Introduction
Colorectal cancer (CRC) is the third most frequent among all types of cancer, comprising an estimated 8-9% of all cancer cases reported in 2020 worldwide (1). In Saudi Arabia, CRC accounts for 14.1% of all cancer-related deaths (2). The local incidence of CRC is on the rise, affecting men more frequently than women (3) and being more frequently diagnosed in young adults (4).
The heterogenous nature of CRC emphasizes the need for a better understanding the of cellular and molecular factors in the tumour microenvironment that regulate tumour cell proliferation and metastasis (5). Alterations in the expression of growth factors and their receptors profoundly affect the proliferation of cancer cells, development of vasculature (angiogenesis) and spread to other organs (6). Signals generated by growth factors modify the tumour microenvironment at various stages of cancer progression (7,8). Low levels of growth factors in biological fluids during the initial stages of cancer development tend to increase rapidly as cancer progresses (9). Certain growth factors, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), exhibit a robust association with cancer progression (10).
Overexpression of EGF receptor (EGFR) in 60-80% of colonic tumours has been associated with poor prognosis (11). The interaction of EGFR with EGF and TGFα promotes tumourigenesis by dysregulation of the cell cycle, resulting in enhanced tumour cell survival (12). Furthermore, TGFα interaction with EGFR has been shown to promote the expression of VEGF (12), and elevated serum levels of VEGF have been associated with shorter disease-free survival among patients with CRC (13); this may be due to VEGF-mediated angiogenesis through the proliferation, differentiation and migration of vascular endothelial cells (14). Increased serum levels of HGF among patients with cancer, including patients with CRC, have been associated with disease progression (15-17), most likely due to its ability to promote angiogenesis in tumours (18).
The literature indicates that the growth factors EGF, VEGF and HGF may play important roles in determining cancer progression. Since EGF induces VEGF production and HGF regulates EGF expression (11-17), simultaneous assessment of these three growth factors may indicate how they vary at different stages of CRC. Therefore, the present study was performed to assess the serum levels of EGF, VEGF and HGF, along with their mRNA and protein expression, in patients with early- and late-stage CRC.
Materials and methods
Study population
The present study was performed between March 2015 and September 2016 at King Khalid University Hospital, King Saud University (Riyadh, Saudi Arabia). The study protocol was approved by the Institutional Review Board of the College of Medicine, King Saud University. After obtaining written informed consent, blood samples and tissue specimens were collected from a total of 30 patients [14 men (46.7%) and 16 women (53.3%); mean age, 60 years; range, 38-80 years]. Early-stage CRC was defined as stages I and II, whereas late-stage CRC was defined as stages III and IV. Patient demographic and clinical characteristics, including the tumour site and type, were recorded. Tumour staging was performed according to the Union for International Cancer Control (UICC)-TNM staging system; tumour localization, UICC stage and tumour grade were classified as described previously (19). Patients receiving neoadjuvant or adjuvant therapy were excluded from the study.
Blood and tissue samples
A 5-ml venous blood sample was obtained under aseptic conditions from each patient and from age- and sex-matched healthy controls (n=30). Healthy blood samples were obtained from Blood Bank Unit, King Khalid University Hospital (Riyadh, Saudi Arabia). The blood samples were allowed to clot for 1 h at room temperature and were centrifuged at 1,000 x g for 10 min at room temperature to collect the serum. All serum samples were then stored at -80˚C until further use. Cancer and adjacent normal tissue samples (at least 10 cm away from the tumour site) were obtained from the patients, freeze-dried in liquid nitrogen and stored at -80˚C until histological examination.
Measurement of serum concentrations of VEGF, EGF and HGF
The serum concentrations of VEGF, EGF and HGF in patients and controls were quantified using a Human Cytokine multiplex panel (LHC6003, Novex, Invitrogen; Thermo Fisher Scientific, Inc.) and the plates were read using Milliplex™ Luminex xMAP technology (EMD Millipore). The assay was performed in accordance with the manufacturer's instructions. Assessments of standards, internal controls and samples were performed in duplicates and the results are expressed in pg/ml.
Preparation of total RNA and reverse transcription (RT)
RNA was extracted from paired tumour and adjacent healthy tissues using PARIS™ kit (Ambion; Thermo Fisher Scientific, Inc.). Reverse transcription was performed as per the manufacturer's instructions using a high-capacity cDNA kit (cat. no. 4368814; Applied Biosystems; Thermo Fisher Scientific, Inc.). The quality of RNA was evaluated by calculating the A260/280 ratio (1.8-2.0).
Quantitative (q)PCR) analysis
qPCR was performed on ViiA™ 7 Real-Time PCR system (Thermo Fisher Scientific, Inc.) using the SYBR Green PCR Master Mix (cat. no. 4385612; Thermo Fisher Scientific, Inc.). The relative mRNA levels of EGF, HGF and VEGF were normalized to GAPDH in order to estimate their expression in tumour and adjacent normal tissues. The qPCR conditions were 95˚C for 15 min, 40 cycles of 94˚C for 15 sec, and 57˚C for 30 sec. The following primers were used: Human EGF, 5'-GGAATTCTACTTGTGTGGGTCCT-3' (sense) and 5'-TCACTGAGACACCAGCATCC-3' (antisense); human HGF, 5'-GACGCAGCTACAAGGGAACA-3' (sense) and 5'-GCTCGAAGGCAAAAAGCTG-3' (antisense); human VEGF, 5'-TGTGAATGCAGACCAAAGAAAGAT-3' (sense) and 5'-GCTCCAGGGCATTAGACAGC-3' (antisense); and human GAPDH, 5'-ACCCATCACCATCTTCCAGGAG-3' (sense) and 5'-GAAGGGGCGGAGATGATGAC-3' (antisense). The results were normalized to GAPDH levels and the relative expression was calculated with the 2-∆∆Cq method (20).
Construction of tissue microarrays (TMAs) and immunohistochemistry
TMAs were constructed and immunohistochemistry was performed as described previously (21). VEGF expression was detected using streptavidin-biotinylated horseradish peroxidase (S-ABC) kit cat. no. 65306; NovoLink Max Polymer Detection System; Leica Microsystems, Ltd.) as per the manufacturer's instructions. Negative controls without the primary antibody were also included. The expression of VEGF in tumour and normal tissue was analyzed with the eSlide capture device (ScanScope CS; Aperio Technologies, Inc.). VEGF staining intensity was evaluated as negative, weak or strong and a semi-quantitative analysis was performed based on the categorization of patient samples by staining intensity (22).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc.). Data are presented as mean ± standard error. A paired t-test was used to compare growth factor serum and mRNA levels between early- and late-stage CRC. P<0.05 was considered to indicate statistically significant differences.
Results
Patients
A total of 30 patients with CRC [14 men (46.7%) and 16 women (53.3%); mean age, 60 years; range, 38-80 years] were included in the study. Histological examination indicated comparable distribution of early-stage [I: n=8 (26.7%) and II: n=7 (23.3%)] and late-stage [III: n=8 (26.7%) and IV: n=7 (23.3%)] CRC. The characteristics of the patients and clinicopathological data are summarised in Table I.
 |
Table I
Clinicopathological characteristics of patients with colorectal cancer.
|
Table I
Clinicopathological characteristics of patients with colorectal cancer.
| Variables |
Number of patients |
Percentage |
| Mean age (years) |
|
|
| ≤60 |
17 |
56.7 |
| >60 |
13 |
33.3 |
| Sex |
|
|
| Male |
14 |
46.7 |
| Female |
16 |
53.3 |
| Primary tumour |
|
|
| Colon |
27 |
90 |
| Rectum |
3 |
10 |
| Tumour stage (UICC 2010) |
|
|
| pT1 |
4 |
13.3 |
| pT2 |
8 |
26.7 |
| pT3 |
15 |
50 |
| pT4 |
3 |
10 |
| Lymph node status (UICC 2010) |
|
|
| pN0 |
15 |
50 |
| pN1 |
13 |
43.3 |
| pN2 |
2 |
6.7 |
| Clinical stage (UICC 2010) |
|
|
| I |
8 |
26.7 |
| II |
7 |
23.3 |
| III |
8 |
26.7 |
| IV |
7 |
23.3 |
| Histological grade (UICC 2010) |
|
|
| G1 |
0 |
0 |
| G2 |
28 |
93.3 |
| G3 |
2 |
6.7 |
Serum levels of VEGF, EGF and HGF
The serum concentrations of VEGF, EGF and HGF were compared between patients with early- and those with late-stage CRC and between patients and controls (Table II). Among patients with CRC, the mean serum concentrations of VEGF during the early (152.9±14.5 vs. 88.39±3.99 pg/ml; P=0.001) and late (182.7±25.8 vs. 88.39±3.99 pg/ml; P=0.002) stages of the disease were significantly higher compared with those in controls. Similarly, the mean serum concentrations of EGF among patients with early-stage (409.4±7.96 vs. 153.7±13.8 pg/ml; P=0.05) and late-stage (669.5±13.1 vs. 153.7±13.8 pg/ml) CRC were higher compared with those controls. The mean serum concentrations of HGF did not differ significantly between patients with early-stage CRC and controls (83.54±14.4 vs. 56.9±4.97 pg/ml; P=0.13); however, the levels of HGF were higher in patients with late-stage CRC compared with controls (90.4±17.4 vs. 56.9±4.97 pg/ml; P=0.05). There was no statistically significant difference in the mean serum concentrations of VEGF, EGF and HGF between early- and late-stage CRC.
 |
Table II
Comparison of serum levels of growth factors between patients with CRC and healthy controls.
|
Table II
Comparison of serum levels of growth factors between patients with CRC and healthy controls.
| |
CRC |
P-value |
| Growth factors |
Controls |
Early-stage |
Late-stage |
Control vs. early-stage CRC |
Control vs. late-stage CRC |
Early- vs. late-stage CRC |
| VEGF |
88.39±3.99 |
152.9±14.5 |
182.7±25.8 |
0.0012c |
0.002b |
0.291 |
| EGF |
153.7±13.8 |
409.4±7.96 |
669.5±13.1 |
0.05a |
0.01b |
0.08 |
| HGF |
56.9±4.97 |
83.54±14.4 |
90.4±17.4 |
0.13 |
0.05a |
0.762 |
Gene expression of VEGF, EGF and HGF in patients with CRC
Relative quantification of growth factor expression in early-stage cancer indicated a substantial upregulation in the levels of VEGF (P<0.001), EGF (P<0.05) and HGF (P<0.01) in early-stage CRC compared to adjacent normal tissues (Fig. 1A). However, decreased expression levels of VEGF and HGF were observed in late-stage CRC compared with the levels observed in the early stages, whereas the levels of EGF remained significantly elevated during both the early and late stages of CRC (P<0.01 vs. control; Fig. 1B).
 |
Figure 1
Relative quantification of growth factor expression normalized to GAPDH in (A) early-stage and (B) late-stage colorectal cancer. *P<0.05, **P<0.01 and ***P<0.001 vs. control. EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor.
|
Protein expression of VEGF in patients with CRC
Immunohistochemical analysis of VEGF expression was performed on TMAs of paired tumour and adjacent normal specimens (n=20). Increased expression of VEGF (brown staining) was confirmed in cancerous tissues compared with that in normal tissues (Fig. 2A-C). Early-stage (I and II) tumour tissues exhibited a higher number of VEGF-positive tumour cells (Fig. 2A) compared with late-stage (III and IV) tumour tissues (Fig. 2B). A semi-quantitative analysis was also performed based on the staining intensity of VEGF as negative, weak or strong. Strong staining was observed in 47% of late-stage CRC samples, whereas 53% of the early-stage samples exhibited strong staining. While all tumours displayed VEGF positivity, the intensity of staining varied from weak to strong between early and late cancer stages, and also within the same stage, demonstrating heterogeneous expression.
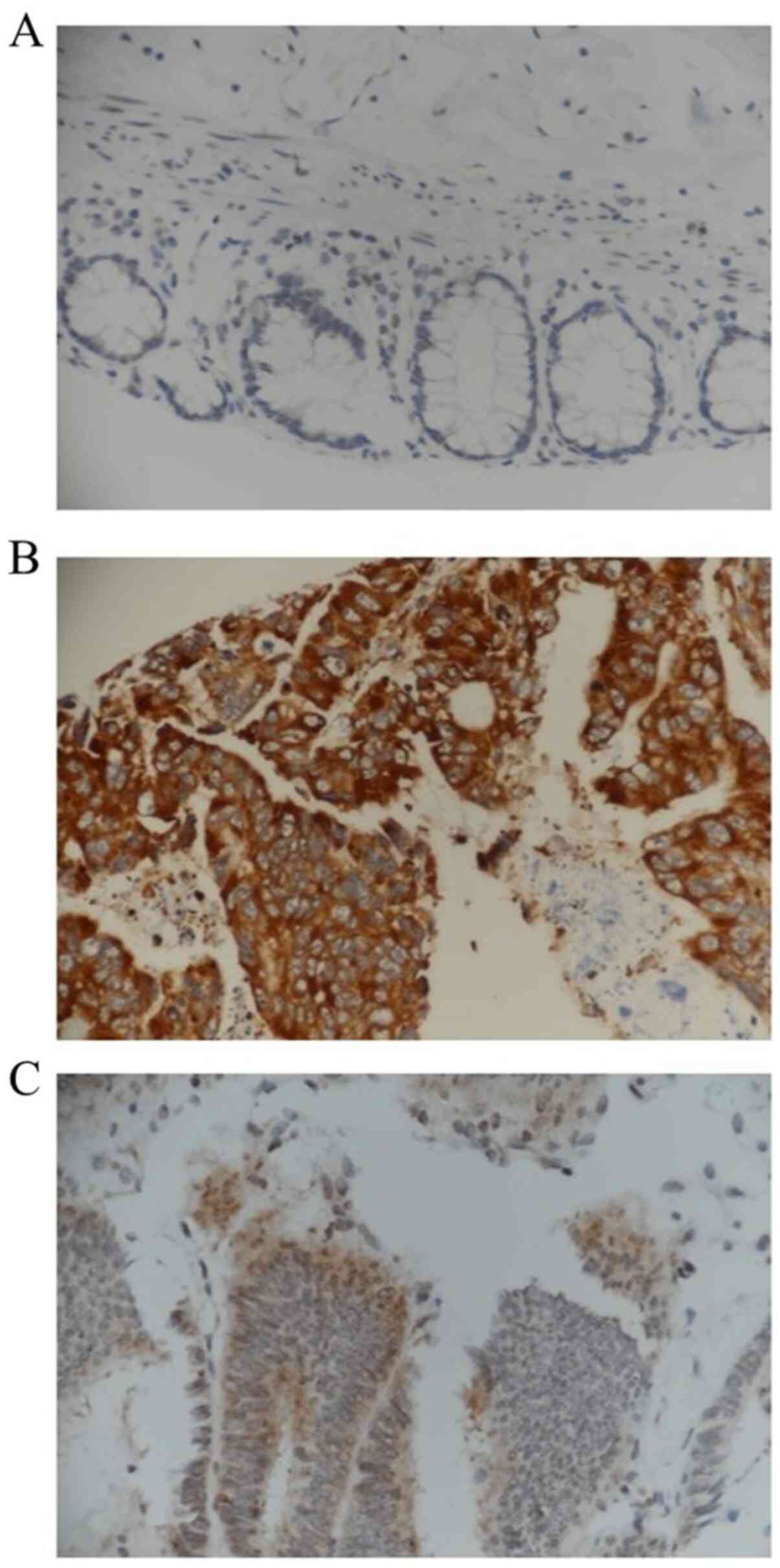 |
Figure 2
Immunohistochemistry staining for VEGF. (A) Photomicrograph showing normal mucosa with negative staining for VEGF. (B) Colonic adenocarcinoma exhibiting strong positive epithelial neoplastic luminal staining for VEGF in a patient with early-stage disease. (C) Colonic adenocarcinoma exhibiting weak staining for VEGF in a patient with late-stage disease. Magnification, x600.
|
Discussion
Growth factors in cancer are well known to stimulate cellular proliferation, tumour progression, angiogenesis and subsequent metastasis (6). Increased expression of growth factors is frequently encountered in neoplastic/cancerous cells (23). This may have potential clinical implications in the management and outcome of CRC, as a number of growth factors have been shown to serve as prognostic markers in monitoring the response to therapeutic interventions (24). Elevated serum levels of tumour growth factors, such as EGF, VEGF and HGF, have been reported among patients with CRC, whereas these levels tend to decline following effective treatment (25). Moreover, higher preoperative levels of VEGF and HGF have been reported to be associated with recurrence of CRC after therapy (26). Targeting these angiogenic factors with biological agents may counteract the tumour-promoting effects of these circulating factors. In the present study, significantly higher serum concentrations of EGF, VEGF and HGF were found among patients with early- and late-stage CRC compared to controls.
Owing to its angiogenic potential, the role of VEGF is considered to be critical in CRC. Significantly higher expression of VEGF has been reported in 50% of patients with CRC compared to its expression in healthy tissues (27). Enhanced expression of VEGF is considered as a poor prognostic marker in CRC (28,29) and is also associated with poor overall survival among affected patients (30). Furthermore, elevated serum levels of VEGF are positively correlated with tumour stage, with significantly higher levels of circulating VEGF observed in advanced stages of CRC compared to early stages (31,32). The results of the present study were consistent with these observations, and the intense expression of VEGF in the later stages of the disease suggested a possible role of VEGF in disease progression. Despite the convincing data linking VEGF with disease progression among patients with CRC, there is however evidence refuting these claims and stating that VEGF has no prognostic significance as a risk factor for CRC progression (33). Moreover, no difference in the serum concentrations of VEGF between patients with CRC and healthy controls has been reported (34). Due to the conflicting reports, investigations of factors, such as the involvement of other members of the VEGF family of growth factors (29,35), the extent of inflammation and the number of tumour-infiltrating lymphocytes in CRC, may be useful in gaining a better understanding of the pathogenesis of CRC.
The serum levels of EGF were found to be significantly elevated in patients with early- and late-stage CRC compared to healthy controls in the present study. Data for EGF among patients with CRC are scarce and, to the best of our knowledge, no previous study has compared EGF levels between the early and late stages of CRC. Elevated serum levels of EGF have been shown to exhibit 80% sensitivity and 65% specificity in the detection of ovarian cancers (36). Increased expression of EGF has not only been associated with various types of cancer, but is also considered to be a poor prognostic marker in terms of adverse clinical outcomes (37-41). By contrast, low levels of EGF have been reported among patients with non-small cell lung cancer and head and neck cancer compared with healthy controls (42). In the present study, however, EGF was found to be markedly elevated in both the early and late stages of CRC, indicating the potential clinical role of this marker. Although studies ascertaining the clinical significance of this finding in CRC are currently lacking, the evidence reported by studies performed on patients with ovarian (36), lung, and head and neck cancers (42) indicate that EGF expression may be of substantial diagnostic value. Further studies are therefore needed to evaluate the diagnostic and/or prognostic significance of this marker in CRC.
HGF and its receptor, c-Met (tyrosine-protein kinase Met or hepatocyte growth factor receptor), are involved in a number of important biological processes (43). HGF/c-Met interaction has been implicated in HGF-activated colonic fibroblast-mediated carcinogenesis of colonic epithelial cancer cells (44,45). The higher levels of c-Met mRNA and protein in CRC liver metastasis, the positive correlation between c-Met expression and liver metastasis, and the association of the downregulation of HGF/c-Met signalling with the reduction in cell proliferation, invasion and metastasis of liver cancer, indicate a potentially crucial role of HGF in CRC (46). Moreover, increased expression of HGF among patients with CRC has been shown to exhibit a positive correlation with disease progression (25). The serum concentration of HGF was found to be higher in the early stages of CRC in the present study, whereas its levels normalized in the late stages. These observations suggest an involvement of HGF in disease pathogenesis in the early stages of CRC, thus highlighting it as a promising target for therapeutic interventions. Furthermore, there appears to be a need for further investigations to elucidate the role of HGF in cancer development, lymphatic invasion and survival in CRC (17).
It is evident that tumour progression from early to late stage involves a number of cellular, biochemical and molecular events, including altered expression of several growth factors (46). However, mRNA expression levels may not be reflective of serum concentrations, possibly owing to post-transcriptional, translational and post-translational modifications, which may determine protein degradation and its regulation (47). Further studies are needed to understand this perspective. Moreover, an increase in the concentrations of growth factors may be associated with an increase in the numbers of cancer niche cells during carcinogenesis, which also needs additional supportive evidence (48). The growth factor expression profile observed in the present study during the early and late stages of CRC offers an opportunity for further investigations to identify targets for predicting the prognosis and establishing therapeutic interventions for the containment of CRC progression.
In conclusion, the elevated levels of growth factors during the early and late stages of CRC observed in the present study highlight the importance of growth factor expression in CRC. However, a major limitation of the present study was its small sample size. Large scale studies are recommended to validate the findings of the present study and to gain a better understanding of the exact role of growth factors in the pathogenesis of CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported and funded by Researchers Supporting Project number (grant no. RSP-2021/344), King Saud University, Riyadh, Saudi Arabia.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
MHA, OAAO and MAVM conceived and designed the study, and drafted the manuscript; MHA, SAS and MAVM performed the statistical data analysis; MAVM, MHA, SAS and TBT have seen and can confirm the authenticity of the raw data; KAAK, TBT, TAJ, ZS and AMZ were involved in data acquisition and interpretation, and critically revised the manuscript for important intellectual content. All the authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of College of Medicine (approval no. 15/0482), King Saud University, and all the participants provided their written informed consent prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ayyub MI, Al-Radi AO, Khazeindar AM, Nagi AH and Maniyar IA: Clinicopathological trends in colorectal cancer in a tertiary care hospital. Saudi Med J. 23:160–163. 2002.PubMed/NCBI
|
|
4
|
Mosli MH and Al-Ahwal MS: Colorectal cancer in the kingdom of Saudi Arabia: Need for screening. Asian Pac J Cancer Prev. 13:3809–3813. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tiash S and Chowdhury EH: Growth factor receptors: Promising drug targets in cancer. J Cancer Metastasis Treat. 1:190–200. 2015.
|
|
6
|
Witsch E, Sela M and Yarden Y: Roles for growth factors in cancer progression. Physiology (Bethesda). 25:85–101. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kantola T, Klintrup K, Vayrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Napankangas J, Mäkelä J, Karttunen TJ, et al: Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 107:1729–1736. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, Bębenek M and Gamian A: Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. Cancer Lett. 337:107–114. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang Y, Liao C, Xu X, Song H, Shi S and Yang S: Th1/Th2 cytokine profiles in G+/G- bacteremia in pediatric hematology/oncology patients. Pediatr Blood Cancer. 58:50–54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Venere M, Lathia JD and Rich JN: Growth factor receptors define cancer hierarchies. Cancer Cell. 23:135–137. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cohen RB: Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2:246–251. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pabla B, Bissonnette M and Konda VJ: Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 6:133–141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cascinu S, Staccioli MP, Gasparini G, Giordani P, Catalano V, Ghiselli R, Rossi C, Baldelli AM, Graziano F, Saba V, et al: Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 6:2803–2807. 2000.PubMed/NCBI
|
|
14
|
Rapisarda A and Melillo G: Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 114:237–267. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gohji K, Nomi M, Niitani Y, Kitazawa S, Fujii A, Katsuoka Y and Nakajima M: Independent prognostic value of serum hepatocyte growth factor in bladder cancer. J Clin Oncol. 18:2963–2971. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Naughton M, Picus J, Zhu X, Catalona WJ, Vollmer RT and Humphrey PA: Scatter factor-hepatocyte growth factor elevation in the serum of patients with prostate cancer. J Urol. 165:1325–1328. 2001.PubMed/NCBI
|
|
17
|
Toiyama Y, Miki C, Inoue Y, Okugawa Y, Tanaka K and Kusunoki M: Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. Int J Cancer. 125:1657–1662. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hanzawa M, Shindoh M, Higashino F, Yasuda M, Inoue N, Hida K, Ono M, Kohgo T, Nakamura M, Notani K, et al: Hepatocyte growth factor upregulates E1AF that induces oral squamous cell carcinoma cell invasion by activating matrix metalloproteinase genes. Carcinogenesis. 21:1079–1085. 2000.PubMed/NCBI
|
|
19
|
Sobin LH and Compton CC: TNM seventh edition: What's new, what's changed: Communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 116:5336–5339. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Al Obeed OA, Alkhayal KA, Al Sheikh A, Zubaidi AM, Vaali-Mohammed MA, Boushey R, McKerrow JH and Abdulla MH: Increased expression of tumour necrosis factor-alpha is associated with advanced colorectal cancer stages. World J Gastroenterol. 20:18390–18396. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Al Obeed OA, Alkhayal KA, Al Sheikh A, Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH and Abdulla MH: Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol. 20:18390–18396. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
De Divitiis C, Nasti G, Montano M, Fisichella R, Iaffaioli RV and Berretta M: Prognostic and predictive response factors in colorectal cancer patients: Between hope and reality. World J Gastroenterol. 20:15049–15059. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Inanc M, Er O, Karaca H, Berk V, Ozkan M, Saraymen R and Elmali F: Prognostic value of tumour growth factor levels during chemotherapy in patients with metastatic colorectal cancer. Med Oncol. 29:3119–3124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoon SS, Kim SH, Gonen M, Heffernan NM, Detwiller KY, Jarnagin WR, D'Angelica M, Blumgart LH, Tanabe KK and Dematteo RP: Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 13:353–362. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bendardaf R, Buhmeida A, Hilska M, Laato M, Syrjänen S, Syrjänen K, Collan Y and Pyrhönen S: VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res. 28:3865–3870. 2008.PubMed/NCBI
|
|
28
|
Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z and Zhu B: IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 407:348–354. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M and Filho AL: Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 10:55–67. 2013.PubMed/NCBI
|
|
30
|
Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL and Perret GY: Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 94:1823–1832. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, Goh RY, Choi HJ, Park KJ, Roh MS, et al: Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 10(203)2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eldesoky A, Shouma A, Mosaad Y and Elhawary A: Clinical relevance of serum vascular endothelial growth factor and interleukin-6 in patients with colorectal cancer. Saudi J Gastroenterol. 17:170–173. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee JC, Chow NH, Wang ST and Huang SM: Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 36:748–753. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Coşkun Ö, Öztopuz Ö and Özkan ÖF: Determination of IL-6, TNF-α and VEGF levels in the serums of patients with colorectal cancer. Cell Mol Biol (Noisy-le-grand). 63:97–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, Eccles SA and Swift RI: VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia. 3:420–427. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Balcan E, Demirkiran F, Aydin Y, Sanioglu C, Bese T, Arvas M, Akçay T and Cift T: Serum levels of epidermal growth factor, transforming growth factor, and c-erbB2 in ovarian cancer. Int J Gynecol Cancer. 22:1138–1142. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF, et al: Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 16:102–108. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheirsilpa A, Ruangvejvorachai P, Karalak A, Sangprakarn S, Pummai S and Sangrajrang S: Determination of epidermal growth factor receptor (EGFR) in patients with colorectal cancer. Cancer Ther. 5:137–142. 2007.
|
|
39
|
Arteaga CL: The epidermal growth factor receptor: From mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 19 (Suppl 18):S32–S40. 2001.PubMed/NCBI
|
|
40
|
Yarden Y: The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 37 (Suppl 4):S3–S8. 2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Goldstein NS and Armin M: Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint committee on cancer stage IV colon adenocarcinoma: Implications for a standardized scoring system. Cancer. 92:1331–1346. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lemos-Gonzalez Y, Rodriguez-Berrocal FJ, Cordero OJ, Gómez C and Paez de la Cadena M: Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br J Cancer. 96:1569–1578. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kermorgant S, Zicha D and Parker PJ: PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J. 23:3721–3734. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li F and Zhu YT: HGF-activated colonic fibroblasts mediates carcinogenesis of colonic epithelial cancer cells via PKC-cMET-ERK1/2-COX-2 signaling. Cell Signal. 27:860–866. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yao JF, Li XJ, Yan LK, He S, Zheng JB, Wang XR, Zhou PH, Zhang L, Wei GB and Sun XJ: Role of HGF/c-Met in the treatment of colorectal cancer with liver metastasis. J Biochem Mol Toxicol. 33(e22316)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Easton DF and Eeles RA: Genome-wide association studies in cancer. Hum Mol Genet. 17:R109–R115. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Vogel C and Marcotte EM: Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 13:227–232. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Plaks V, Kong N and Werb Z: The cancer stem cell niche: How essential is the niche in regulating stemness of tumour cells? Cell Stem Cell. 16:225–238. 2015.PubMed/NCBI View Article : Google Scholar
|
















