Introduction
Malignant melanoma is the result of cancerous
transformation of melanin pigment cells. The molecular mechanisms
of carcinogenesis and progression of malignant melanoma have been
reported to include cyclin-dependent kinase (CDK) inhibitor 2A
deletion, NRAS and BRAF mutations, and activation of melanocyte
inducing transcription factor and CDK2(1). Therefore, it is essential to develop
therapeutic strategies using these molecular targets. Combination
therapy with BRAF and MEK inhibitors is already in clinical use for
malignant melanoma with BRAF V600E mutation (2). In addition, the US Food and Drug
Administration approved the combination of dabrafenib and
trametinib for unresectable or metastatic malignant melanoma in
January 2014(2).
By contrast, the standard of care for malignant
melanoma without specific genetic mutations has been systemic
chemotherapy. Dacarbazine has long been the systemic chemotherapy
of choice for metastatic or inoperable malignant melanoma. However,
the response rate to dacarbazine as a single agent is ~10% and the
complete response rate is <5% (3). Various multidrug combinations,
including dacarbazine, have been devised, but none of them have
achieved a survival benefit. Hence, the development of novel
treatment strategies is highly encouraged. ICIs have markedly
improved treatment outcomes for melanoma (4). Prolonged progression-free survival
(PFS) has been observed with nivolumab monotherapy, and
furthermore, combination therapy with ipilimumab led to
improvements in PFS more than ipilimumab monotherapy (5); thus, nivolumab monotherapy and
combination with ipilimumab are considered one of the standards of
care as first-line therapies for advanced melanoma today. However,
as the patients experience more adverse events, combination therapy
is difficult to be adapted to elderly patients. Treatment for
elderly patients with melanoma tends to be difficult, as the
response rate to nivolumab monotherapy is only 20-40% (6,7). The
present study reported on an elderly case of locally advanced
mucosal melanoma in the head and neck that was successfully treated
with nivolumab and resection of one of the tumors that
progressively grew despite nivolumab administration.
Case report
Case presentation
In July 2019, an 85-year-old female patient visited
Kindai University Nara Hospital (Ikoma, Japan) with a 1-month
history of a buccal mucosa mass. The patient had no particular
complications or diseases under treatment and no oral medications.
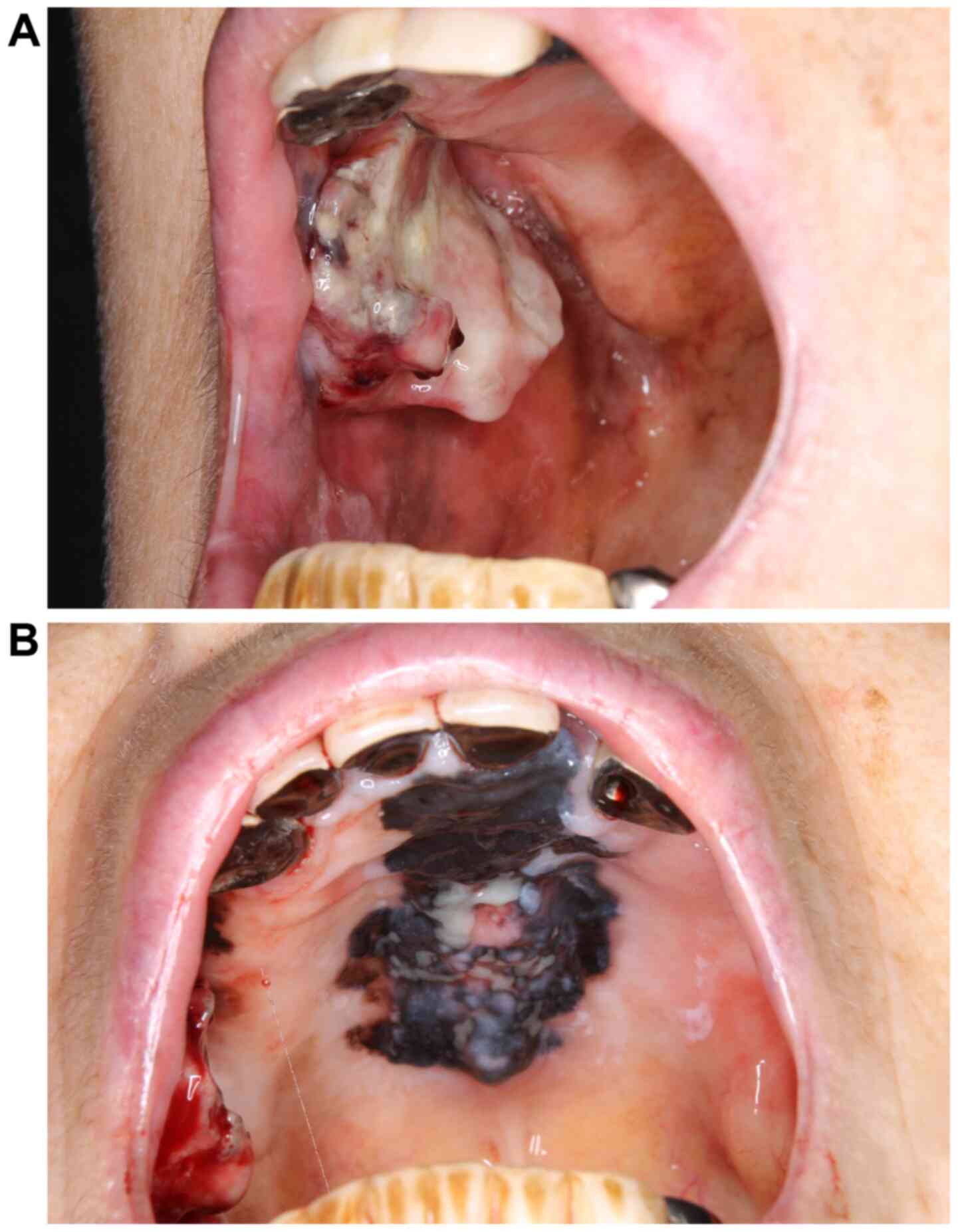
Initial physical findings included conjoined black area in the hard
palate and gums and a protruding hemorrhagic lesion in the right
buccal mucosa (Fig. 1A and
B). No cervical lymph node
metastasis or distant metastases were present. Serum lactate
dehydrogenase was 148 U/l (normal range, 129-221 U/l). Biopsies of
the hard palate and buccal mucosal lesions were performed and both
of them were diagnosed as malignant melanoma. The mutation status
of BRAF was investigated with the Cobas® 4800 BRAF V600
Mutation Test (Roche Diagnostics) and the result was negative.
Treatment
The tumor of the patient was deemed unresectable.
Due to its location in the oral cavity, functions such as
mastication and deglutition would not be preserved after resection.
Considering the patient's age and adverse events, nivolumab
monotherapy was administered instead of ipilimumab + nivolumab
combination therapy. The patient received nivolumab (Ono Pharma)
240 mg in total every two weeks. After two months, the tumor in the
buccal mucosa had grown larger and bled, compromising oral intake
(Fig. 2A). The tumor in the buccal
mucosa did not invade the skin of the cheek; therefore, it appeared
that the skin of the cheek was able to be preserved after tumor
resection. During nivolumab treatment, the already present tumor
exhibited enlargement. Of note, the growing oral mass impaired the
patient's oral intake ability and bleeding from the tumor became
more prominent. These conditions impacted the patient's quality of
life and as the tumor hemorrhage was uncontrolable, palliative
local treatment was deemed necessary. Palliative buccal mucosa
tumor resection was thus performed.
The right buccal mucosal tumor was resected under
local anesthesia. Macroscopically, resection was possible without
residual tumor tissues by partially resecting the buccinator muscle
(Fig. 2B). The resected specimen
was examined pathologically and the sample exhibited malignant
melanoma growth (Fig. 3A). There
were no findings suggestive of pseudoprogression, such as
infiltration of immune cells, so-called tumor-infiltrating
lymphocytes (8). Almost all tumor
cells were viable on histological examination and there was no
necrosis, apoptosis or infarction. A certain amount of
CD45-positive lymphocytes were present in the submucosal layer, but
inside the tumor nodule, no lymphocytes were present (Fig. 3B).
The wound had healed 1 month postoperatively,
leaving only a mild cicatricial contracture and no trismus.
Nivolumab therapy was re-administered after exacerbation and was
able to be continued for >5 months without any discontinuation
due to adverse events. The buccal mucosal tumor did not recur and
the black lesion on the hard palate mildly decreased in size
without the formation of any new lesions. Eventually, the patient
was able to eat unobstructedly without any tumor bleeding (Fig. 4A and B).
Immunohistochemical analysis
The resected tissues were fixed with
phosphate-buffered 10% neutral formalin and processed routinely for
paraffin-embedded tissue sections and with H&E staining.
Immunohistochemical staining for Leukocyte Common
Antigen (CD45) was performed with the Histofine Histostainer 36A
(Nichirei Biosciences, Inc.) using primary antibodies against CD45
(cat. no. 422071; clone no. PD7/26,2B11; pre-diluted working
solution for Histostainer; Nichirei Biosciences, Inc.) according to
manufacturer's protocol.
Discussion
Mucosal melanoma of the head and neck is more
aggressive than melanoma of the skin (9). Complete resection with adequate
margins is required for good outcomes; however, there are numerous
important organs in the head and neck region and therefore, it is
difficult to perform resection with adequate margins, resulting in
a variety of functional disorders (9). Until recently, chemotherapy with
dacarbazine was the main first-line therapy for unresectable
melanomas. The overall response rate of dacarbazine was only 13.4%
and the median survival duration ranged from 5.6 to 11 months
(3). Thus, the response rate of
treatment with dacarbazine is low and treatment efficacy is
limited. The situation has changed drastically with the advent of
ICIs. Today, ipilimumab + nivolumab combination therapy and
nivolumab monotherapy are the standard of care as first-line
therapies (10). The results of a
pooled analysis of trials of immune checkpoint inhibitors in
malignant mucosal melanoma have indicated that, while combination
therapy is more likely to result in a high response, it is also
associated with a higher frequency of serious adverse events
leading to treatment discontinuation (7). As combination therapy has an
increased incidence of adverse events, nivolumab monotherapy is
thought to be an appropriate treatment option for elderly patients,
such as the present case. However, good outcomes may not be
achieved in over half of the patients, as the response rate of
nivolumab monotherapy is only 20-40% (6,7).
Several factors, such as PD-L1 status or tumor
burden, have been proposed as predictors of the efficacy of
nivolumab (6,10). In the present case, surgical
debulking may have influenced the lasting effectiveness of
nivolumab by two factors. The first is the reduction of the tumor
burden and the second is the abscopal effect associated with the
surgery. In ICI therapy, better therapeutic outcomes have been
achieved when the ratio of activated T-cells to tumor burden was
increased (11). In other words, a
higher tumor burden would exhaust activated T-cells, which may
prevent a better therapeutic response. Debulking surgery increased
the number of activated T-cells, thereby contributing to a better
clinical response. It was also reported that the tumor burden was
related to the efficacy of ICIs in the treatment of small-cell lung
cancer; they also noted that larger tumor volumes tended to have
more robust T-regulatory cell infiltration, which induces immune
tolerance (12). Furthermore,
baseline tumor size is a prognosticator during treatment with
pembrolizumab for melanoma (13).
The abscopal effect is frequently reported as a
distant anti-tumor effect in radiotherapy with an immune checkpoint
inhibitor. Regulating the tumor microenvironment or cancer antigen
release may cause a cancer immune response at distant sites
(14). Oronsky et al
(15) reported an abscopal effect
in patients with unresectable cancers treated by tumor reduction
surgery or resection of metastatic lesions undergoing PD-L1
inhibitor therapy. It may also be inferred that surgical maneuvers
are able to lead to release of cancer antigens, which mobilizes
effector T-cells to generate the abscopal effect. This suggests
that reducing systemic tumor burden or large resection of masses
may be expected to enhance the effect of ICIs or lead to the
abscopal effect.
The application of ICIs for post-operative adjuvant
therapy of melanomas has been established. Pembrolizumab or
nivolumab were reported as an effective post-operative adjuvant
therapy for stage III advanced melanoma (16-18).
These results suggested that tumor load reduction and
post-operative adjuvant chemotherapy may prolong PFS.
The present case report has a limitation: It is a
single case report and the disease course success may not be
translatable to other cases. Therefore, it cannot be concluded that
local treatment should be used in all cases where ICI treatment has
failed.
In the future, it may be worthwhile to perform a
prospective study or an observational study to evaluate a strategy
of adding local treatment to cases in which the general condition
is maintained during ICI treatment.
In conclusion, while surgery of the primary head and
neck mucosal melanoma with adequate margins is not easy, resecting
the tumor for palliation may be beneficial in certain cases, as
long as the patient's general physical condition is sufficient to
withstand surgery, as was demonstrated in the present study. Tumor
burden reduction by surgery, particularly for bulky mass or mass
continuing growth despite ICI administration, may bring about a
better ICI response or long-term duration of response. The
combination of ICIs and surgery may become one of the treatment
options in patients with locally advanced mucosal melanoma.
Acknowledgements
The authors thank Dr Takafumi Okabe (Department of
Medical Oncology, Kindai University Nara Hospital, Ikoma, Japan)
and Dr Katsuyuki Eto (Department of Otorhinolaryngology, Kindai
University Nara Hospital, Ikoma, Japan) for performing patient
care.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
TK, KY and TTak: Conception and design of the study
and drafting of the manuscript. TW made the pathological diagnosis.
SA, YA and TTam: Management of the patient. TTak and TK checked and
approved the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tucker MA: Melanoma epidemiology. Hematol
Oncol Clin North Am. 23:383–395. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated Melanoma. N Engl J Med. 373:23–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'Angelo SP, Larkin J, Sosman JA, Lebbé C,
Brady B, Neyns B, Schmidt H, Hassel JC, Hodi FS, Lorigan P, et al:
Efficacy and safety of nivolumab alone or in combination with
ipilimumab in patients with mucosal melanoma: A pooled analysis. J
Clin Oncol. 35:226–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ozaki Y, Shindoh J, Miura Y, Nakajima H,
Oki R, Uchiyama M, Masuda J, Kinowaki K, Kondoh C, Tanabe Y, et al:
Serial pseudoprogression of metastatic malignant melanoma in a
patient treated with nivolumab: A case report. BMC Cancer.
17(778)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
López F, Rodrigo JP, Cardesa A,
Triantafyllou A, Devaney KO, Mendenhall WM, Haigentz M Jr, Strojan
P, Pellitteri PK, Bradford CR, et al: Update on primary head and
neck mucosal melanoma. Head Neck. 38:147–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall Survival with Combined Nivolumab and
Ipilimumab in Advanced Melanoma. N Engl J Med. 377:1345–1356.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guisier F, Cousse S, Jeanvoine M,
Thiberville L and Salaun M: A rationale for surgical debulking to
improve anti-PD1 therapy outcome in non small cell lung cancer. Sci
Rep. 9(16902)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Joseph RW, Elassaiss-Schaap J, Kefford R,
Hwu WJ, Wolchok JD, Joshua AM, Ribas A, Hodi FS, Hamid O, Robert C,
et al: Baseline tumor size is an independent prognostic factor for
overall survival in patients with melanoma treated with
pembrolizumab. Clin Cancer Res. 24:4960–4967. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11(104)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oronsky B, Larson C, Reid TR and Carter
CA: Case series: Abscopal benefit of surgery in 3
immunotherapy-treated patients with unresectable cancer. J Investig
Med High Impact Case Rep: Jul 6, 2018 (Epub ahead of print). doi:
10.1177/2324709618786319.
|
|
16
|
Eggermont AMM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Prolonged survival in stage III melanoma with
ipilimumab adjuvant therapy. N Engl J Med. 375:1845–1855.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: CheckMate 238 collaborators. Adjuvant
nivolumab versus ipilimumab in resected stage III or IV melanoma. N
Engl J Med. 377:1824–1835. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eggermont AMM, Blank CU, Mandala M, Long
GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A,
Carlino MS, et al: adjuvant pembrolizumab versus placebo in
resected stage III melanoma. N Engl J Med. 378:1789–1801.
2018.PubMed/NCBI View Article : Google Scholar
|