Introduction
Lung cancer is usually diagnosed at a late stage due
to lack of early disease symptoms. Currently we are trying to
direct all high risk patients to have computed tomography scans of
the thorax with low dose radiation (1). We have novel tools for diagnosis such
as the radial endobronhial ultrasound, endobronchial ultrasound
with convex probe, electromagnetic navigation and
ARCHIMEDES® fused navigation system. Moreover; we can
perform transthoracic biopsy with convex probe or computed
tomography guided biopsies (2-7).
Currently we have several treatments for non-operable non-small
cell lung cancer (NSCLC) with chemotherapy, radiotherapy, tyrosine
kinase inhibitors, immunotherapy or combinations (8-11).
Moreover; after we acquire biopsy we investigate for
the following gene expressions growth factor receptor (EGFR) or
T790M, anaplastic lymphoma kinase (ALK), Programmed death-ligand 1
(PD-L1), Proto-oncogene tyrosine-protein kinase ROS-1, RET
proto-oncogene encodes and proto-oncogene B-Raf (9,12).
All these treatments of course have their adverse effects that the
treating physician has to overcome (13,14).
Moreover; usually patients upon diagnosis have
recently lost weight and have cancer cachexia. All patients which
are going to receive chemotherapy or radiotherapy must have good
nutritional status. It has been previously observed that cachexia
is an important independent factor for survival and treatment
efficiency (15). It is known that
malnutrition frequently coexists in cancer patients. Several
studies demonstrated that the incidence of malnutrition among
cancer patients can be up to 31-97% (16,17).
Most studies have been done in gastrointestinal tumors and very few
in lung cancer patients. Xara et al (18) observed that the incidence of
malnutrition among patients with non-small-cell lung cancer is up
to 35.7%. The consequences of malnutrition may reduce immune
function, decrease treatment response, increase infection rates,
and tolerance to treatment, lower quality of life, increase health
care costs, and reduce survival time (19,20).
Moreover; the relative risk of death from malnutrition was observed
to be 1.8 times higher for cancer patients without malnutrition
(21). It is absolutely important
that the nutritional status of cancer patients is included in the
treatment management, since it decides the patient's tolerance for
curative treatment (22).
Nutritional support will maintain current health status but also,
will improve patient satisfaction, quality of life, and treatment
outcomes.
Malnutrition has been observed to be prevalent in
advanced lung cancer patients. These patients require timely
nutrition support and guidance, management of treatment symptoms
mainly with drug interventions. Regarding lung cancer, the
nutritional status of patients with advanced lung cancer,
particularly those at a higher risk of malnutrition, such as
elderly patients, female patients, smokers, poor nutritional status
has been associated with worse clinical outcome. Moreover; patients
with malnutrition should be given more attention; their nutritional
status should be evaluated and re-evaluated at least after 2 months
and they should be given nutritional support in time. It is known
that in almost all patients taste is altered and nausea is
observed, especially in chemotherapy treatment. There are special
tools to evaluate these alterations.
Improvement of the nutritional status of patients
with advanced lung cancer may have beneficial effects on their
quality of life and treatment effectiveness. The same has been
observed with chronic obstructive pulmonary disease (COPD)
(23). We investigated in our
study non-small cell lung cancer patients and recorded several body
and metabolism characteristics and associated them with treatment
benefit and survival.
Patients and methods
Patients
We collected data from 82 NSCLC patients stage IV.
Our study was conducted in the ‘Theageneio’ Cancer Hospital,
Thessaloniki, Greece. Our study was also approved by our
investigational review board (‘Theageneio’ Cancer Hospital,
Thessaloniki, Greece) and was performed according to the principles
of the Declaration of Helsinki. Written informed consent was
acquired from the patients. All patients were diagnosed and had
first line treatment. Patients cheracteristics were as follows: 40
adenocarcinomas, 42 squamous cell carcinoma, 40 male, 42 female,
Male mean age 65, Female mean age 62. The only criteria was all
newly diagnosed patients at that time point where our study was
initiated. Patients received chemotherapy, immunotherapy,
radiotherapy or combination of these treatments. Several data for
all patients were recorded upon the first day of diagnosis and last
follow up. In specific: Survival, treatment, weight, height, age,
body mass index (BMI), waist and hips length, waist/hip ratio
(WHR), lean body mass, total body water, extracellular water,
intracellular water, body cell mass (BCM), basic metabolism (RMR),
Harris Benedict (%Pred), VO2 (ml/min), Ve (lt/min),
VarVe (%), VarVO2 (%), Rf (1/min), FeO2 (%),
Mediterranean diet score, physical activity level and number of
years spent smoking.
Statistical procedure
Regarding survival, it was represented as the time
of diagnosis and death or last follow up. The BMI is a measure used
to determine obesity. It is the ratio of weight to the square of a
person's height. BMI <18.5 indicates that the person is
underweight 18.5-24.9 indicates that the person is normal weight
25-29.9 indicates that the person is overweight >30 indicates
that the person is obese. The WHR shows the relative distribution
of fat in adults and the risk of disease. It is the ratio of the
waist circumference (cm) to the hip circumference (cm). WHR is
strongly associated with visceral (or abdominal) obesity, which
significantly increases the likelihood of developing chronic
diseases such as cardiovascular disease and type II diabetes.
Increased risk when WHR >1.0 (in men). Total body water; the
ideal percentage for adult men is 50-65%. BCM (kg); the total mass
of cellular elements in the body that make up the whole of
metabolically active tissue. There is a depletion of BCM that is
characteristic of chronic conditions such as AIDS and end-stage
cancer. RMR (kcal/day); basic metabolism (RMR) is the calories
consumed by the body at rest for its basic vital functions. Harris
Benedict (%Pred); the percentage of RMR, obtained from the Harris
Benedict equation. VO2 (ml/min); the volume (ml) of
oxygen consumed per minute. Ve (lt/min); ventilation is the volume
of air that is exhaled from the lungs over a period of one minute.
The normal adult at rest breathes about 5-10 l/min. VarVe (%); the
percentage variance of Ve. VarVO2 (%); the percentage of
the average VO variation. Rf (1/min); respiratory frequency is the
number of breaths per minute. Normal Rf for healthy adults is
between 12 and 20 breaths per minute. FeO2 (%); the
concentration of OD in the exhaled air. Mediterranean diet score;
this score ranges from 0-55 and results from a questionnaire of 11
questions about the frequency of food consumption, which assesses
adherence to the Mediterranean diet and the risk of heart disease.
The higher the score, the greater the attachment to the
Mediterranean diet. 0-20: Low attachment, 21-35: Moderate
attachment and 36-55: High attachment (All patients said that they
did not change anything in their eating habits from the first to
the second measurement). Physical activity level; Level of physical
activity evaluated through 8 questions and patients were
categorized into 3 categories: i) Low, ii) moderate and iii) high
physical activity (also all patients said that they did not change
anything in their physical activity from the first to the second
measurement). Number of years spent smoking; ‘Pack years’ is a
clinical quantification of smoking used to measure a person's
exposure to tobacco. It is the product of the packs of cigarettes
smoked during the day the smoking years.
Patients were subjected to various treatments such
as chemotherapy (chemo) or immunotherapy (immune), a combination of
those (combo) and finally radiation treated in combination with
either one as previously explained or combined additionally with
drug administration (radiation+). Under the four treatment
regimens, patients were additionally cross-tabulated with their
survival status. The Kaplan-Meier survival rates were also produced
and differences among groups were examined using log-rank and
Kruskal-Wallis test. The well-being of patients was recorded in two
periods, the initial commenced upon diagnosis and the final date
upon death or last follow-up, measuring at that interval the
weight, fat, lean and BMI, relative metabolic rate RMR and breath
index (VO2). Their response, treated as paired final
minus initial difference, was questioned to reflect the impact of
survival status and treatment type, thus a two-factor analysis of
variance (ANOVA) was conducted. Pairwise group mean differences
were tested via Tukey method. Normality and variance homogeneity
was checked in all cases.
Results
Survival status and treatment
type
Table I shows the
cross-tabulation between survival status and treatment type.
Interestingly, only one death was recorded when immunotherapy
interacted with, whereas the survival ratio was greater in patients
treated with radiation+ (24/8) although not statistically
supported. Few patients were combo treated (13.41%) reasonably
putting under question for possible synergistic results. The lack
of observations in the cell immune-death did not permit us to
include the interaction term in ANOVA due to possible errors in the
calculation of sum of squares.
 | Table ICross-tabulated data according to
survival status and treatment type. |
Table I
Cross-tabulated data according to
survival status and treatment type.
| Treatment | 0 | 1 | All | % |
|---|
| Chemotherapy | 8 | 10 | 18 | 21.95 |
| Combined | 8 | 13 | 21 | 13.41 |
| Immunotherapy | 1 | 10 | 11 | 25.61 |
| Radiation+ | 8 | 24 | 32 | 39.02 |
| All | 25 | 57 | 82 | 100.00 |
| Percentage of
patients receiving treatment | 30.48 | 69.52 | | |
The combined effect of chemotherapy and
immunotherapy revealed higher survival rates as Kaplan-Meier graph
clearly indicated (Fig. 1),
supported also by the statistically significantly result of
log-rank (P=0.0053) and Kruskal-Wallis test (P=0.007) in Tables II and III. Patients treated with the combined
protocol increased 2.5 times higher their mean survival rate
(828.75) as compared to those treated with chemotherapy (337.75)
and 1.6 times to those treated with radiation+ (510). Considering
the median survival time, the above multipliers are reformed to
1.76 (670 days vs. 380.5) and 1.97 670 vs. 339.5) respectively.
 | Table IIParametric and quantile data of
survival time according to treatment effects. |
Table II
Parametric and quantile data of
survival time according to treatment effects.
| | Parametric
data | Quantiles |
|---|
| Group | Number failed | Number
censored | Mean (days) | Standard error | Median time
(days) | Lower 95% | Upper 95% | 25% Failures | 75% Failures |
|---|
| Chemotherapy | 8 | 0 | 337.75 | 39.221 | 380.5 | 172 | 425 | 237.0 | 420.5 |
| Combined | 8 | 0 | 828.75 | 141.305 | 670.0 | 439 | 1,056 | 580.5 | 1,006.5 |
| Radiation+ | 8 | 0 | 510.00 | 141.079 | 339.5 | 230 | 748 | 273.0 | 609.5 |
 | Table IIIBetween group test data of survival
time according to treatment effects. |
Table III
Between group test data of survival
time according to treatment effects.
| Test | χ2 | DF |
Prob>χ2 |
|---|
| Log-rank | 10.4696 | 2 | 0.0053 |
| Kruskal-Wallis | 9.9218 | 2 | 0.0070 |
Data were further investigated using one-factor
ANOVA (treatment effect) on the log survival days.
Survival
The survival days of 25 patients (recorded as dead)
were affected by the treatment type (P=0.006) in a way as shown in
Fig. 2.
The combo treatment prolongs the patients' life
nearly double as compared to the chemotherapy and radiation+
therapy (761 days vs. 418 and 318, in antilog values), whose
effects become equal on surviving according to Fisher's LSD
comparisons (Table IV). The mean
metabolic rate RMR was higher in the initial stage of study (117.8%
vs. 110.3%) but the mean breath index was found similar between
stages (10.0% vs. 10.3%).
 | Table IVGrouping information using the
Fisher's LSD on treatment type and mean survival (log10
values). |
Table IV
Grouping information using the
Fisher's LSD on treatment type and mean survival (log10
values).
| Treatment | N | Mean | Grouping |
|---|
| Combined | 8 | 2.881 | A | - |
| Radiation+ | 8 | 2.6222 | - | B |
| Chemotherapy | 8 | 2.5026 | - | B |
Parameters
The parameters RMR and breath condition were
negatively affected by the survival status (P=0.012 and 0.043,
respectively). Fig. 3 reveals an
abrupt drop of RMR mean difference for the survived group (-217.9),
probably as a result of stressed conditions subjected to patients
under therapy.
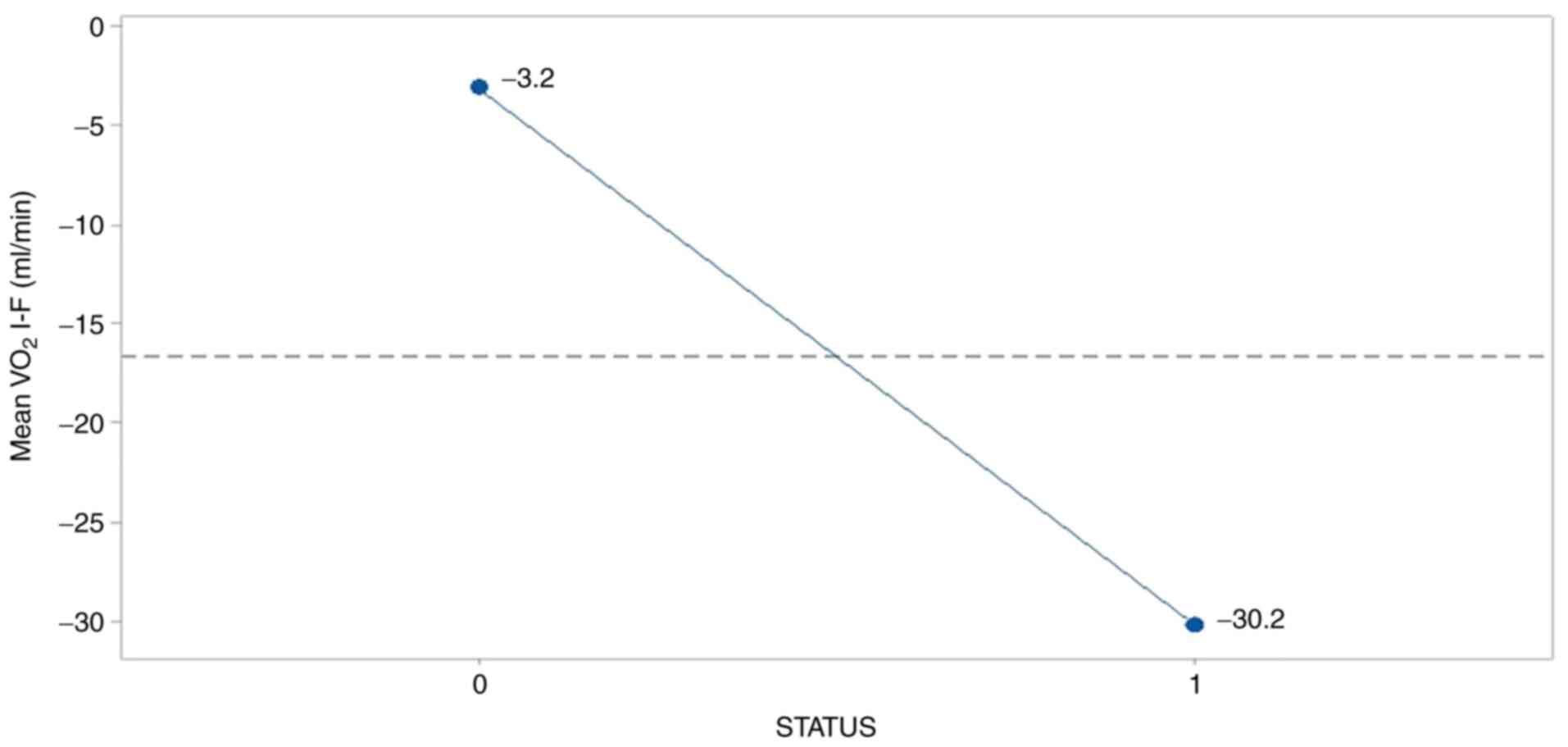
In a similar manner, breath index was deteriorated
in the survived group (-30.2) as Fig.
4 illustrates so, resulting presumably from the adverse effects
of treatments operation.
The treatment effect was significantly present
(P=0.044) only on the fat condition of patients (Fig. 5). Although the mean fat% in the
patients was similar in both stages (initial 27.9% and final
28.6%), mean fat difference was higher and positive in the patients
treated with immunity and lower and negative for those combo
treated as as Tukey's mean comparisons demonstrate in Table V. Radiation+ and chemotherapy did
not clearly differentiated among treatments due to letters overlap
in the table.
 | Table VGrouping information using the Tukey
test on treatment type and mean fat difference (final minus
initial). |
Table V
Grouping information using the Tukey
test on treatment type and mean fat difference (final minus
initial).
| Treatment | N | Mean | Grouping |
|---|
| Immunotherapy | 11 | 3.18 | A | - |
| Chemotherapy | 18 | 2.31 | A | B |
| Radiation+ | 32 | 0.48 | A | B |
| Combined | 21 | -1.16 | - | B |
Discussion
It has been clinically observed that advanced lung
cancer patients experience various degrees of weight loss. During a
six month period of time severe weight loss was observed in 7% of
the patients (weight loss of >10% in 1 month or >20% in 6
months). Most of cancer patient survivors keep losing weight after
six months although they are under treatment. Usually continuous
weight loss indicates poor treatment response and finally
contributes to mortality in lung cancer (24,25).
It is absolutely important that the nutritional status of cancer
patients is included in the treatment management, since it decides
the patient's tolerance for curative treatment (22).
Nutritional support will maintain current health
status but also, will improve patient satisfaction, quality of
life, and treatment outcomes. Malnutrition has been observed to be
prevalent in advanced lung cancer patients. These patients require
timely nutrition support and guidance, management of treatment
symptoms, with drug interventions. There was a study where 58.8% of
the patients had difficulty with eating food, including 6.5% who
could consume food only in puree form and 2.2% who could consume
food only in liquid form. Many cancer patients with advanced lung
cancer have been consuming diets that would likely be insufficient
to maintain weight even in healthy individuals. Results from recent
studies presented data where 61.8 to 11.7% of the patients had a
reduction in fat. It has been observed that patients with lung
cancer which have higher consumption of protein and fat, which
could lead to increased weight loss.
It is known that increased weight loss may result in
increased rate of complications; impaired wound healing; reduced
immune function, and decreased tolerance to surgery, radiotherapy,
and of course chemotherapy. Most importantly reduced quality of
life (26,27). More than 82.8% of the advanced lung
cancer patients have nutrition impact symptoms, appetite loss,
vomiting, including nausea or fullness (9.1%), choking (57.2%), and
diarrhea (59.0%). In all cases these symptoms are disease related
by advanced lung cancer. In our study the most important factors
affecting survival and treatment efficiency were fat, RMR and
VO2 consumption. Meaning that patients with severe
emphysema (COPD) and cachexia upon diagnosis had the worst survival
and treatment efficiency due to their low cell metabolism. Our
findings agree with previous studies in the field. In our study we
included more parameters than other studies, however; our main
findings remain the same.
The sooner a patient is diagnosed with low tumor
burden, the higher the survival will be (28-32).
Early stage lung cancer patients have by definition low tumor
burden since the main lesion is ≤3 cm and infiltrated lymphnodes
are N0-N1 disease. Non-operable patients usually have a main lesion
≥3 cm with several infiltrated lymphnode stations N2-N3 disease or
evan distant metastasis. Therefore the extended tumor burden
consumes several nutritional elements in different parts of the
body and cachexia is extensive. We should focus on adding
supplements and special nutrition to these patients. In our study
we investigated the following parameters for the first time RMR
(basic metabolism), %Pred (Harris Benedict), VO2
(ml/min), Ve (lt/min), VarVe (%), VarVO2 (%), Rf
(1/min), FeO2 (%), Mediterranean diet score, physical
activity level and we believe data and information should be
included as they are currently. We believe that the additional
measurements that we included enlighten more aspects of lung cancer
cachexia. Moreover, we enlightened the association between lung
cancer cachexia and treatment benefit. The RMR which is the basic
metabolism was found to be lower in the survivors because the
survivors have depleted their fat and muscle reservoir (cachexia).
These factors are associated directly with the metabolism of a
patient.
Our findings agree with previous studies. However;
since we have investigated different treatments, our new findings
are mostly with immunotherapy patients. In conclusion, the combined
treatment with chemotherapy and immunotherapy is a superior
protocol for prolonging (twice) the survival rate and reducing the
fat condition of patients. On the other hand, Harris Benedict RMR%
and breath condition (VO2) were found distantly lower in
the survivors.
Major limitation of our study is the small number of
patients and that all patients recruited were only lung cancer
patients. Moreover; patients received different types of treatment,
therefore the number of patients for each treatment was even
smaller. In conclusion, the combined treatment with chemotherapy
and immunotherapy appears a superior protocol for prolonging
(twice) the survival rate and reducing the fat condition of
patients. On the other hand, Harris Benedict RMR% and breath
condition (VO2) were found distantly lower in the
survivors. Moreover; Our study included the findings from previous
studies, but also added novel measurements such as: RMR (basic
metabolism), %Pred (Harris Benedict), VO2 (ml/min), Ve
(lt/min), VarVe (%), VarVO2 (%), Rf (1/min),
FeO2 (%), Mediterranean diet score, physical activity
level and we believe data and information should be included as
they are currently. We believe that the additional measurements
that we included enlighten more aspects of lung cancer cachexia.
Moreover, we enlightened the association between lung cancer
cachexia and treatment benefit. The RMR which is the basic
metabolism was found to be lower in the survivors because the
survivors have depleted their fat and muscle reservoir (cachexia).
The VO2 consumption was also found to be lower in lung
cancer survivors because of COPD status and disease within the
lung, most of these patients used oxygen supplement due emphysema
and lung cancer disease. Moreover, this is the first study to
include different treatment options.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
MP, FP, SP, TT, ET, KA, AM, ZT, GA, RN, KB, AAT, AC,
DP and PZ collected the data and wrote the manuscript. DP and PZ
performed the statistics analysis. All authors read and approved
the final manuscript. PZ, DP and TT confirm the authenticity of the
raw data.
Ethics approval and consent to
participate
The current study was conducted in the ‘Theageneio’
Cancer Hospital, Thessaloniki, Greece and was approved by the
investigational review board of (‘Theageneio’ Cancer Hospital,
Thessaloniki, Greece) and was performed according to the principles
of the Declaration of Helsinki. Written informed consent was
acquired from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi HK and Mazzone PJ: Lung cancer
screening. Surg Oncol Clin N Am. 29:509–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zaric B, Stojsic V, Sarcev T, Stojanovic
G, Carapic V, Perin B, Zarogoulidis P, Darwiche K, Tsakiridis K,
Karapantzos I, et al: Advanced bronchoscopic techniques in
diagnosis and staging of lung cancer. J Thorac Dis. 5 (Suppl
4):S359–S370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zaric B, Stojsic V, Carapic V, Kovacevic
T, Stojanovic G, Panjkovic M, Kioumis I, Darwiche K, Zarogoulidis
K, Stratakos G, et al: Radial endobronchial ultrasound (EBUS)
guided suction catheter-biopsy in histological diagnosis of
peripheral pulmonary lesions. J Cancer. 7:7–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darwiche K, Zarogoulidis P, Baehner K,
Welter S, Tetzner R, Wohlschlaeger J, Theegarten D, Nakajima T and
Freitag L: Assessment of SHOX2 methylation in EBUS-TBNA specimen
improves accuracy in lung cancer staging. Ann Oncol. 24:2866–2870.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oezkan F, Khan A, Zarogoulidis P,
Hohenforst-Schmidt W, Theegarten D, Yasufuku K, Nakajima T, Freitag
L and Darwiche K: Efficient utilization of EBUS-TBNA samples for
both diagnosis and molecular analyses. Onco Targets Ther.
7:2061–2065. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sapalidis K, Zarogoulidis P, Petridis D,
Kosmidis C, Fyntanidou B, Tsakiridis K, Maragouli E, Amaniti A,
Giannakidis D, Koulouris C, et al: EBUS-TNBA 22G samples:
Comparison of PD-L1 expression between DAKO and
BIOCARE®. J Cancer. 10:4739–4746. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Boskovic T, Stanic J, Pena-Karan S,
Zarogoulidis P, Drevelegas K, Katsikogiannis N, Machairiotis N,
Mpakas A, Tsakiridis K, Kesisis G, et al: Pneumothorax after
transthoracic needle biopsy of lung lesions under CT guidance. J
Thorac Dis. 6 (Suppl 1):S99–S107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Domvri K, Darwiche K, Zarogoulidis P and
Zarogoulidis K: Following the crumbs: from tissue samples, to
pharmacogenomics, to NSCLC therapy. Transl Lung Cancer Res.
2:256–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Domvri K, Zarogoulidis P, Darwiche K,
Browning RF, Li Q, Turner JF, Kioumis I, Spyratos D, Porpodis K,
Papaiwannou A, et al: Molecular targeted drugs and biomarkers in
NSCLC, the evolving role of individualized therapy. J Cancer.
4:736–754. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5 (Suppl
4):S389–S396. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zarogoulidis P, Huang H, Bai C, Petridis
D, Papadopoulou S, Faniadou E, Eleftheriadou E, Trakada G,
Cristoforos K, Rapti A, et al: Nab-paclitaxel as first line
treatment for NSCLC in elderly patients more than 75 years old. J
Cancer. 8:1673–1678. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tsoulos N, Papadopoulou E, Metaxa-Mariatou
V, Tsaousis G, Efstathiadou C, Tounta G, Scapeti A, Bourkoula E,
Zarogoulidis P, Pentheroudakis G, et al: Tumor molecular profiling
of NSCLC patients using next generation sequencing. Oncol Rep.
38:3419–3429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zarogoulidis P, Huang H, Tsiouda T,
Sardeli C, Trakada G, Veletza L, Kallianos A, Kosmidis C, Rapti A,
Papaemmanouil L, et al: Immunotherapy ‘Shock’ with vitiligo due to
nivolumab administration as third line therapy in lung
adenocarcinoma. Respir Med Case Rep. 22:283–286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sapalidis K, Kosmidis C, Michalopoulos N,
Koulouris C, Mantalobas S, Giannakidis D, Munteanu A, Surlin V,
Laskou S, Zarogoulidis P, et al: Psoriatic arthritis due to
nivolumab administration a case report and review of the
literature. Respir Med Case Rep. 23:182–187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Conigliaro T, Boyce LM, Lopez CA and
Tonorezos ES: Food intake during cancer therapy: A systematic
review. Am J Clin Oncol. 43:813–819. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huhmann MB and Cunningham RS: Importance
of nutritional screening in treatment of cancer-related weight
loss. Lancet Oncol. 6:334–343. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Segura A, Pardo J, Jara C, Zugazabeitia L,
Carulla J, de Las Peñas R, García-Cabrera E, Luz Azuara M, Casadó J
and Gómez-Candela C: An epidemiological evaluation of the
prevalence of malnutrition in Spanish patients with locally
advanced or metastatic cancer. Clin Nutr. 24:801–814.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xara S, Amaral TF and Parente B:
Undernutrition and quality of life in non small cell lung cancer
patients. Rev Port Pneumol. 17:153–158. 2011.PubMed/NCBI View Article : Google Scholar : (In Portuguese).
|
|
19
|
Lis CG, Gupta D, Lammersfeld CA, Markman M
and Vashi PG: Role of nutritional status in predicting quality of
life outcomes in cancer-a systematic review of the epidemiological
literature. Nutr J. 11(27)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Laky B, Janda M, Kondalsamy-Chennakesavan
S, Cleghorn G and Obermair A: Pretreatment malnutrition and quality
of life-association with prolonged length of hospital stay among
patients with gynecological cancer: A cohort study. BMC Cancer.
10(232)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Datema FR, Ferrier MB and Baatenburg de
Jong RJ: Impact of severe malnutrition on short-term mortality and
overall survival in head and neck cancer. Oral Oncol. 47:910–914.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Van Cutsem E and Arends J: The causes and
consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 9
(Suppl 2):S51–S63. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Szmidt MK, Kaluza J, Harris HR, Linden A
and Wolk A: Long-term dietary fiber intake and risk of chronic
obstructive pulmonary disease: A prospective cohort study of women.
Eur J Nutr. 59:1869–1879. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohan A, Singh P, Kumar S, Mohan C, Pathak
AK, Pandey RM and Guleria R: Effect of change in symptoms,
respiratory status, nutritional profile and quality of life on
response to treatment for advanced non-small cell lung cancer.
Asian Pac J Cancer Prev. 9:557–562. 2008.PubMed/NCBI
|
|
25
|
Martin L, Birdsell L, Macdonald N, Reiman
T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB and
Baracos VE: Cancer cachexia in the age of obesity: skeletal muscle
depletion is a powerful prognostic factor, independent of body mass
index. J Clin Oncol. 31:1539–1547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van Bokhorst-de van der Schueren MA, van
Leeuwen PA, Sauerwein HP, Kuik DJ, Snow GB and Quak JJ: Assessment
of malnutrition parameters in head and neck cancer and their
relation to postoperative complications. Head Neck. 19:419–425.
1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Van Bokhorst-de Van der Schuer MA,
Langendoen SI, Vondeling H, Kuik DJ, Quak JJ and Van Leeuwen PA:
Perioperative enteral nutrition and quality of life of severely
malnourished head and neck cancer patients: A randomized clinical
trial. Clin Nutr. 19:437–444. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barreira JV: The role of nutrition in
cancer patients. Nutr Cancer. 1–2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Inglis JE, Lin PJ, Kerns SL, Kleckner IR,
Kleckner AS, Castillo DA, Mustian KM and Peppone LJ: Nutritional
interventions for treating cancer-related fatigue: A qualitative
review. Nutr Cancer. 71:21–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Campbell TC: Nutrition and cancer: An
historical perspective. The past, present, and future of nutrition
and cancer. Part 2. Misunderstanding and ignoring nutrition. Nutr
Cancer. 69:962–968. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kiss NK, Krishnasamy M and Isenring EA:
The effect of nutrition intervention in lung cancer patients
undergoing chemotherapy and/or radiotherapy: A systematic review.
Nutr Cancer. 66:47–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lindstad T, Jin Y, Wang L, Qu S and
Saatcioglu F: STAMPs at the crossroads of cancer and nutrition.
Nutr Cancer. 62:891–895. 2010.PubMed/NCBI View Article : Google Scholar
|