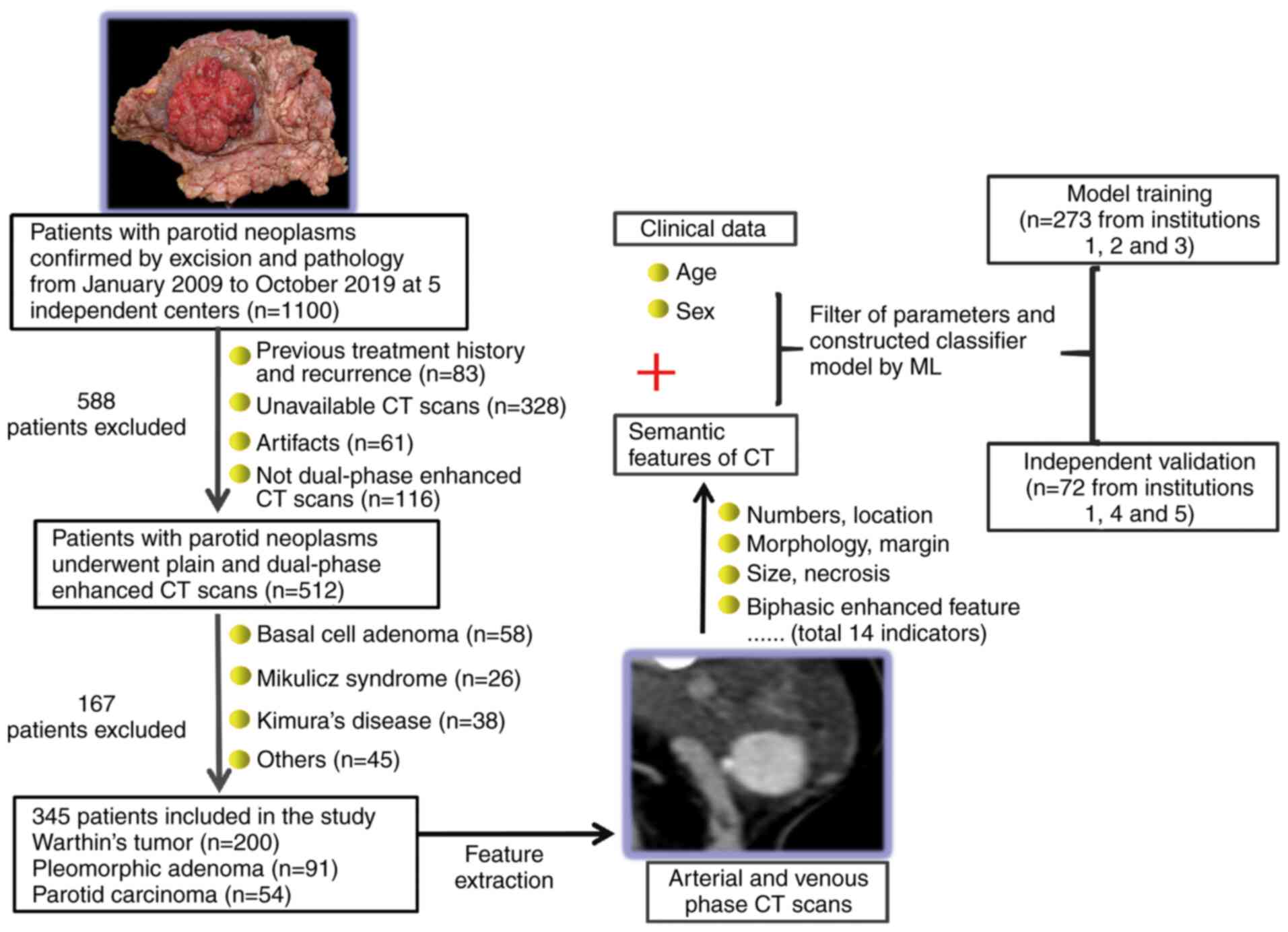
Introduction
Characterization of parotid tumors (PTs) is
important for treatment planning and prognosis, and PT
discrimination has recently been developed at the molecular level.
Diffusion-weighted imaging (DWI)- and dynamic contrast-enhanced
(DCE)-MRI are the most important methods described to date, with a
single or multiple technology/parameter combination (1-3),
largely expanding predictive power. Additionally, molecular
imaging, such as diffusion tensor imaging, has been used to
differentiate malignant from benign tumors, as well as Warthin
tumors (WTs) from pleomorphic adenomas (PAs), both with an accuracy
of 86% (4). Unfortunately, such
measurements based on molecular biomarkers are expensive,
time-consuming, involve complicated analyses and are available at
few facilities. Therefore, it is of paramount importance to explore
simple and cost-effective approaches to improve the accuracy of
differentiation among different PTs. Compared with MRI, multislice
computed tomography (MSCT) has the characteristics of wide
application and simple generalization of the results (5). The effectiveness of machine learning
(ML) methods for high-throughput extraction of quantitative
features from clinical images has recently been demonstrated in
multiple disciplinary fields, including tumor identification
(6) and clinical outcome prediction
(7). However, there are few
published reports using ML-based multiparametric CT radiomics to
study PTs, which represents a knowledge gap. The aim of the present
study was to establish a model through analysis of clinical data
and dual contrast-enhanced CT coupled with ML-based algorithms to
improve the accuracy of PT differential diagnosis and to test its
efficacy upon biopsy completion to enable timely decisions for
treatment.
Materials and methods
Patients
A total of 1,100 patients (427 women and 683 men;
mean age, 54.5 years; age range, 19-78 years) with pathologically
confirmed PTs who were treated by curative resection between
January 2010 and May 2019 at five independent institutions [i) The
First People's Hospital of Foshan; ii) The Sanshui People's
Hospital; iii) The Foshan Chancheng District Central Hospital; iv)
The Foshan Medicine Hospital; and v) The Second People's Hospital
of Nanhai District; all in Foshan, China] were reviewed. Of the
subjects reviewed, 345 were included in the final analysis
according to the study criteria shown in Fig. 1. A total of 273 patients recruited
from institutions 1, 2 and 3 were randomly assigned to the training
model. The independent validation set consisted of 72 patients
treated at institutions 1, 4 and 5.
Imaging feature extraction
This retrospective study was approved by the
Institutional Review Board (Ethics Committee of The First People's
Hospital of Foshan; approval no. FSYYY-EC-SOP-008-02.0-A09).
Informed consent was obtained from all participants included in the
study. Two CT radiologists with 10 years (reader 1) and 15 years
(reader 2) of experience in PT imaging who were blinded to all
clinical data independently reviewed baseline CT images to evaluate
the following characteristics: Location, number, boundary,
calcification, morphology, vascular marginalization (VM; small,
newly formed blood vessels around tumors), enlarged lymph nodes
(ELN) around tumors (diameter ≥5 mm), density (Hounsfield Units;
HU) in plain phase (PP), arterial phase (AP) and venous phase (VP),
perfusion rate (PR) in AP
[PR=(HUAP-HUPP)/HUpp] and
clearance [(HUVP-HUAP)/HUAp)]
(Figs. S1-S2). The tumor was deemed to have a clear
boundary if it was well-demarcated along its entire circumference
and to have an unclear boundary otherwise. When the assessment was
inconsistent, consensus was reached through discussion. To assess
tumor attenuation, a circular region of interest (ROI) that
excluded obvious cystic and necrotic areas was identified, and the
two averages of the ROI were taken as the final value. Moreover,
patient age and sex were considered. A total of 16 indicators were
examined in the statistical analysis.
ML models and statistical
analysis
The Kruskal-Wallis H test and χ2 test
were used to compare parameters among WT, PA and parotid carcinoma
(PCa). Parameters with significant differences among the three
groups were used to construct a classifier model, and the other
parameters were filtered. The R package MASS (https://CRAN.R-project.org/package=MASS)
was utilized to construct a classifier model by linear discriminant
analysis (LDA) using the training data. The model was analyzed as a
classifier for the three tumors in the training cohort
(institutions 1, 2 and 3) and validated in a combination cohort
from three hospitals (institutions 1, 4 and 5). The effect of the
classifier model was based on accuracy. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of different PTs
Compared with patients with PA and PCa, the majority
of patients with WT had multiple lesions in the bilateral parotid
glands, and the difference was statistically significant
(P<0.01). A total of 79.5 and 74.4% of lesions in PCa patients
displayed an unclear boundary and morphological irregularity,
respectively, which was higher compared with the respective
percentages in patients with WT and PA (P<0.01). Additionally,
ELN signs were observed around the lesions in ~64.7% of WT and in
64.1% of PCa cases, which was significantly higher compared with
the percentage for PA (30.8%; P<0.01). The majority of WTs
(~66.7%) displayed VM signs (P<0.01). Regarding quantitative CT
characteristics, the Hu and arterial perfusion rate of WTs were
both significantly higher compared with those of other tumors in
plain and dual-enhanced scans (P<0.01), and the majority of
patients with WT exhibited characteristics of fast forward and fast
retreat, with a high clearance rate (Figs. S1-S2). These features were selected for
predictive ML model establishment.
Construction of the ML classifier
model
An ML classifier model was successfully constructed
based on the selected clinical and CT characteristics. These
characteristics differed significantly among WT, PA and PCa. The
results regarding the ability of ML-based CT characteristic and
clinical data analysis to discriminate the three subtypes of PTs in
the training and validation cohorts are summarized in Table I and Fig. 2. In general, this classifier
achieved satisfactory performance, with a total accuracy of 82.1%
in the training cohort and 80.5% in the validation cohort. The
cases of 3 representative patients with WT, PA and PCa for whom the
proposed ML model correctly predicted pathology are presented in
Fig. 3.
 | Table IDiagnostic performance of machine
learning-based CT features and clinical data classifiers for
discriminating WT, PA and PCa in the training and validation
cohorts. |
Table I
Diagnostic performance of machine
learning-based CT features and clinical data classifiers for
discriminating WT, PA and PCa in the training and validation
cohorts.
| Training group | Pathological
diagnosis |
|---|
| Predictive | PCa | PA | WT |
|---|
| PCa | 27 | 12 | 4 |
| PA | 6 | 42 | 5 |
| WTs | 6 | 14 | 147 |
| Accuracy | 82.1% | | |
| Validation group |
| Predictive | PCa | PA | WT |
| PCa | 12 | 1 | 1 |
| PA | 1 | 13 | 2 |
| WTs | 2 | 9 | 41 |
| Accuracy | 80.5% | | |
Discussion
The characterization of PTs is important for
preoperative treatment planning, as well as assessment of
therapeutic responsiveness and prognosis (1). In the present study, LDA-based ML with
selected clinical data and CT radiomics were applied to
discriminate between benign (PA and WT) and malignant (PCa) PTs
preoperatively, with satisfactory results. The results demonstrated
that the ML approach is a promising non-invasive method that is
feasible and reliable for the evaluation of PTs.
In general, the clinical symptoms of PTs are
non-specific, and preoperative imaging is crucial for diagnosis. CT
and MRI are promising examination techniques for PTs based on the
analysis of various morphological parameters and molecular imaging.
Over the last 5 years, CT multiphase enhancement (8), CT perfusion (9), DCE-MRI (1,3,10) and
apparent diffusion coefficients derived from DWI (1-3,10)
have been applied to improve the diagnostic efficiency of PTs, with
diagnostic accuracies ranging from 70 to 97% (3,8-12).
However, the large influence of subjective factors, the small case
sample size and a single evaluation index are the main limitations
of these studies, and results among studies have not been
replicated. In ML, the predominant task is predictive modeling,
namely, the creation of models to characterize new examples. In
terms of the clinical demand discussed above, the histopathology of
PT must be predicted accurately and preoperatively, for which there
is an opportunity to apply ML technology. As our sample number was
lower compared with that in common industrial cases, 2 clinical
indices and 14 CT features were selected; 16 indicators were
included in the statistical analysis according to prior clinical
knowledge for prediction. ML has been shown to have a great
advantage in tumor diagnosis as well as prognosis and recurrence
prediction, with an increasing number of reports involving hepatic
carcinoma (13), thyroid nodules
(14), renal tumors (15) and colon cancer (16), among others. Previous studies have
demonstrated that ML-based texture analysis has diagnostic accuracy
ranging from 98.3 to 100% for neoplastic lesions of the abdomen. To
the best of our knowledge, this is the first ML study for PT
discrimination, revealing powerful diagnostic performance.
The key to building a powerful predictive model is
to select efficient indicators and appropriate modeling methods. In
the present study, radiomic features were extracted from plain
scans and dual-phase enhancement of CT examinations. The analysis
mainly included the location, number, boundary, morphology, VM,
ELN, density and enhancement pattern, among others, which should
represent the underlying tumor biology. Previous studies have
reported that feature optimization can enhance the predictive value
of radiomic features (6,7,17).
Therefore, statistical analysis was first used to eliminate
indicators without significant differences, and among the tumor
categories, no significant differences were observed regarding
characteristics such as tumor location or nodule-in-nodule sign.
Finally, 14 CT characteristics that were valuable in the
differential diagnosis of PA, WT and PCa were selected, which was
consistent with the results of our previous studies (3,5,9). In
addition, given the importance of sex and age in the identification
of PTs found in previous studies, these characteristics were
included as modeling indicators to improve the success of the
model. It was previously demonstrated that LDA-based models had
slightly better diagnostic performance compared with support vector
machine-based models (6,18). LDA is a representative linear
classifier that uses a straight line (a vector) to separate three
classes (19), namely PA, WT and
PCa in the present study. In general, this classifier achieved
satisfactory performance, with a total accuracy of 82.1% in the
training cohort and 80.5% in the validation cohort. Our previous
study (3) established a simple
model for PT discrimination based on multiparametric analysis
derived from DCE-MR and DWI, and LDA analysis was used. Our results
revealed the biomarker that appeared to be the best indicator
(extracellular extravascular space volume fraction + time-intensity
curve), with a prediction accuracy of 75%, while the predictive
model in the present study exhibited a higher accuracy at 80.5%.
Therefore, we believe that the model established in the present
study is more valuable. First, dual-phase enhanced CT scanning has
a wide range of applications and is relatively simple and
practical. Although CT involves exposure to radiation, the progress
of low-dose scanning and image reconstruction algorithms has
greatly reduced the radiation dose (20). Second, the modeling indicators
combined with clinical and CT characteristics in the present study
were efficient and simple. Additionally, the number of cases in
this study was large (345 cases) and, as the data from the training
and prediction models were both derived from multiple centers, the
model is considered as reliable and accurate.
There were three primary limitations to the present
study. First, as this was a retrospective study, selection bias
could not be fully avoided, and the number of patients with each
subtype of PT was not balanced in the validation set, which may
have influenced the performance metrics to a certain extent.
However, it is not clear whether this had a significant impact on
the modeling. Second, a manual ROI method was used for the
determination of tumor enhancement indicators. Although multiple
point and multiple site measurements were adopted to reduce errors,
the enhancement characteristics of the tumors could not be fully
and accurately reflected. We are confident that, in the future,
semiautomated software will be able to recognize PTs with CT
images. Finally, only three types of tumors (WTs, PA and PCa) were
analyzed in the present study. Other types of tumors, such as basal
cell adenoma, eosinophiloma and oncocytic adenoma, are rare tumors
with a small number of cases. Therefore, these pathological types
of PTs were not included in the diagnostic scope in this study. The
current diagnostic model still needs to be further improved.
In summary, we herein established an ML classifier
based on LDA analysis with selected CT characteristics derived from
dual-enhanced parotid CT examination and clinical characteristics
(sex and age) that achieved 80.5% diagnostic accuracy for
differentiating among PA, WT and PCa. To the best of our knowledge,
this is the first successful ML classifier established for PT
discrimination. This predictive model has high diagnostic accuracy
and can be quickly translated into clinical applications and
popularized in primary hospitals. Therefore, this classifier will
benefit patients with PTs and help in providing appropriate
treatment.
Supplementary Material
Sex and CT categorical characteristics
were analyzed and compared in PAs, WTs and PCas.
*P<0.05; **P<0.01;
***P<0.001. PA, pleomorphic adenoma; WT, Warthin
tumor; PCa, parotid carcinoma; U/B, unilateral/bilateral; SL/DL,
superficial lobe/deep lobe; VM, vascular marginalization; NINS,
nodule-in-nodule sign; ELN, enlarged lymph nodes.
age and CT continuous characteristics
were analyzed and compared among PAs, WTs and PCas.
*P<0.05; **P<0.01;
***P<0.001. PA, pleomorphic adenoma; WT, Warthin
tumor; PCa, parotid carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Innovative
Project of Foshan (grant no. FSOAA-KJ218-1301-0021), the Foshan
Ascending Peak Plan Project (grant no. 2020B003), and the Medical
Scientific Research Foundation of Guangdong Province of China
(grant no. A2021493).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX performed the majority of experiments and wrote
the manuscript; YJ wrote the manuscript and prepared the graphs;
WW, JW, BL, CZ, XG, MG and SG collected the data and analyzed
images; AP designed the study and was involved in editing the
manuscript. ZX and AP have seen and can confirm the authenticity of
the raw data. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the
Institutional Review Board (Ethics Committee of The First People's
Hospital of Foshan; approval no. FSYYY-EC-SOP-008-02.0-A09).
Written consent was obtained from all participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eissa L, Seif SA, Desooky SE, Eid M and
Koraitim T: Accuracy assessment of combined diffusion weighed and
dynamic gadolinium MR sequences in characterization of salivary
gland tumors. Egypt J Radiol Nucl Med. 47:127–139. 2016.
|
|
2
|
Razek AAK, Samir S and Ashmalla GA:
Characterization of parotid tumors with dynamic susceptibility
contrast perfusion weighted magnetic resonance imaging and
diffusion-weighted MR imaging. J Comput Assist Tomogr. 41:131–136.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu Z, Zheng S, Pan A, Cheng X and Gao M: A
multiparametric analysis based on DCE-MRI to improve the accuracy
of parotid tumor discrimination. Eur J Nucl Med Mol Imaging.
46:2228–22344. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Razek AA: Characterization of salivary
gland tumours with diffusion tensor imaging. Dentomaxillofac
Radiol. 47(20170343)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu ZF, Yong F, Yu T, Chen YY, Gao MY, Zhou
T, Pan AZ and Wu RH: Different histological subtypes of parotid
gland tumors: CT findings and diagnostic strategy. World J Radiol.
5:313–320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fan Y, Chen C, Zhao F, Tian Z, Wang J, Ma
X and Xu J: Radiomics-based machine learning technology enables
better differentiation between glioblastoma and anaplastic
oligodendroglioma. Front Oncol. 9(1164)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hua X, Huang X, Huang ZZ, Song CG, Deng
JP, Long ZQ, Lin HX and Yuan ZY: Establishment of prognostic
nomograms based on skeletal muscle index and serum biomarker in
breast cancer patients receiving radiotherapy. Clin Transl Med.
10(e115)2020.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Jin GQ, Su DK, Xie D, Zhao W, Liu LD and
Zhu XN: Distinguishing benign from malignant parotid gland tumours:
Low-dose multiphasic CT protocol with 5-minute delay. Eur Radiol.
21:1692–1698. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xu Z, Rong F, Yu T, Chen YY, Gao Q, Zhou T
and Pan AZ: Pleomorphic adenoma versus Warthin tumor of the parotid
gland: Diagnostic value of CT perfusion imaging and its pathologic
explanation:CT Perfusion of pleomorphic adenoma versus Warthin. J
Tumor. 4:419–425. 2016.
|
|
10
|
Yuan Y, Tang W and Tao X: Parotid gland
lesions: Separate and combined diagnostic value of conventional
MRI, diffusion-weighted imaging, and dynamic contrast-enhanced MRI.
Br J Radiol. 89(20150912)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsusue E, Fujihara Y, Matsuda E,
Tokuyasu Y, Nakamoto S, Nakamura K and Ogawa T: Vanishing parotid
tumors on MR imaging. Yonago Acta Med. 61:33–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kato H, Kanematsu M, Watanabe H, Mizuta K
and Aoki M: Salivary gland tumors of the parotid gland: CT and MR
imaging findings with emphasis on intratumoral cystic components.
Neuroradiology. 56:789–795. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang
WW, Li XC and Wang XH: Machine-learning analysis of
contrast-enhanced CT radiomics predicts recurrence of
hepatocellular carcinoma after resection: A multi-institutional
study. EBioMedicine. 50:156–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Colakoglu B, Alis D and Yergin M:
Diagnostic value of machine learning-based quantitative texture
analysis in differentiating benign and malignant thyroid nodules. J
Oncol. 31(6328329)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ding J, Xing Z, Jiang Z, Chen J, Pan L,
Qiu J and Xing W: CT-based radiomic model predicts high grade of
clear cell renal cell carcinoma. Eur J Radiol. 103:51–56.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gessert N, Bengs M, Wittig L, Drömann D,
Keck T, Schlaefer A and Ellebrecht DB: Deep transfer learning
methods for colon cancer classification in confocal laser
microscopy images Int J Comput Assist Radiol. Surg. 14:1837–1845.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Griethuysen JJ, Fedorov A, Parmar C,
Hosny A, Aucoin N, Narayan V, Beets-Tan RG, Fillion-Robin JC,
Pieper S and Aerts HJ: Computational radiomics system to decode the
radiographic phenotype. Cancer Res. 77:e104–e107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bellingegni AD, Gruppioni E, Colazzo G,
Davalli A, Sacchetti R, Guglielmelli E and Zollo L: NLR, MLP, SVM,
and LDA: A comparative analysis on EMG data from people with
trans-radial amputation. J Neuroeng Rehabil. 15(82)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiao qing D and Hong cai C: Feature
extraction using a fusion method based on sub-pattern row-column
two-dimensional linear discriminant analysis. Journal of Computer
Applications. 12:3593–3598. 2014.
|
|
20
|
Zhu Y and Ding Y: Auto-optimized
paralleled sinogram noise reduction method based on relative
quality assessment for low-dose X-ray computed tomography. J Med
Imaging Health Informatics. 7:278–282. 2017.
|