RRS/LP_2016.pdf.
|
|
2
|
Argiris A, Harrington KJ, Tahara M,
Schulten J, Chromette P, Ferreira Castro A and Licitra L:
Evidence-based treatment options in recurrent and/or metastatic
squamous cell carcinoma of the head and neck. Front Oncol.
7(72)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vermorken J, Mesia R, Rivera F, Remenar E,
Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D,
et al: Platinum-based chemotherapy plus cetuximab in head and neck
cancer. N Engl J Med. 339:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gatta G, Botta L, Sánchez MJ, Anderson LA,
Pierannunzio D and Licitra L: EUROCARE Working Group. Prognoses and
improvement for head and neck cancers diagnosed in Europe in early
2000s: The EUROCARE-5 population-based study. Eur J Cancer.
51:2130–2143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gregoire V, Lefebvre JL, Licitra L and
Felip E: EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21
(Suppl 5):v184–v186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Strojan P, Zakotnik B, Žumer B, Karner K,
Dremelj M, Jančar B, Jereb S and Grašič Kuhar C: Skin reaction to
cetuximab as a criterion for treatment selection in head and neck
cancer. Anticancer Res. 38:4213–4220. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
The RECIST Working Group. RECIST 1.1.
Available from: https://project.eortc.org/recist/wp-content/uploads/sites/4/2015/03/RECISTGuidelines.pdf.
|
|
9
|
US Department of Health and Human
Services: Common Terminology Criteria for Adverse Events. Version
5.0. Published November 27, 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
Accessed December 12, 2020.
|
|
10
|
Depenni R, Cossu Rocca M, Ferrari D,
Azzarello G, Baldessari C, Alu M, Nole F, Codeca C, Boscolo G,
Piccininni M, et al: Clinical outcomes and prognostic factors in
recurrent and/or metastatic head and neck cancer patients treated
with chemotherapy plus cetuximab as first-line therapy in a
real-world setting. Eur J Cancer. 115:4–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Magnes T, Melchardt T, Weiss L, Mittermair
C, Neureiter D, Klieser E, Gampenrieder S, Moser G, Gaggl A, Griel
R and Egle A: Prognostic score in patients with recurrent or
metastatic carcinoma of the head and neck treated with cetuximab
and chemotherapy. PLoS One. 12(e180995)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sano D, Fujisawa T, Tokuhisa M, Shimizu M,
Sakagami T, Hatano T, Nishimura G, Ichikawa Y, Iwai H and Oridate
N: Real-world treatment outcomes of the EXTREME regimen as
first-line therapy for recurrent/metastatic squamous cell carcinoma
of the head and neck: A multi-center retrospective cohort study in
Japan. Anticancer Res. 39:6819–6827. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Le Tourneau C, Ghiani M, Cau MC, Depenni
R, Ronzino G, Bonomo P, Montesarchio V, Leo L, Schulten J,
Messinger D, et al: Cetuximab plus platinum-based therapy (PBT) as
a first-line treatment for patients with recurrent and/or
metastatic squamous cell carcinoma of the head and neck (R/M
SCCHN): An observational study (ENCORE). Ann Oncol. 29 (Suppl
8):viii372–viii399. 2018.
|
|
14
|
Grünwald V, Chirovsky D, Cheung WY,
Bertolini F, Ahn MJ, Yang MH, Castro G, Berrocal A, Sjoquist K,
Kuyas H, et al: Global treatment patterns and outcomes among
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results of the GLANCE H&N study. Oral Oncol.
102(104526)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vermorken JB and Specenier P: Optimal
treatment for recurrent/metastatic head and neck cancer. Ann Oncol.
21 (Suppl 7):S252–S261. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Argiris A, Li Y and Forastiere A:
Prognostic factors and long-term survivorship in patients with
recurrent or metastatic carcinoma of the head and neck. Cancer.
101:2222–2229. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Anderson JR, Cain KC and Gelber RD:
Analysis of survival by tumor response and other comparisons of
time-to-event by outcome variables. J Clin Oncol. 26:3913–3915.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alshadwi A, Nadershah M, Carlson ER, Young
LS, Burke PA and Daley BJ: Nutritional considerations for head and
neck cancer patients: A review of the literature. J Oral Maxillofac
Surg. 71:1853–1860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Greenlee H, Unger JM, LeBlanc M, Ramsey S
and Hershman DL: Association between body mass index (BMI) and
cancer survival in a pooled analysis of 22 clinical trials. Cancer
Epidemiol Biomarkers Prev. 26:21–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gama RR, Song Y, Zhang Q, Brown MC, Wang
J, Habbous S, Tong L, Huang SH, O'Sullivan B, Waldron J, et al:
Body mass index and prognosis in patients with head and neck
cancer. Head Neck. 39:1226–1233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guigay J, Feyette J, Dillies AF, Sire C,
Kerger JN, Tennevet I, Machiels JP, Zanetta S, Pointreau Y, Bozec
Le Moal L, et al: Cetuximab, docetaxel and cisplatin in first-line
treatment in patients with recurrent or metastatic head and neck
squamous cell carcinoma: A multicenter, phase II GORTEC study. Ann
Oncol. 26:1941–1947. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hitt R, Irigoyen A, Cortes-Funes H, Grau
JJ, García-Sáenz JA and Cruz-Hernandez JJ: Spanish Head and Neck
Cancer Cooperative Group (TTCC). Phase II study of the combination
of cetuximab and weekly paclitaxel in the first-line treatment of
patients with recurrent an/or metastatic squamous cell carcinoma of
head and neck. Ann Oncol. 23:1016–1022. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cramer JD, Burtness B and Ferris RL:
Immunotherapy for head and neck cancer: Recent advances and future
directions. Oral Oncol. 99(104460)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Machiels JP, Rene Leemans C, Golunski W,
Grau C, Licitra L and Gregoire C: EHNS Executive Board. Squamous
cell carcinoma of the oral cavity, larynx, oropharynx and
hypopharynx: EHNS-ESMO-ESTO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 31:1462–1475.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Baste N, Prakash
N, Bratland A, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928.
2019.PubMed/NCBI View Article : Google Scholar
|