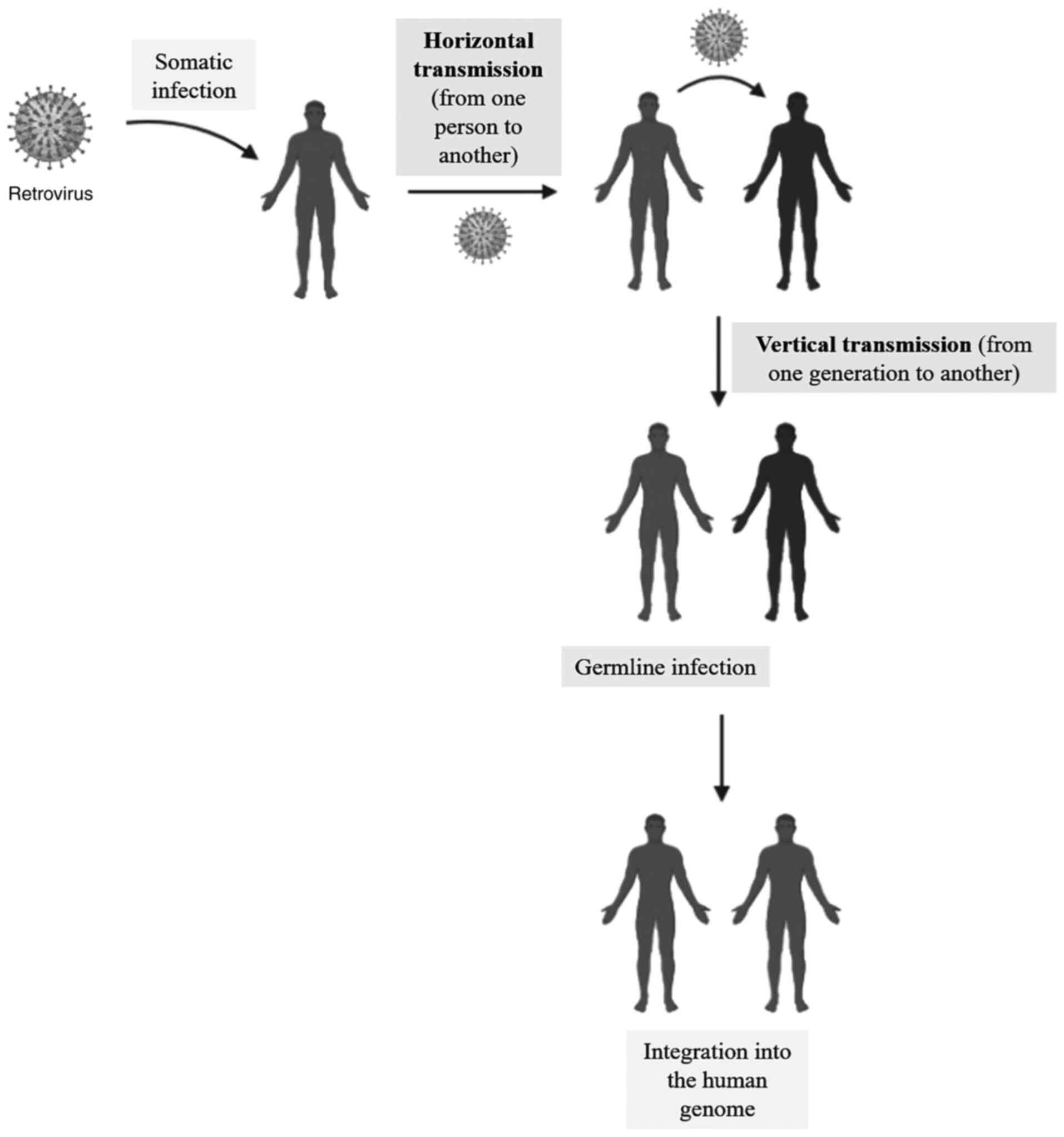
Human endogenous retroviruses (HERVs) are molecular
remnants of exogenous retroviruses that infected the human germline
millions of years ago (horizontal transmission). These genetic
fragments are stably integrated into the human genome; they are
called endogenous retroviruses (1). They are often considered
‘non-functional DNA’ and account for approximately 8.29% of the
human genome (2). They are
inherited from parents to the offspring like any other gene
(vertical transmission) (Fig. 1).
The retroviruses are known for their transforming potential in
their animal host via reverse transcription, but the HERVs are
typically silenced or non-productive due to the accumulation of
mutations and therefore rendered inadequate to produce virions
(3). However, due to epigenetic
dysregulation, some HERVs can retain their potency, produce
virus-like particles, and express some immunogenic protein
products, such as Syncytins (4), Np9, and Rec (5). The production of these proteins by
the retroelements affects the biological functions and cancer
immunoregulation (2).
HERVs are not usually expressed in normal cells.
Nevertheless, some of their gene products and viral components can
be expressed in human cells as antigens in some instances. Their
expression has been reported to have a dual impact on human
physiology (2). They aid in human
physiological functions like regulating pluripotency of embryonic
stem cells (6), involvement in
placental morphogenesis (7),
modulating the innate immune response (8), and regulating gene expression
(9). On the contrary, they are
also involved in the pathogenesis of multiple sclerosis (10), rheumatoid arthritis (11), schizophrenia (12), AIDS (13), cellular senescence (14), and diabetes (15). HERVs have gained a significant
attraction due to their association with various cancers and their
progression (3). Due to their
abnormal expression in multiple malignancies and their pleiotropic
role in oncogenesis, extensive research has targeted HERV antigens
for immunotherapy by triggering both innate and adaptive immune
response (16,17). Blocking the expression and function
of HERVs in tumor cells via small interfering RNA (siRNA) or CRISPR
(18), and anti-viral drugs
(19) have also been studied by
researchers (20). Since HERVs are
extensively present in the human genome, their gene products can
also be used as biomarkers to detect cancer progression (21).
HERVs have diverse structures ranging from solo LTR
(long terminal repeats) to partially or fully intact open reading
frames (ORFs) (22). The most
active HERV group reported having a relatively intact ORF is the
HERV-K HML-2 subtype (23).
Classical HERVs structure contains the general components of the
retroviruses, including protein-encoding sequences, GAG
(gene-specific antigen), PRO (protease gene), POL (polymerase
gene), and ENV (envelope gene) flanked by non-coding LTRs (Fig. 2) which are the regulatory region
and can act as a promoter or an enhancer. Until now, 31 discrete
groups of HERVs have been discovered. They can be categorized into
three classes of retroviruses based on their similar phylogenetic
origin to exogenous viruses (24)
(Fig. 3).
Generally, HERVs, having intact ORF, remain inactive
due to CpG hypermethylation of their sequence which is catalyzed by
DNA methylase-1(25). However,
they can be activated by multiple factors like exogenous viruses
such as human immunodeficiency virus (HIV) (26), Kaposi sarcoma-associated
herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8)
(27), Epstein-Barr virus
(28), and human T-cell leukemia
virus-1(29). Also, epigenetic
modifications (DNA demethylation, histone modification) (30), chemical substances (hydroquinone
(31),
phorbol-12-myristate-13-acetate (PMA) (32), phytohemagglutinin (PHA) (33), cupric salt (34)), physical factors (X-rays, UV-B)
(35,36), and cytokines (37) (Fig.
4).
On the contrary, HERVs can exert suppressive effects
on cancer instead of promoting it. The HERV protein products have
been reported to stimulate innate, humoral, and cellular immune
responses against malignant tumors by acting as an antigen like
PAMP (pathogen-associated molecular patterns) recognized by pattern
recognition receptors (PRRs) of immune cells (68). This triggers an immune response and
causes the pro-inflammatory signals to exert an anti-viral effect
against the HERV antigens by treating them as exogenous infections
(2,69). This phenomenon has been reported in
the case of clear cell renal cell carcinoma (ccRCC), where the
infiltration of CD8+ cytotoxic T-cells triggered by HERV-E antigen
was increased in ccRCC patients with hematopoietic stem cell
transfer, which negatively affected the cancer progression
(70). It might happen due to
viral mimicry, which causes the activation of the interferon
signaling pathway to upregulate the antitumor immune responses
(3,16,71)
like HERV-W interacts explicitly with the TLR4 and CD14 receptors,
inducing the production of IL-1β, IL-6, and TNF-α pro-inflammatory
cytokines. These cytokines further activate the dendritic cells,
resulting in a Th-1 response (72). Different HERV Env peptides
activate specific cytotoxic T-cells and dendritic cells (DCs) in
cancers like ovarian, breast, and colorectal cancer (2). Activation of B-cells and production
of antibodies have been seen in the case of breast cancer (73). Hence, it can be concluded that
triggering the viral mimicry pathway and targeting the HERV
proteins/transcripts can be a potential anti-cancer therapy.
Most germ cell tumors (GCTs) like teratocarcinoma,
multiple GCTs, and testicular cancers are known to express HERV-K
for a long time. Mueller et al (74) performed a study on different stages
of GCTs and suggested that the expression of HERV-K is regulated by
the epigenetic mechanisms occurring during different stages of
cellular development, which also affects the neighboring cells. Its
expression is linked with oncogenesis, migration, and resistance to
chemotherapy and correlates with poor prognosis (42). A variety of HERV-K viral particles
promote tumor development in multiple GCTs (75). Teratocarcinoma is known to be a
classical model for the study of HERV-K. An increase in the
Np9 accessory protein in teratocarcinoma has the oncogenic
potential (42,64). An increased expression of
Gag protein in teratocarcinoma was also seen due to
hypomethylation of the HERV-K sequence (76). Likewise, an increase in
syncytin-1 transcribed by HERV-W Env was seen in
seminoma patients, which may be involved in oncogenesis (2).
HERV-K is the most reported and studied ERV in
breast cancer. It is associated with tumor metastasis and invasion.
It is also involved in cancer cell stemness and endothelial to
mesenchymal transition (EMT) (41). HERV-K Env is involved in the
carcinogenesis of breast cancer. The Env proteins
downregulate the p53 cancer suppressor gene, causing the induction
of cancer (77). They are also
involved in the activation and upregulation of the RAS/ERK pathway,
thus, causing the growth and proliferation of tumor cells (78). Anti-HERV-K Env antibody was
able to inhibit tumor growth and induced breast cancer cells
apoptosis, thus, showing an anti-tumor response (73). Increased HERV ENV, GAG mRNA, and RT
(reverse transcriptase) expression in breast cancer are associated
with poor prognosis (79,80). Detection of Env proteins in
the early stages suggests that they may be involved in initiating
oncogenesis in breast cancer (81). Thus, understanding the downstream
function of Env may offer a new therapeutic target besides
improving our knowledge. Besides, using vaccines against HERV-K
Env may prevent breast cancer (82). Also, HERV-K RT can be used as an
early prognostic biomarker for breast cancer as its expression was
found in patients who develop cancer (79).
HERV-K protein expression and HERV-K specific
antibodies are found in different melanoma cell lines, assisting in
cell-to-cell fusion. HERV-K proteins are immunogenic; therefore,
antibodies are generated against them, resulting in increased
antibody titer, which is correlated with poor prognosis. The
Env protein maintains the tumor cell stemness and promotes
phenotypic switching of tumorigenic cells, making them non-adherent
and malignant (3,20). The overall expression of
Env, Rec, Np9 and Gag has been reported
in melanoma patients (85,86). Similarly, HERV-H is also found in a
cell line Hs294T of melanoma which promotes dedifferentiation of
tumor cells and helps them escape the immune cells (87). Further, HERV-W Env is
expressed in cutaneous T cell lymphoma (CTCL), which promotes cell
fusion (88), similar to its
function in mediating trophoblast fusion during placental
development (89).
Until now, only HERV-K expression has been reported
in the case of prostate cancer. HERV-K Env protein was
upregulated in prostate cancer patients (90). Targeting the Env protein via
CRISPR/Cas9 downregulated the proto-oncogene SF2/ASF and RAS
pathway expression in prostate cancer cell lines (44). Likewise, HERV-K Gag protein
expression was also upregulated in prostate cancer due to
demethylation and androgen stimulation (91). Gag protein expressions are
also associated with smoking, old age, and disease status, leading
to more aggressive prostate cancer (90). Anti-HERV-K Gag antibody
titer was increased in stage III and stage IV of cancer compared to
stage I and II, promoting carcinogenesis and depicting worse
survival (91). Both HERV mRNA and
anti-HERV antibodies have been reported to be used as potential
biomarkers in prostate cancer (90).
Cytotoxic T-cells were involved in the regression of
kidney cancer in clear cell renal cell carcinoma (ccRCC) patients
undergoing hematopoietic stem cell transfer. After the
investigation, it was found that CT-RCC, a highly immunogenic
antigen encoded by HERV-E, induces the activation of CD8+ T-cells
and, therefore, triggers an immune response against the RCC cells.
This led to tumor regression in-vitro and in-vivo
(70). Further, it was found that
an increase in the HERV-E expression was strongly correlated with
the non-functional von Hippel Lindau (VHL) tumor suppressor gene.
Absence of VHL protein induced the expression of HIF-2α, which
regulated the expression of HERV-E due to the presence of hypoxia
regulatory element (HRE) on the 5' LTR of HERV-E (98-100).
A full-length protein of HERV-E, Env expression, was also
selectively expressed in ccRCC patients, which can serve as a
biomarker for ccRCC (101).
Kaposi's sarcoma is caused by the infection of human
herpesvirus 8 (HHV8), also known as Kaposi's sarcoma-associated
herpesvirus (KSHV), and is the leading cause of mortality in HIV
infection (102). It is
characterized by the most common AIDS-related malignancies, which
still require effective treatment options. Kaposi's sarcoma is a
classic example of activation of HERV through exogenous viral
infection. KSHV infection was found to upregulate the production of
HERV-K Np9 protein, which advanced the invasiveness of
primary endothelial cells by the action of disintegrins and
metalloproteinases, contributing to carcinogenesis increasing the
morbidity among Kaposi's sarcoma patients (27).
Various HERV expression in colorectal cancer (CRC)
has been reported, including HERV-K, HERV-W, HERV-H, HERV-FRD, and
HERV-3. HERV-K is involved in cell growth, proliferation,
migration, and colonization (20).
Expression of HERV-W is correlated with poor prognosis in
syncytintal cancer (104). HERV-H
Env exerts an immune-modulatory effect (40). HERVs are also suggested to be used
as a biomarker and clinical examination for better predicting CRC
patient survival (105).
HERVs have been associated with cancer for a long
time. Their abnormal level of expression has been found in a
variety of cancers. Different groups of HERV are found to be
overexpressed in different cancers. Multiple factors are
responsible for their activation like epigenetic dysregulation
(30), exogenous infections
(26-29),
radiations (35,36), cytokines (37), chemical induction (31-34),
etc. They encode highly immunogenic antigens whose expression can
promote or inhibit cancer advancement by modulating the immune
system. HERVs are correlated with tumor cell proliferation,
migration, decreased apoptosis, endothelial to mesenchymal
transition (EMT), and immune suppression, thus initiating and
promoting oncogenesis (20). Since
the expression of HERV is a natural phenomenon, each HERV protein
must be characterized separately to elucidate its role in the
pathogenesis of different cancer and other diseases. Future studies
may shed light on the effect of vaccination against a specific
epitope of HERV elements and monoclonal antibody (MAB) on the
control and prevention of certain cancers. It suggests the need to
develop an onco-immunotherapy approach for rapidly evolving cancer
types.
Not applicable.
Funding: No funding was received.
Not applicable.
SS was involved in conceptualization, wrote the
original draft and was involved in visualization. BS reviewed and
edited the manuscript. AKR was involved in conceptualization and
provided supervision. Data authentication is not applicable. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wang X, Huang J and Zhu F: Human
endogenous retroviral envelope protein syncytin-1 and inflammatory
abnormalities in neuropsychological diseases. Front Psychiatry.
9(422)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gao Y, Yu XF and Chen T: Human endogenous
retroviruses in cancer: Expression, regulation and function. Oncol
Lett. 21(121)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grandi N and Tramontano E: HERV envelope
proteins: physiological role and pathogenic potential in cancer and
autoimmunity. Front Microbiol. 9(462)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Durnaoglu S, Lee SK and Ahnn J: Syncytin,
envelope protein of human endogenous retrovirus (HERV): No longer
‘fossil’in human genome. Anim Cells Syst (Seoul). 25:358–368.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salavatiha Z, Soleimani-Jelodar R and
Jalilvand S: The role of endogenous retroviruses-K in human cancer.
Rev Med Virol. 30:1–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Santoni FA, Guerra J and Luban J: HERV-H
RNA is abundant in human embryonic stem cells and a precise marker
for pluripotency. Retrovirology. 9(111)2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mi S, Lee X, Li X, Veldman GM, Finnerty H,
Racie L, LaVallie E, Tang XY, Edouard P, Howes S, et al: Syncytin
is a captive retroviral envelope protein involved in human
placental morphogenesis. Nature. 403:785–789. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alcazer V, Bonaventura P and Depil S:
Human endogenous retroviruses (HERVs): Shaping the innate immune
response in cancers. Cancers (Basel). 12(610)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim HS: Genomic impact, chromosomal
distribution and transcriptional regulation of HERV elements. Mol
Cells. 33:539–544. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kremer D, Gruchot J, Weyers V, Oldemeier
L, Göttle P, Healy L, Ho Jang J, Kang T, Xu Y, Volsko C, et al:
pHERV-W envelope protein fuels microglial cell-dependent damage of
myelinated axons in multiple sclerosis. Proc Natl Acad Sci USA.
116:15216–15225. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dervan E, Bhattacharyya DD, McAuliffe JD,
Khan FH and Glynn SA: Ancient adversary-HERV-K (HML-2) in cancer.
Front Oncol. 11(658489)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang WJ, Liu ZC, Wei W, Wang GH, Wu JG
and Zhu F: Human endogenous retroviral pol RNA and protein detected
and identified in the blood of individuals with schizophrenia.
Schizophr Res. 83:193–199. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Monde K, Terasawa H, Nakano Y, Soheilian
F, Nagashima K, Maeda Y and Ono A: Molecular mechanisms by which
HERV-K Gag interferes with HIV-1 Gag assembly and particle
infectivity. Retrovirology. 14(27)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu X, Liu Z, Sun L, Ren J, Wu Z, Jiang X,
Ji Q, Wang Q, Fan Y, Cai Y, et al: Resurrection of human endogenous
retroviruses during aging reinforces senescence. bioRxiv:
2021.02.22.432260, 2021.
|
|
15
|
Levet S, Charvet B, Bertin A, Deschaumes
A, Perron H and Hober D: Human endogenous retroviruses and type 1
diabetes. Curr Diab Rep. 19(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hurst TP and Magiorkinis G: Activation of
the innate immune response by endogenous retroviruses. J Gen Virol.
96:1207–1218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smith CC, Beckermann KE, Bortone DS, De
Cubas AA, Bixby LM, Lee SJ, Panda A, Ganesan S, Bhanot G, Wallen
EM, et al: Endogenous retroviral signatures predict immunotherapy
response in clear cell renal cell carcinoma. J Clin Invest.
128:4804–4820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mangeney M, Pothlichet J, Renard M, Ducos
B and Heidmann T: Endogenous retrovirus expression is required for
murine melanoma tumor growth in vivo. Cancer Res. 65:2588–2591.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Díaz-Carballo D, Acikelli AH, Klein J,
Jastrow H, Dammann P, Wyganowski T, Guemues C, Gustmann S,
Bardenheuer W, Malak S, et al: Therapeutic potential of antiviral
drugs targeting chemorefractory colorectal adenocarcinoma cells
overexpressing endogenous retroviral elements. J Exp Clin Cancer
Res. 34(81)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Müller MD, Holst PJ and Nielsen KN: A
systematic review of expression and immunogenicity of human
endogenous retroviral proteins in cancer and discussion of
therapeutic approaches. Int J Mol Sci. 23(1330)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu CH, Grandi N, Palanivelu L, Tramontano
E and Lin LT: Contribution of human retroviruses to disease
development-A focus on the HIV- and HERV-cancer relationships and
treatment strategies. Viruses. 12(852)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kassiotis G: Endogenous retroviruses and
the development of cancer. J Immunol. 192:1343–1349.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Subramanian RP, Wildschutte JH, Russo C
and Coffin JM: Identification, characterization, and comparative
genomic distribution of the HERV-K (HML-2) group of human
endogenous retroviruses. Retrovirology. 8(90)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Curty G, Marston JL, de Mulder Rougvie M,
Leal FE, Nixon DF and Soares MA: Human endogenous retrovirus K in
cancer: A potential biomarker and immunotherapeutic target.
Viruses. 12(726)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lavie L, Kitova M, Maldener E, Meese E and
Mayer J: CpG methylation directly regulates transcriptional
activity of the human endogenous retrovirus family HERV-K(HML-2). J
Virol. 79:876–883. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Contreras-Galindo R, López P, Vélez R and
Yamamura Y: HIV-1 infection increases the expression of human
endogenous retroviruses type K (HERV-K) in vitro. AIDS Res Hum
Retroviruses. 23:116–122. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dai L, Del Valle L, Miley W, Whitby D,
Ochoa AC, Flemington EK and Qin Z: Transactivation of human
endogenous retrovirus K (HERV-K) by KSHV promotes Kaposi's sarcoma
development. Oncogene. 37:4534–4545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sutkowski N, Conrad B, Thorley-Lawson DA
and Huber BT: Epstein-Barr virus transactivates the human
endogenous retrovirus HERV-K18 that encodes a superantigen.
Immunity. 15:579–589. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Toufaily C, Landry S, Leib-Mosch C,
Rassart E and Barbeau B: Activation of LTRs from different human
endogenous retrovirus (HERV) families by the HTLV-1 tax protein and
T-cell activators. Viruses. 3:2146–2159. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Romanish MT, Cohen CJ and Mager DL:
Potential mechanisms of endogenous retroviral-mediated genomic
instability in human cancer. In: Seminars in cancer biology.
Elsevier, pp246-253, 2010.
|
|
31
|
Conti A, Rota F, Ragni E, Favero C, Motta
V, Lazzari L, Bollati V, Fustinoni S and Dieci G: Hydroquinone
induces DNA hypomethylation-independent overexpression of
retroelements in human leukemia and hematopoietic stem cells.
Biochem Biophys Res Commun. 474:691–695. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Johnston JB, Silva C, Holden J, Warren KG,
Clark AW and Power C: Monocyte activation and differentiation
augment human endogenous retrovirus expression: Implications for
inflammatory brain diseases. Ann Neurol. 50:434–442.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kelleher CA, Wilkinson DA, Freeman JD,
Mager DL and Gelfand EW: Expression of novel transposon-containing
mRNAs in human T cells. J Gen Virol. 77:1101–1110. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Karimi A, Sheervalilou R and Kahroba H: A
new insight on activation of human endogenous retroviruses (HERVs)
in malignant melanoma upon exposure to CuSO4. Biol Trace Elem Res.
191:70–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JR, Ahn K, Kim YJ, Jung YD and Kim HS:
Radiation-induced human endogenous retrovirus (HERV)-R env gene
expression by epigenetic control. Radiat Res. 178:379–384.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Reiche J, Pauli G and Ellerbrok H:
Differential expression of human endogenous retrovirus K
transcripts in primary human melanocytes and melanoma cell lines
after UV irradiation. Melanoma Res. 20:435–440. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Katsumata K, Ikeda H, Sato M, Ishizu A,
Kawarada Y, Kato H, Wakisaka A, Koike T and Yoshiki T: Cytokine
regulation of env gene expression of human endogenous retrovirus-R
in human vascular endothelial cells. Clin Immunol. 93:75–80.
1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Montesion M, Bhardwaj N, Williams ZH,
Kuperwasser C and Coffin JM: Mechanisms of HERV-K (HML-2)
transcription during human mammary epithelial cell transformation.
J Virol. 92:e01258–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Barth M, Gröger V, Cynis H and Staege MS:
Identification of human endogenous retrovirus transcripts in
Hodgkin lymphoma cells. Mol Biol Rep. 46:1885–1893. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liang Q, Xu Z, Xu R, Wu L and Zheng S:
Expression patterns of non-coding spliced transcripts from human
endogenous retrovirus HERV-H elements in colon cancer. PLoS One.
7(e29950)2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou F, Li M, Wei Y, Lin K, Lu Y, Shen J,
Johanning GL and Wang-Johanning F: Activation of HERV-K Env protein
is essential for tumorigenesis and metastasis of breast cancer
cells. Oncotarget. 7:84093–84117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chan SM, Sapir T, Park SS, Rual JF,
Contreras-Galindo R, Reiner O and Markovitz DM: The HERV-K
accessory protein Np9 controls viability and migration of
teratocarcinoma cells. PLoS One. 14(e0212970)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sauter M, Roemer K, Best B, Afting M,
Schommer S, Seitz G, Hartmann M and Mueller-Lantzsch N: Specificity
of antibodies directed against Env protein of human endogenous
retroviruses in patients with germ cell tumors. Cancer Res.
56:4362–4365. 1996.PubMed/NCBI
|
|
44
|
Ibba G, Piu C, Uleri E, Serra C and Dolei
A: Disruption by SaCas9 endonuclease of HERV-Kenv, a retroviral
gene with oncogenic and neuropathogenic potential, inhibits
molecules involved in cancer and amyotrophic lateral sclerosis.
Viruses. 10(412)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rycaj K, Plummer JB, Yin B, Li M, Garza J,
Radvanyi L, Ramondetta LM, Lin K, Johanning GL, Tang DG and
Wang-Johanning F: Cytotoxicity of human endogenous retrovirus
K-specific T cells toward autologous ovarian cancer cells. Clin
Cancer Res. 21:471–483. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zare M, Mostafaei S, Ahmadi A, Azimzadeh
Jamalkandi S, Abedini A, Esfahani-Monfared Z, Dorostkar R and
Saadati M: Human endogenous retrovirus env genes: Potential blood
biomarkers in lung cancer. Microb Pathog. 115:189–193.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Krishnamurthy J, Rabinovich BA, Mi T,
Switzer KC, Olivares S, Maiti SN, Plummer JB, Singh H, Kumaresan
PR, Huls HM, et al: Genetic engineering of T cells to target
HERV-K, an ancient retrovirus on melanoma. Clin Cancer Res.
21:3241–3251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kreimer U, Schulz WA, Koch A, Niegisch G
and Goering W: HERV-K and LINE-1 DNA methylation and reexpression
in urothelial carcinoma. Front Oncol. 3(255)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grabski DF, Hu Y, Sharma M and Rasmussen
SK: Close to the bedside: A systematic review of endogenous
retroviruses and their impact in oncology. J Surg Res. 240:145–155.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Giebler M, Staege MS, Blauschmidt S, Ohm
LI, Kraus M, Würl P, Taubert H and Greither T: Elevated HERV-K
expression in soft tissue sarcoma is associated with worsened
relapse-free survival. Front Microbiol. 9(211)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Galli UM, Sauter M, Lecher B, Maurer S,
Herbst H, Roemer K and Mueller-Lantzsch N: Human endogenous
retrovirus rec interferes with germ cell development in mice and
may cause carcinoma in situ, the predecessor lesion of germ cell
tumors. Oncogene. 24:3223–3228. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Aagaard L, Bjerregaard B, Kjeldbjerg AL,
Pedersen FS, Larsson LI and Rossi JJ: Silencing of endogenous
envelope genes in human choriocarcinoma cells shows that envPb1 is
involved in heterotypic cell fusions. J Gen Virol.
93(1696)2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bergallo M, Montanari P, Mareschi K,
Merlino C, Berger M, Bini I, Daprà V, Galliano I and Fagioli F:
Expression of the pol gene of human endogenous retroviruses HERV-K
and -W in leukemia patients. Arch Virol. 162:3639–3644.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mangeney M, Renard M, Schlecht-Louf G,
Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B and
Heidmann T: Placental syncytins: Genetic disjunction between the
fusogenic and immunosuppressive activity of retroviral envelope
proteins. Proc Natl Acad Sci USA. 104:20534–20539. 2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cianciolo GJ, Copeland TD, Oroszlan S and
Snyderman R: Inhibition of lymphocyte proliferation by a synthetic
peptide homologous to retroviral envelope proteins. Science.
230:453–455. 1985.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lemaître C, Tsang J, Bireau C, Heidmann T
and Dewannieux M: A human endogenous retrovirus-derived gene that
can contribute to oncogenesis by activating the ERK pathway and
inducing migration and invasion. PLoS Pathog.
13(e1006451)2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bjerregaard B, Holck S, Christensen I and
Larsson LI: Syncytin is involved in breast cancer-endothelial cell
fusions. Cell Mol Life Sci. 63:1906–1911. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Argaw-Denboba A, Balestrieri E, Serafino
A, Cipriani C, Bucci I, Sorrentino R, Sciamanna I, Gambacurta A,
Sinibaldi-Vallebona P and Matteucci C: HERV-K activation is
strictly required to sustain CD133+ melanoma cells with stemness
features. J Exp Clin Cancer Res. 36(20)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen T, Meng Z, Gan Y, Wang X, Xu F, Gu Y,
Xu X, Tang J, Zhou H, Zhang X, et al: The viral oncogene Np9 acts
as a critical molecular switch for co-activating β-catenin, ERK,
Akt and Notch1 and promoting the growth of human leukemia
stem/progenitor cells. Leukemia. 27:1469–1478. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Li M, Radvanyi L, Yin B, Rycaj K, Li J,
Chivukula R, Lin K, Lu Y, Shen J, Chang DZ, et al: Downregulation
of human endogenous retrovirus type K (HERV-K) viral env RNA in
pancreatic cancer cells decreases cell proliferation and tumor
growth. Clin Cancer Res. 23:5892–5911. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang M, Liang JQ and Zheng S:
Expressional activation and functional roles of human endogenous
retroviruses in cancers. Rev Med Virol. 29(e2025)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Cherkasova E, Malinzak E, Rao S, Takahashi
Y, Senchenko VN, Kudryavtseva AV, Nickerson ML, Merino M, Hong JA,
Schrump DS, et al: Inactivation of the von Hippel-Lindau tumor
suppressor leads to selective expression of a human endogenous
retrovirus in kidney cancer. Oncogene. 30:4697–4706.
2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Rai AK, Singh A, Saxena A, Seth T, Raina V
and Mitra DK: Exonal switch down-regulates the expression of CD5 on
blasts of acute T cell leukaemia. Clin Exp Immunol. 190:340–350.
2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Denne M, Sauter M, Armbruester V, Licht
JD, Roemer K and Mueller-Lantzsch N: Physical and functional
interactions of human endogenous retrovirus proteins Np9 and rec
with the promyelocytic leukemia zinc finger protein. J Virol.
81:5607–5616. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Strissel PL, Ruebner M, Thiel F, Wachter
D, Ekici AB, Wolf F, Thieme F, Ruprecht K, Beckmann MW and Strick
R: Reactivation of codogenic endogenous retroviral (ERV) envelope
genes in human endometrial carcinoma and prestages: Emergence of
new molecular targets. Oncotarget. 3:1204–1219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yu H, Liu T, Zhao Z, Chen Y, Zeng J, Liu S
and Zhu F: Mutations in 3'-long terminal repeat of HERV-W family in
chromosome 7 upregulate syncytin-1 expression in urothelial cell
carcinoma of the bladder through interacting with c-Myb. Oncogene.
33:3947–3958. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li S, Liu ZC, Yin SJ, Chen YT, Yu HL, Zeng
J, Zhang Q and Zhu F: Human endogenous retrovirus W family envelope
gene activates the small conductance Ca2+-activated K+ channel in
human neuroblastoma cells through CREB. Neuroscience. 247:164–174.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Barbalat R, Ewald SE, Mouchess ML and
Barton GM: Nucleic acid recognition by the innate immune system.
Annu Rev Immunol. 29:185–214. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bannert N, Hofmann H, Block A and Hohn O:
HERVs new role in cancer: From accused perpetrators to cheerful
protectors. Front Microbiol. 9(178)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Panda A, de Cubas AA, Stein M, Riedlinger
G, Kra J, Mayer T, Smith CC, Vincent BG, Serody JS, Beckermann KE,
et al: Endogenous retrovirus expression is associated with response
to immune checkpoint blockade in clear cell renal cell carcinoma.
JCI Insight. 3(e121522)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cao W, Kang R, Xiang Y and Hong J: Human
endogenous retroviruses in clear cell renal cell carcinoma:
Biological functions and clinical values. Onco Targets Ther.
13:7877–7885. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rolland A, Jouvin-Marche E, Viret C, Faure
M, Perron H and Marche PN: The envelope protein of a human
endogenous retrovirus-W family activates innate immunity through
CD14/TLR4 and promotes Th1-like responses. J Immunol.
176:7636–7644. 2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wang-Johanning F, Li M, Esteva FJ, Hess
KR, Yin B, Rycaj K, Plummer JB, Garza JG, Ambs S and Johanning GL:
Human endogenous retrovirus type K antibodies and mRNA as serum
biomarkers of early-stage breast cancer. Int J Cancer. 134:587–595.
2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mueller T, Hantsch C, Volkmer I and Staege
MS: Differentiation-dependent regulation of human endogenous
retrovirus K sequences and neighboring genes in germ cell tumor
cells. Front Microbiol. 9(1253)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kleiman A, Senyuta N, Tryakin A, Sauter M,
Karseladze A, Tjulandin S, Gurtsevitch V and Mueller-Lantzsch N:
HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in
patients with germ cell tumors. Int J Cancer. 110:459–461.
2004.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Götzinger N, Sauter M, Roemer K and
Mueller-Lantzsch N: Regulation of human endogenous retrovirus-K Gag
expression in teratocarcinoma cell lines and human tumours. J Gen
Virol. 77:2983–2990. 1996.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang Z, Zheng Y, Park HJ, Li J, Carr JR,
Chen YJ, Kiefer MM, Kopanja D, Bagchi S, Tyner AL and Raychaudhuri
P: Targeting FoxM1 effectively retards p53-null lymphoma and
sarcoma. Mol Cancer Ther. 12:759–767. 2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
von Lintig FC, Dreilinger AD, Varki NM,
Wallace AM, Casteel DE and Boss GR: Ras activation in human breast
cancer. Breast Cancer Res Treat. 62:51–62. 2000.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Golan M, Hizi A, Resau JH, Yaal-Hahoshen
N, Reichman H, Keydar I and Tsarfaty I: Human endogenous retrovirus
(HERV-K) reverse transcriptase as a breast cancer prognostic
marker. Neoplasia. 10:521–533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Johanning GL, Malouf GG, Zheng X, Esteva
FJ, Weinstein JN, Wang-Johanning F and Su X: Expression of human
endogenous retrovirus-K is strongly associated with the basal-like
breast cancer phenotype. Sci Rep. 7(41960)2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kim HJ, Moon BI, Lee JW, Kim SC and Kim
HJ: Age-related reduction of antibody response against the human
endogenous retrovirus K envelope in women. Oncotarget.
7:17327–17337. 2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhou F, Krishnamurthy J, Wei Y, Li M, Hunt
K, Johanning GL, Cooper LJ and Wang-Johanning F: Chimeric antigen
receptor T cells targeting HERV-K inhibit breast cancer and its
metastasis through downregulation of Ras. Oncoimmunology.
4(e1047582)2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Jin X, Xu XE, Jiang YZ, Liu YR, Sun W, Guo
YJ, Ren YX, Zuo WJ, Hu X, Huang SL, et al: The endogenous
retrovirus-derived long noncoding RNA TROJAN promotes
triple-negative breast cancer progression via ZMYND8 degradation.
Sci Adv. 5(eaat9820)2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Li N, Li Y, Lv J, Zheng X, Wen H, Shen H,
Zhu G, Chen TY, Dhar SS, Kan PY, et al: ZMYND8 reads the dual
histone mark H3K4me1-H3K14ac to antagonize the expression of
metastasis-linked genes. Mol Cell. 63:470–484. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Büscher K, Hahn S, Hofmann M, Trefzer U,
Ozel M, Sterry W, Löwer J, Löwer R, Kurth R and Denner J:
Expression of the human endogenous retrovirus-K transmembrane
envelope, Rec and Np9 proteins in melanomas and melanoma cell
lines. Melanoma Res. 16:223–234. 2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Hahn S, Ugurel S, Hanschmann KM, Strobel
H, Tondera C, Schadendorf D, Löwer J and Löwer R: Serological
response to human endogenous retrovirus K in melanoma patients
correlates with survival probability. AIDS Res Hum Retroviruses.
24:717–723. 2008.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kudo-Saito C, Yura M, Yamamoto R and
Kawakami Y: Induction of immunoregulatory CD271+ cells by
metastatic tumor cells that express human endogenous retrovirus H.
Cancer Res. 74:1361–1370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Maliniemi P, Vincendeau M, Mayer J, Frank
O, Hahtola S, Karenko L, Carlsson E, Mallet F, Seifarth W,
Leib-Mösch C and Ranki A: Expression of human endogenous
retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma.
PLoS One. 8(e76281)2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Blond JL, Lavillette D, Cheynet V, Bouton
O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F and Cosset FL:
An envelope glycoprotein of the human endogenous retrovirus HERV-W
is expressed in the human placenta and fuses cells expressing the
type D mammalian retrovirus receptor. J Virol. 74:3321–3329.
2000.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Wallace TA, Downey RF, Seufert CJ,
Schetter A, Dorsey TH, Johnson CA, Goldman R, Loffredo CA, Yan P,
Sullivan FJ, et al: Elevated HERV-K mRNA expression in PBMC is
associated with a prostate cancer diagnosis particularly in older
men and smokers. Carcinogenesis. 35:2074–2083. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Reis BS, Jungbluth AA, Frosina D, Holz M,
Ritter E, Nakayama E, Ishida T, Obata Y, Carver B, Scher H, et al:
Prostate cancer progression correlates with increased humoral
immune response to a human endogenous retrovirus GAG protein. Clin
Cancer Res. 19:6112–6125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Saini SK, Ørskov AD, Bjerregaard AM,
Unnikrishnan A, Holmberg-Thydén S, Borch A, Jensen KV, Anande G,
Bentzen AK, Marquard AM, et al: Human endogenous retroviruses form
a reservoir of T cell targets in hematological cancers. Nat Commun.
11(5660)2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Alqahtani S, Promtong P, Oliver AW, He XT,
Walker TD, Povey A, Hampson L and Hampson IN: Silver nanoparticles
exhibit size-dependent differential toxicity and induce expression
of syncytin-1 in FA-AML1 and MOLT-4 leukaemia cell lines.
Mutagenesis. 31:695–702. 2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Sun Y, Ouyang DY, Pang W, Tu YQ, Li YY,
Shen XM, Tam SC, Yang HY and Zheng YT: Expression of syncytin in
leukemia and lymphoma cells. Leuk Res. 34:1195–1202.
2010.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Contreras-Galindo R, Kaplan MH, Leissner
P, Verjat T, Ferlenghi I, Bagnoli F, Giusti F, Dosik MH, Hayes DF,
Gitlin SD and Markovitz DM: Human endogenous retrovirus K (HML-2)
elements in the plasma of people with lymphoma and breast cancer. J
Virol. 82:9329–9336. 2008.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Tatkiewicz W, Dickie J, Bedford F, Jones
A, Atkin M, Kiernan M, Maze EA, Agit B, Farnham G, Kanapin A and
Belshaw R: Characterising a human endogenous retrovirus
(HERV)-derived tumour-associated antigen: Enriched RNA-Seq analysis
of HERV-K(HML-2) in mantle cell lymphoma cell lines. Mob DNA.
11(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Morgan D and Brodsky I: Human endogenous
retrovirus (HERV-K) particles in megakaryocytes cultured from
essential thrombocythemia peripheral blood stem cells. Exp Hematol.
32:520–525. 2004.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Takahashi Y, Harashima N, Kajigaya S,
Yokoyama H, Cherkasova E, McCoy JP, Hanada K, Mena O, Kurlander R,
Tawab A, et al: Regression of human kidney cancer following
allogeneic stem cell transplantation is associated with recognition
of an HERV-E antigen by T cells. J Clin Invest. 118:1099–1109.
2008.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Rao S, Abdul T, Kurlander R, Harashima N,
Lundqvist A, Hong J, Malinzak E, Smith A, Cherkasova E, McCoy P, et
al: The human endogenous retrovirus (HERV) derived kidney cancer
antigen CT-RCC1 induces proliferation of CD8+ antigen-specific
T-cells in vitro that kill renal cell carcinoma (RCC) and is
up-regulated by inhibiting histone deacetylase. Cancer Res. 68 (9
Suppl)(S1033)2008.
|
|
100
|
Haruta M, Gray WM and Sussman MR:
Regulation of the plasma membrane proton pump (H(+)-ATPase) by
phosphorylation. Curr Opin Plant Biol. 28:68–75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Weyerer V, Strissel PL, Stöhr C, Eckstein
M, Wach S, Taubert H, Brandl L, Geppert CI, Wullich B, Cynis H, et
al: Endogenous retroviral-K envelope is a novel tumor antigen and
prognostic indicator of renal cell carcinoma. Front Oncol.
11(657187)2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Gabaev I, Williamson JC, Crozier TWM,
Schulz TF and Lehner PJ: Quantitative proteomics analysis of lytic
KSHV infection in human endothelial cells reveals targets of viral
immune modulation. Cell Rep. 33(108249)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Chiappinelli KB, Strissel PL, Desrichard
A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, et
al: Inhibiting DNA methylation causes an interferon response in
cancer via dsRNA including endogenous retroviruses. Cell.
162:974–986. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Larsen JM, Christensen IJ, Nielsen HJ,
Hansen U, Bjerregaard B, Talts JF and Larsson LI: Syncytin
immunoreactivity in colorectal cancer: Potential prognostic impact.
Cancer Lett. 280:44–49. 2009.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Golkaram M, Salmans ML, Kaplan S,
Vijayaraghavan R, Martins M, Khan N, Garbutt C, Wise A, Yao J,
Casimiro S, et al: HERVs establish a distinct molecular subtype in
stage II/III colorectal cancer with poor outcome. NPJ Genom Med.
6(13)2021.PubMed/NCBI View Article : Google Scholar
|