Introduction
Distal bile duct cancer is rare and surgical
resection is the only treatment that offers the opportunity for a
cure. However, the 5-year survival rate after surgical resection is
as low as 40% (1). To improve the
surgical outcomes, appropriate neoadjuvant therapy or adjuvant
chemotherapy would be desired as an alternative approach.
Neoadjuvant chemotherapy (NAC) or neoadjuvant chemo-radiation
therapy (NACRT) has been widely adopted for the treatment of
various solid cancers and has been reported to improve the
prognosis after surgery. We previously reported that surgery
following NACRT was associated with a good prognosis in patients
with pancreatic cancer or bile duct cancer (2,3).
Several body composition parameters, for example the
body mass index (BMI), skeletal muscle mass, and total fat mass
have been reported to be associated with postoperative morbidity or
the prognosis of various types of cancer. Some reports showed that
the body composition changed during neoadjuvant treatment and that
the body composition parameters of were associated with
postoperative complications. However, the change in body
composition during NACRT for patients with distal bile duct cancer
and its influence on the prognosis has not been clarified.
The aim of this study was to investigate the impact
of NACRT on the body composition of patients with distal bile duct
cancer, and to evaluate the influence of body composition and its
changes on postoperative complications and the prognosis.
Patients and methods
Patients and data collection
This study retrospectively analyzed 16 patients with
distal bile duct cancer who underwent chemoradiation therapy
followed by surgery between March 2006 and December 2015 in our
institute. The clinicopathological characteristics of these
patients are shown in Table I. All
patients needed preoperative biliary drainage due to obstructive
jaundice. Four patients underwent percutaneous transhepatic
cholangiodrainage and twelve underwent endoscopic retrograde
biliary drainage. They were diagnosed with resectable distal
extrahepatic bile duct cancer, and received gemcitabine-based
neoadjuvant chemoradiation therapy after histological or
cytological confirmation of bile duct cancer. After the completion
of NACRT, pancreaticoduodenectomy with extensive regional
lymphadenectomy was performed. We collected the following data at
the diagnosis and after NACRT of white blood cell count, neutrophil
count, lymphocyte count, hemoglobin, platelet count, and the serum
levels of total protein, albumin, blood urea nitrogen (BUN),
creatinine and C reactive protein (CRP). The prognostic nutrition
index (PNI) and the neutrophil-to-lymphocyte ratio (NLR) were
calculated according to these results. The PNI was calculated using
the following equation: PNI = 10 x serum albumin (mg/dl) + 0.005 x
total lymphocyte count. In the present study, the cutoff values for
the PNI and NLR were 40 and 2.5, respectively.
 | Table IClinical characteristics of 16
patients who received preoperative chemoradiation. |
Table I
Clinical characteristics of 16
patients who received preoperative chemoradiation.
| Patient
characteristics (n=16) | Values |
|---|
| Age, median years
(range) | 64.5 (40-76) |
| Gender
(male:female) | 11:5 |
| Tumor depth
(T1:T2:T3:T4) | 1:5:10:0 |
| Nodal status
(N0:N1) | 14:2 |
| Stage
(IA:IB:IIA:IIB) | 1:5:8:2 |
| Tumor differentiation
(well:moderately:poorly) | 7:5:4 |
| Residual tumor
(R0:R1) | 16:0 |
| CA19-9, median
(range) (U/ml) | 15 (2-154) |
| CEA, median (range)
(mg/dl) | 2.65 (1.3-6) |
| Cycle of chemo Tx
(2:3) | 6:10 |
| Dosages of RT (50
Gy:60 Gy) | 12:4 |
| Period of CRT to
operation (day) | 100 (70-121) |
| Chemotherapy-induced
toxicity (≥ grade 3) | |
|
Hematological | 9 |
|
Non-hematological | 2 |
| Postoperative
complication | |
|
Clavien-Dindo
grade ≥2 | 10 |
|
POPF (ISGPF
grade ≥B) | 6 |
Definition of adverse events during
chemo-radiation therapy and surgical complication
We retrospectively collected information related to
adverse events during NACRT and the complications after surgery.
Adverse events during NACRT were graded according to the Common
Terminology Criteria for Adverse Events version 4.0, and
postoperative complications were defined as any complication
classified as grade 2 (requiring pharmacological treatment) or
higher, according to the Clavien-Dindo classification (4).
Preoperative gemcitabine-based
chemoradiation therapy and adjuvant chemotherapy
The protocol of gemcitabine-based chemoradiation
therapy has reported previously (1). In brief, the intravenous
administration of gemcitabine (1,000 mg/m2) was
concurrently initiated on days 1, 8, and 15 during each four-week
cycle, and repeated for two or three cycles. 3 dimensional
radiation was administered at a total dosage of 50-60 Gy with a
daily fraction of 2-2.4 Gy 5 times per week, which targeted the
primary distal bile tumor with the surrounding lymph nodes it, the
para-aortic region, and the retroperitoneal soft tissue. All
protocols were conducted after obtaining written informed consent
from all patients in accordance with the approved procedure at our
hospital. Patients eligible for enrollment were confirmed before
the initiation of NACRT. The criteria for the patients were as
follows: resectable distal bile duct cancer, subserosal or deeper
tumor invasion, and no evidence of distant metastases. The
introduction of adjuvant chemotherapy and the regimens of
anticancer drugs were decided based on the evaluation of the
attending physicians and the patient's performance status. Among
the 16 patients, 6 (37%) received gemcitabine, and 3(19%) received
TS-1 after surgery. The adjuvant S-1 group received 4 cycles of
oral TS-1 chemotherapy at the dose of 40 mg/m2 twice
daily for 4 weeks, followed by 2 weeks of rest.
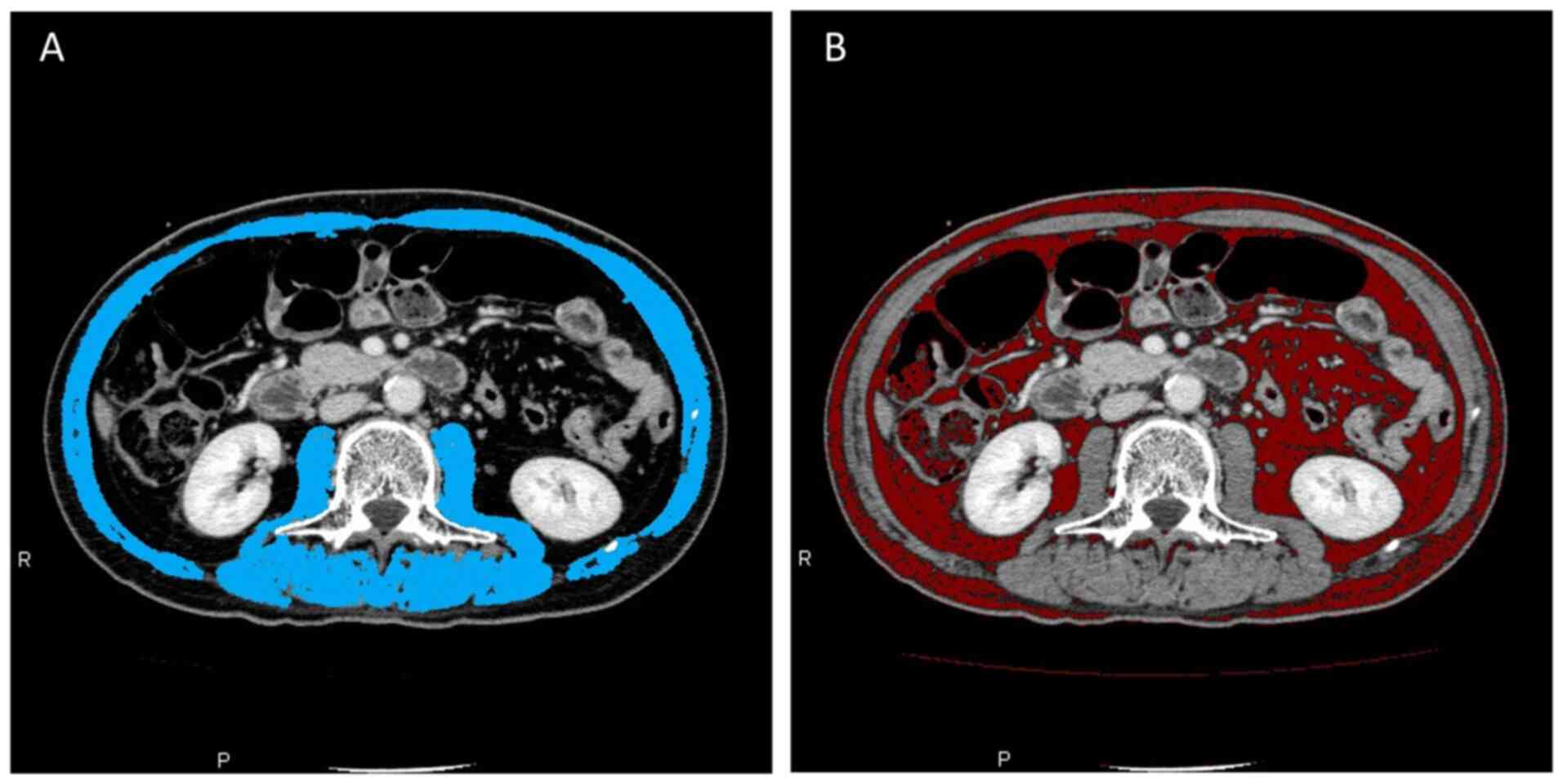
Evaluation of skeletal muscle,
visceral fat, and subcutaneous fat by computed tomography
The skeletal muscle area, visceral fat area and
subcutaneous fat area were assessed by computed tomography before
and after NACRT using the Zio station 2 (Ziosoft, Inc.) software
program (Fig. 1). The skeletal
muscle area was measured as the cross-sectional area of the total
skeletal muscle area at the level of middle portion of the 3rd
lumbar vertebra by manual tracing. The visceral fat and
subcutaneous fat area were also measured at the level of middle
portion of the 3rd lumbar vertebra by applying a threshold with an
attenuation range of -60 to -120 Hounsfield units. The skeletal
muscle index (SMI) was calculated with the following equation:
(cm2/m2) = skeletal muscle area
(cm2)/height (m2). Sarcopenia was defined as
an SMI of 42 cm2/m2 in males and 38
cm2/m2 in females according to Japan Society
of Hepatology guidelines for sarcopenia in liver disease (1st
edition) (5).
Statistical analysis
The data were analyzed using JMP® 13 (SAS
Institute Inc.). Categorical variables were reported as numbers and
percentages, while continuous variables were reported as the median
and range. The Fisher's exact test was used for the comparison of
categorical variables, and the Mann-Whitney U test was used for the
comparison of continuous variables. The Wilcoxon signed-rank test
was used for the comparison between paired samples. Pearson's
correlation analysis was performed to analyze the correlation
between SMI and total fat area. Survival rates were calculated
using the Kaplan-Meier methods and compared using a log-rank test.
P-values of <0.05 were considered to indicate statistical
significance.
Results
The clinical course and short-term
outcomes
All 16 patients completed NACRT followed by
pancreaticoduodenectomy without mortality. The median period from
NACRT to operation was 100 days (range, 70-121). Six patients (37%)
experienced grade ≥3 adverse events. Grade ≥2 complications were
observed in 10 patients (62%). The most common complication was
postoperative pancreatic fistula (POPF) which occurred in six
patients (37%) (Table I).
Change in laboratory test parameters,
immunological and nutritional indices, and the body
composition
The results of laboratory tests, immunological and
nutritional indices, and the body composition of before and after
NACRT are shown in Table II.
There was no significant change in the neutrophil count, platelet
count, total protein, albumin, BUN, creatinine, CRP from before to
after NACRT. The lymphocyte count (P=0.0076) and hemoglobin level
(P=0.001) significantly decreased after NACRT. The median PNI and
NLR were 42.7 (range, 30.8-50.3) and 1.89 (range, 1.06-5.07) before
NACRT, and 40.8 (range, 32.4-50.1) and 2.46 (range, 1.0-5.12) after
NACRT. There was no significant difference in the pre-NACRT and
post-NACRT values.
 | Table IIClinical characteristics of patients
before (pre-NACRT) and after (post-NACRT) neoadjuvant
chemoradiation therapy. |
Table II
Clinical characteristics of patients
before (pre-NACRT) and after (post-NACRT) neoadjuvant
chemoradiation therapy.
| Patient
Characteristics | Pre-NACRT | Post-NACRT | P-value |
|---|
| Weight (kg) | 57.9 (78.0-44.6) | 58.7 (76.7-43.3) | 0.368 |
| SMI (cm²/m²) | 37.2 (57.6-26.2) | 38.9 (51.2-28.5) | 0.899 |
| Total fat area
(cm²) | 168.8
(25.9-296.6) | 130.9
(14.6-276.4) | 0.211 |
| Subcutaneous fat area
(cm²) | 72.8
(19.2-140.8) | 67.1 (8.3-152.9) | 0.159 |
| Visceral fat area
(cm²) | 63.5
(6.6-195.1) | 67.4
(4.2-143.6) | 0.211 |
| Neutrophil count
(10³/µl) | 2067
(1216-5176) | 1860
(1018-3880) | 0.322 |
| Lymphocyte count
(10³/µl) | 1363
(762-2302) | 772 (458-2025) | 0.007 |
| Hemoglobin
(g/dl) | 12.0
(10.3-14.3) | 11.0
(9.4-12.1) | 0.001 |
| Platelet count
(10³/µl) | 239 (171-411) | 256 (107-597) | 0.355 |
| Albumin (g/dl) | 3.4 (2.5-4.0) | 3.6 (2.8-4.4) | 0.392 |
| CRP (mg/dl) | 0.18
(0.01-2.66) | 0.30
(0.04-5.49) | 0.426 |
| PNI | 42.7
(30.8-50.3) | 40.8
(32.4-50.1) | 0.705 |
| NLR | 1.89
(1.06-5.07) | 2.46
(1.00-5.12) | 0.322 |
The median SMI in males was 44.6
cm2/m2 (range, 26.2-57.6) before NACRT and
44.4 cm2/m2 (range, 29.6-51.2) after NACRT.
The median SMI in females was 30.5 cm2/m2
(range, 27.2-33.6) before NACRT, and 31.5
cm2/m2 (range, 28.5-39.0) after NACRT. The
total SMI before and after NACRT did not differ to a statistically
significant extent (Fig. 2A). In
male patients, there was a significant correlation between total
fat area and SMI after NACRT (R=0.602 and P=0.049). Nine (56%) of
the 16 patients were identified as sarcopenic before NACRT. Eight
(50%) patients met the criteria for sarcopenia after NACRT. Among
all nine patients with sarcopenia before NACRT, only one who had
sarcopenia before NACRT recovered after NACRT, whereas the other
eight patients remained sarcopenic after NACRT. Only two patients
required a reduction in the dosage of NACRT, including one with
sarcopenia. After NACRT, the median BMI, subcutaneous fat area and
total fat area at the level of the middle portion of the 3rd lumbar
vertebra were smaller in comparison to before NACRT; however, the
differences were not statistically significant (Fig. 2B).
Association of the body composition
and immunological and nutritional indices with postoperative
complications
POPF, intra-abdominal abscess, chylous ascites,
diarrhea, and delayed gastric empty were observed in this study. We
also focused on POPF because it is one of the most important and
frequent complications after pancreaticoduodenectomy. The patients
with POPF (ISGPF B or C) had a significantly greater SMI and total
fat area than patients without POPF (P=0.019, and P=0.007,
respectively). There was no significant association between the
incidence of postoperative complications and the NLR, PNI, or
sarcopenia after NACRT (Tables
III and IV).
 | Table IIIRelationship between complications
and inflammation-based prognostic scores or body composition. |
Table III
Relationship between complications
and inflammation-based prognostic scores or body composition.
| Post-NACRT | Complication | No
complication | P-value |
|---|
| PNI
(<40:≥40) | 4:6 | 2:4 | 0.789 |
| NLR
(<2.5:≥2.5) | 6:4 | 3:3 | 0.696 |
| Sarcopenia (yes:
no) | 3:7 | 5:1 | 0.118 |
| SMI (cm²/m²) | 44.9
(29.6-51.2) | 34.8
(28.5-44.4) | 0.115 |
| Total fat area
(cm²) | 163.4
(14.6-276.4) | 130.9
(20.8-221.1) | 0.704 |
 | Table IVRelationship between POPF and the
inflammation-based prognostic scores or body composition. |
Table IV
Relationship between POPF and the
inflammation-based prognostic scores or body composition.
| Post-NACRT | POPF | Non-POPF | P-value |
|---|
| PNI
(<40:≥40) | 2:4 | 4:6 | 1 |
| NLR
(<2.5:≥2.5) | 3:3 | 6:4 | 1 |
| Sarcopenia (yes:
no) | 1:5 | 7:3 | 0.118 |
| SMI (cm²/m²) | 48.8
(32.3-51.2) | 34.8
(28.5-45.8) | 0.019 |
| Total fat area
(cm²) | 237.5
(112.6-276.4) | 93.0
(14.6-213.5) | 0.007 |
Association of the body composition
and immunological and nutritional indices with the long-term
outcomes
Patients with sarcopenia had significantly shorter
disease-free survival in comparison to those without sarcopenia
(P=0.025). The 3-year overall survival rate of patients without
sarcopenia was 100%, while that of patients with sarcopenia was
71%; however, the difference was not statistically significant
(Fig. 3). The SMI, total fat area,
PNI, and NLR were not correlated with the overall and disease-free
survival rates. Adjuvant chemotherapy was introduced in 9 (56%) of
16 patients, and only 2 needed to have the dosage of adjuvant
chemotherapy reduced, while 1 required cessation of therapy. There
was no significant difference between receiving a reduced dose of
adjuvant chemotherapy and the presence of sarcopenia (Table V). The induction rate of adjuvant
chemotherapy was not significantly different between the patients
with and without sarcopenia and was also not associated with the
disease-free survival (DFS) or overall survival (OS).
 | Table VRelationship between sarcopenia and
dose intensity in NACRT and adjuvant chemotherapy. |
Table V
Relationship between sarcopenia and
dose intensity in NACRT and adjuvant chemotherapy.
| Type of
chemotherapy | Sarcopenia | Non-sarcopenia | P-value |
|---|
| NACRT | | | |
|
Dose
intensity 100:80% | 7:1 | 7:1 | >0.999 |
| Adjuvant
chemotherapy | | | |
|
Dose
intensity 100:80% | 4:1 | 3:1 | >0.999 |
|
Induction
rate | 62.5% | 50% | >0.999 |
Discussion
In this study, we assessed the changes in body
composition during NACRT for distal bile duct cancer, and
investigated its influence on postoperative complications,
disease-free and, overall survival rates. Extrahepatic bile duct
cancer was divided into two major categories; proximal and distal.
The surgical procedure for proximal bile duct cancer is major liver
resection. On the other hand, pancreaticoduodenectomy is applied
for distal bile duct cancer. The profiles of postoperative
complication between major liver resection and
pancreaticoduodenectomy are quite different. Therefore, we selected
patients with distal bile duct for limiting the surgical procedure
to pancreaticoduodenectomy only.
Our study showed that body weight, the SMI, and the
total fat area were not significantly changed after NACRT. The
proportion of sarcopenic patients was also unchanged after NACRT,
and only one patient recovered from sarcopenia after NACRT. No
significant changes in the immunological or nutritional indices
were observed although the lymphocyte counts and serum hemoglobin
level were decreased after NACRT. Some reports showed that the SMI
was significantly decreased during NACRT or NAC, others did not.
The proportion of sarcopenic patients significantly increased after
NAC in patients with esophageal cancer (6,7).
Reisinger et al demonstrated that the SMI reduction during
NACRT was larger in patients with stage III-IV than in those with
stage I-II, and was a predictor of postoperative mortality
(8). In gastric cancer, the
proportion of patients with sarcopenia was also increased after NAC
(9). On the other hand, the mean
SMI remained unchanged after NACRT in colorectal cancer (10). These previous studies would
indicate that patients with upper gastrointestinal tract cancer
probably became sarcopenic during NAC because of changes of in
their dietary habitat due to obstruction or stenosis by the tumor.
Another possibility is that severe anorexia and nausea were only
seen in 2.5-7.5 and 3.5-5%, of patients who received gemcitabine
monotherapy (11,12). The change in body composition and
incidence of sarcopenia during NAC or NACRT may depend on the type
of cancer and the chemotherapeutic regimen. Gemcitabine monotherapy
and gemcitabine plus radiation therapy for patients with distal
bile duct cancer did not induce severe anorexia or nausea, and then
their body compositions were not significantly changed.
In this study, body compression, sarcopenia, and
immunological and nutritional index values were not associated with
the total incidence of postoperative complications, although the
presence of sarcopenia or lower SMI values has been associated with
postoperative complications in various types of cancer including
biliary tract cancer (9,13,14).
Our study also found that a greater SMI and total fat area before
surgery were associated with the occurrence of POPF. POPF is one of
the most frequent postoperative complications after
pancreaticoduodenectomy. This finding was consistent with previous
studies. Mathur et al reported that patients with a
pancreatic fistula after pancreaticoduodenectomy had significantly
more intralobular and interlobular fat in the pancreas (15). Sandini et al also reported
that the total fat volume and visceral fat volume were
significantly correlated with POPF (16). In our study, the SMI was
significantly associated with the total fat area in males; thus, in
addition to the total fat area, the SMI was associated with
POPF.
Another finding of our study is that the
disease-free survival rates were significantly correlated with
sarcopenia after NACRT. Sarcopenia after NACRT has been reported to
be independently associated with a poor prognosis in various
cancers (8,17-20).
Chakedis et al carried out a study that included 117
patients with biliary tract cancers to assess the impact of
sarcopenia after NACRT, and reported that 41 (35%) patients had
sarcopenia, and that sarcopenia was associated with an increased
risk of death among patients who underwent resection (17). Eriksson et al reported that
the association between NAC and the SMI reduction in patients with
resectable colorectal liver metastasis, and concluded that the
patients a ≥5% SMI reduction during NAC were less likely to undergo
adjuvant chemotherapy, and showed a shorter overall survival time
(21). Cooper et al carried
out a study to determine the relationship between sarcopenia after
NAC or NACRT in patients with resectable pancreatic cancer and
their survival rates. They reported that the degree of skeletal
muscle loss was correlated with disease-free survival. They
reported that pre-existing sarcopenia was associated with the
dose-limiting toxicity of NAC and adjuvant chemotherapy and can
have a negative impact on treatment efficacy and the prognosis
(18). Another possible
explanation for the negative impact of sarcopenia on long-term
outcomes is that some chemokines that are secreted from muscle
cells, called myokines, may prevent cancer cell growth, invasion
and metastasis (22). In this
study, there was a significant correlation with sarcopenia only in
the DFS, not the OS. Our previous report showed that the three-year
overall survival rate of the patients who received the preoperative
chemo-radiation therapy was 81% (2). On the other hand, the five-year
survival rate after surgical resection was 33.1% from the Japanese
Biliary Tract Cancer Statistics Registry (1). We suspect that this was because of
the small number of cases and the high OS rate after NACRT and
surgery.
The present study was associated with some
limitations. Firstly, this was a nonrandomized and retrospective
study, that included a small number of patients from a single
institution. Secondly, the definitions of sarcopenia were those
included in the Japan Society of Hepatology Guidelines for
Sarcopenia in Liver Disease (1st edition) as no uniformly agreed
upon definition of sarcopenia exists.
In conclusion, gemcitabine plus radiotherapy for
initially resectable distal bile duct cancer was safe, and did not
significantly affect to the body composition, or immunological or
nutritional indices during NACRT. We clarified that pretreatment
sarcopenia after NACRT was significantly associated with early
recurrence after pancreaticoduodenectomy. We should attempt to
maintain an appropriate SMI during NACRT. Further accumulation of
cases is necessary to reveal the impact of sarcopenia after
preoperative therapy on overall survival in biliary tract
cancer.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by JSPS KAKENHI (grant no.
JP20K09069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WF, HW, HM, MO, MS and HT conceived and designed the
study. WF, SH, YM, KA, HA, TS, MYamamoto, TT, NS, HH and TK
acquired the data. WF, HW, NH, JN, MYasui, CM, TO and HT performed
statistical analysis and interpreted the data. WF, HW, and HT
drafted the manuscript. WF and HW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Osaka International Cancer Institute (reference no.
1503315263).
Patient consent for publication
The patient, or parent/guardian/next of kin provided
written informed consent for the publication of any data and/or
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miyakawa S, Ishihara S, Horiguchi A,
Takada T, Miyazaki M and Nagakawa T: Biliary tract cancer
treatment: 5,584 Results from the biliary tract cancer statistics
registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg.
16:1–7. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kobayashi S, Gotoh K, Takahashi H, Akita
H, Marubashi S, Yamada T, Teshima T, Nishiyama K, Yano M, Ohigashi
H, et al: Clinicopathological features of surgically-resected
biliary tract cancer following chemo-radiation therapy. Anticancer
Res. 36:335–342. 2016.PubMed/NCBI
|
|
3
|
Ohigashi H, Ishikawa O, Eguchi H,
Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H,
Tomita Y and Nishiyama K: Feasibility and efficacy of combination
therapy with preoperative full-dose gemcitabine, concurrent
three-dimensional conformal radiation, surgery, and postoperative
liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg.
250:88–95. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan society of hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yip C, Goh V, Davies A, Gossage J,
Mitchell-Hay R, Hynes O, Maisey N, Ross P, Gaya A, Landau DB, et
al: Assessment of sarcopenia and changes in body composition after
neoadjuvant chemotherapy and associations with clinical outcomes in
oesophageal cancer. Eur Radiol. 24:998–1005. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Awad S, Tan BH, Cui H, Bhalla A, Fearon
KC, Parsons SL, Catton JA and Lobo DN: Marked changes in body
composition following neoadjuvant chemotherapy for oesophagogastric
cancer. Clin Nutr. 31:74–77. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reisinger KW, Bosmans JW, Uittenbogaart M,
Alsoumali A, Poeze M, Sosef MN and Derikx JP: Loss of skeletal
muscle mass during neoadjuvant chemoradiotherapy predicts
postoperative mortality in esophageal cancer surgery. Ann Surg
Oncol. 22:4445–4452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mirkin KA, Luke FE, Gangi A, Pimiento JM,
Jeong D, Hollenbeak CS and Wong J: Sarcopenia related to
neoadjuvant chemotherapy and perioperative outcomes in resected
gastric cancer: A multi-institutional analysis. J Gastrointest
Oncol. 8:589–595. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Levolger S, Van Vledder MG, Alberda WJ,
Verhoef C, De Bruin RWF, Ijzermans JNM and Burger JW: Muscle
wasting and survival following pre-operative chemoradiotherapy for
locally advanced rectal carcinoma. Clin Nutr. 37:1728–1735.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okusaka T, Ishii H, Funakoshi A, Yamao K,
Ohkawa S, Saito S, Saito H and Tsuyuguchi T: Phase II study of
single-agent gemcitabine in patients with advanced biliary tract
cancer. Cancer Chemother Pharmacol. 57:647–653. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Otsuji H, Yokoyama Y, Ebata T, Igami T,
Sugawara G, Mizuno T and Nagino M: Preoperative sarcopenia
negatively impacts postoperative outcomes following major
hepatectomy with extrahepatic bile duct resection. World J Surg.
39:1494–1500. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pamoukdjian F, Bouillet T, Levy V, Soussan
M, Zelek L and Paillaud E: Prevalence and predictive value of
pre-therapeutic sarcopenia in cancer patients: A systematic review.
Clin Nutr. 37:1101–1113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mathur A, Pitt HA, Marine M, Saxena R,
Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ and Lillemoe KD: Fatty
pancreas: A factor in postoperative pancreatic fistula. Ann Surg.
246:1058–1064. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sandini M, Bernasconi DP, Ippolito D,
Nespoli L, Baini M, Barbaro S, Fior D and Gianotti L: Preoperative
computed tomography to predict and stratify the risk of severe
pancreatic fistula after pancreatoduodenectomy. Medicine
(Baltimore). 94(e1152)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chakedis J, Spolverato G, Beal EW, Woelfel
I, Bagante F, Merath K, Sun SH, Chafitz A, Galo J, Dillhoff M, et
al: Pre-operative sarcopenia identifies patients at risk for poor
survival after resection of biliary tract cancers. J Gastrointest
Surg. 22:1697–1708. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cooper AB, Slack R, Fogelman D, Holmes HM,
Petzel M, Parker N, Balachandran A, Garg N, Ngo-Huang A,
Varadhachary G, et al: Characterization of anthropometric changes
that occur during neoadjuvant therapy for potentially resectable
pancreatic cancer. Ann Surg Oncol. 22:2416–2423. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shachar SS, Williams GR, Muss HB and
Nishijima TF: Prognostic value of sarcopenia in adults with solid
tumours: A meta-analysis and systematic review. Eur J Cancer.
57:58–67. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Okumura S, Kaido T, Hamaguchi Y, Fujimoto
Y, Kobayashi A, Iida T, Yagi S, Taura K, Hatano E and Uemoto S:
Impact of the preoperative quantity and quality of skeletal muscle
on outcomes after resection of extrahepatic biliary malignancies.
Surgery. 159:821–833. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eriksson S, Nilsson JH, Strandberg Holka
P, Eberhard J, Keussen I and Sturesson C: The impact of neoadjuvant
chemotherapy on skeletal muscle depletion and preoperative
sarcopenia in patients with resectable colorectal liver metastases.
HPB (Oxford). 19:331–337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pratesi A, Tarantini F and Di Bari M:
Skeletal muscle: An endocrine organ. Clin Cases Miner Bone Metab.
10:11–14. 2013.PubMed/NCBI View Article : Google Scholar
|