Introduction
Laryngeal cancer constitutes 1.1% of all new cancer
diagnoses worldwide; there are ~177,000 new laryngeal cancer cases
and ~94,000 deaths annually (1).
In India, laryngeal cancer is the seventh most common cancer,
whereas it ranks ninth in Asia (2). The incidence of laryngeal cancer is
1.26-8.18 per 100,000 population in different regions in India
(3). Laryngeal cancer is divided
into supraglottic (epiglottis, false vocal cords, ventricles,
aryepiglottic folds, arytenoids), glottic (true vocal cords,
anterior commissure), and subglottic (located below the vocal
cords) cancer for staging and prognostication purposes. Of these
types of cancer, glottic cancer has the best 5-year relative
survival rate (77%), due to a higher percentage of patients
presenting with localized disease (83%) (4). The 5-year relative survival rate of
subglottic cancer is 53% (4).
Furthermore, supraglottic primary tumors more often recur when
compared with glottic primary tumors (5,6).
Supraglottic tumors are also associated with higher rates of
regional nodal metastasis, whereas the glottic site is less prone
to nodal spread as the lymphatic drainage is sparse at this
site.
It is estimated that 75% of laryngeal cancer cases
are attributable to cigarette smoking and alcohol use. For several
years, alcohol and tobacco were thought to act synergistically
(7); however, more recent data
have suggested that the two are independent risk factors (8). The effect of smoking and alcohol is
greater for supraglottic cancer than glottic cancer (9). People who employ their voices
extensively in their work also appear to be at a higher risk of
developing laryngeal cancer. In addition, occupational exposure to
asbestos, diesel fumes, rubber and wood dust (9), vitamin and nutrient deficiencies
(10), and gastroesophageal reflux
disease (11,12) may also lead to the development of
laryngeal cancer. A molecular etiology for laryngeal cancer is
emerging (13,14) and mutations in p53, Ki-67, Chek-2,
EGFR, h-TERT, cyclin D1, cathepsin D and TGF-β have been identified
(15-17).
Locally advanced cancer of the larynx includes TNM
stages T3, T4 and N1-N3. Until the early 1990s, the standard
treatment for locally advanced disease was total laryngectomy
followed by adjuvant radiation. Crucial changes in the treatment
approaches have come about in the management of these types of
cancer as a result of definitive evidence supporting the role of
organ preservation (18-20).
The role of radiotherapy (RT) was established with the publishing
of the Veterans Affair trial in 1991(18). With the use of induction
chemotherapy (IC), and subsequently concurrent chemoradiation
therapy (CCRT), organ preservation approaches have become the
standard of care in stage III and stage IV laryngeal cancer with
intact cartilage and functional larynx (21-28).
Although these changes have been incorporated in the treatment of
laryngeal cancer worldwide, their clinical outcomes and tolerance
in the Indian population have not been adequately quantified. The
present retrospective study aimed at analyzing the overall survival
(OS) and disease-free survival (DFS) of patients with locally
advanced (stage III and stage IV) squamous cell carcinoma of the
larynx who have been treated with definitive radical RT with or
without chemotherapy in a tertiary cancer center between January 1,
2006 and December 31, 2015. The results may provide detailed
insight on the success rates of the current treatment protocols in
laryngeal cancer, which may in turn open up areas of focused
research aiming at improving the outcome further.
Materials and methods
Ethics approval
The retrospective study protocol was approved by the
scientific review committee institutional review board of Regional
Cancer Centre, Thiruvananthapuram (IRB no. 09/2019/04). Data were
retrieved from case files using a structured proforma.
Patient cohort
A retrospective analysis was conducted on 630
patients with biopsy-proven locally advanced (stage III and IV)
squamous cell carcinoma of the larynx who were treated with
definitive RT with or without chemotherapy between the period
January 1, 2006 and December 31, 2015 in Regional Cancer Centre
(Thiruvananthapuram, India) Patients with histology other than
squamous cell carcinoma, patients who presented after primary
treatment elsewhere for salvage procedures, patients with stage T4a
disease with cartilage destruction who underwent primary surgical
management and patients having received palliative treatment were
excluded from the study.
The work-up after flexible endoscopy and biopsy
included routine hemogram, creatinine clearance test, dental
checkup, neck CT, chest X-ray and baseline cardiac evaluation. The
patients were staged according to American Joint Committee on
Cancer (AJCC) TNM staging 7 edition (29).
Treatment
The patients who received radiotherapy (RT) alone,
CCRT, IC followed by RT, or IC followed by CCRT were included in
the present study.
RT. All patients receiving RT were treated
with either a two-dimensional (2D) technique using X-ray simulator
with customized MLC shielding with 6 MV photons or cobalt 60 or
with intensity-modulated RT. The different dose fractionations used
were 60 Gy/26 fractions, 66 Gy/33 fractions, 55 Gy/20 fractions or
66 Gy/30 fractions. Patients were reviewed weekly during RT for any
acute complications and were managed accordingly. Any interruption
in treatment was corrected after giving adequate gap
correction.
IC. The IC regimens used were 5-fluorouracil
(5-FU) + cisplatin (PF) or PF + docetaxel (TPF). The IC regimens
were administered every 3 weeks. The CCRT regimens were 3-weekly
cisplatin (80-100 mg/m2), 3-weekly carboplatin (area
under the curve, 5) or weekly cisplatin (40 mg/m2).
Follow up of patients
Patients underwent the first clinical review at 2
months after completion of RT, and response assessment was done at
3-4 months. Further follow-up was done every 3 months in the first
year of completion of treatment, every 4 months in the second year,
followed by every 6 months until 5 years post-RT and annually
thereafter. Patients were clinically examined and an evaluation
with indirect laryngoscopy/70-degree laryngeal endoscopy was done
at each visit. If patients were lost to follow-up, details were
updated through telephonic conversation. Salvage surgery was
planned for patients who had residual disease or who had developed
locoregional relapse.
Statistical analysis
Details of the patients, their tumor and their
treatment-related characteristics were retrieved from the hospital
database using a structured proforma. Follow-up data were updated
until October 30, 2020. The primary endpoints analyzed were
disease-free survival (DFS) and overall survival (OS). DFS was
defined as the period from the date of registration to the date of
locoregional relapse, distant relapse or death, whichever occurred
earlier. OS was defined as the period from the date of registration
to the date of death from any cause. Compliance to treatment was
assessed based on whether the patient completed the planned course
of treatment or whether there were any interruptions. Evaluation of
toxicity was not included in the present study.
Survival curves were generated using the
Kaplan-Meier method and statistical significance was assessed using
the log-rank test. For univariate analysis, the patient factors
with potential prognostic value with respect to OS and DFS were
recorded and analyzed. Age, sex, smoking and drinking habits,
comorbidity, T stage, N stage, composite stage, and sequencing of
chemotherapy were tested for statistical significance. Prognostic
factors were assessed using Cox proportional hazards regression
model. Patients were stratified into three age groups (<50,
50-70 and >70 years) for analysis of outcomes. With respect to
chemotherapy, the use of IC alone, IC followed by CCRT, CCRT alone
or no chemotherapy were separately analyzed for any significant
association with outcomes.
Results
Baseline characteristics
A total of 630 patients were included in the present
retrospective study; the baseline characteristics of the study
population are provided in Table
I. The median age of the target population was 61 years (range,
30-89 years), and the majority of the patients were in the
50-70-years age group (n=477; 75.7%). Most of the patients were
male (n=601; 95.4%). In addition, 61% (n=384) of the patients were
habituated to smoking and 41% (n=263) to alcohol consumption.
 | Table IBaseline clinicopathological
characteristics of the patients with laryngeal carcinoma. |
Table I
Baseline clinicopathological
characteristics of the patients with laryngeal carcinoma.
| Clinicopathological
characteristic | Patients,
n=630 | % |
|---|
| Age, years | | |
|
<50 | 67 | 10.6 |
|
50-70 | 477 | 75.7 |
|
>70 | 86 | 13.7 |
| Sex | | |
|
Male | 601 | 95.4 |
|
Female | 29 | 4.6 |
| Habit | | |
|
Smoking | 384 | 61.0 |
|
Alcohol
consumption | 263 | 41.0 |
| Comorbidity | | |
|
Diabetes
mellitus | 274 | 43.5 |
|
Hypertension | 156 | 24.8 |
|
Heart
disease | 112 | 17.8 |
| Disease
characteristic (laryngeal subsite) | | |
|
Supraglottis | 496 | 78.7 |
|
Glottis | 126 | 20.0 |
|
Subglottis | 8 | 1.3 |
| T stage | | |
|
T1 | 23 | 3.7 |
|
T2 | 93 | 14.8 |
|
T3 | 413 | 65.6 |
|
T4a | 81 | 12.9 |
|
T4b | 20 | 3.2 |
| N stage | | |
|
N0 | 257 | 40.8 |
|
N1 | 162 | 25.7 |
|
N2a | 66 | 10.5 |
|
N2b | 85 | 13.5 |
|
N2c | 57 | 9.0 |
|
N3 | 3 | 0.5 |
| Composite
stage | | |
|
Stage
III | 367 | 58.1 |
|
Stage
IVa | 240 | 38.3 |
|
Stage
IVb | 23 | 3.6 |
| Tracheostomy | | |
|
No | 482 | 76.5 |
|
Yes | 148 | 23.5 |
| Dose of RT | | |
|
60 Gy/26
fractions | 483 | 76.7 |
|
66 Gy/33
fractions | 38 | 6.0 |
|
55 Gy/20
fractions | 86 | 13.7 |
|
66 Gy/30
fractions | 23 | 3.7 |
| Technique | | |
|
2D | 606 | 96.2 |
|
IMRT | 24 | 3.8 |
| Sequencing of
chemotherapy | | |
|
CCRT | 295 | 46.8 |
|
IC followed
by CCRT | 139 | 22.1 |
|
IC followed
by RT | 17 | 2.6 |
|
RT alone (no
chemotherapy) | 177 | 28.1 |
|
Unknown | 2 | 0.4 |
| Induction
chemotherapeutic agent | | |
|
No IC | 455 | 72.2 |
|
PF | 160 | 25.4 |
|
TPF | 9 | 1.4 |
|
5-FU +
carboplatin | 5 | 0.8 |
|
MTX | 1 | 0.2 |
| Treatment
interruption | | |
|
No | 576 | 91.4 |
|
Yes | 54 | 8.6 |
In the majority of the patients, the supraglottis
was the primary site of disease (n=496; 78.7%). A total of 257
patients (40.8%) were N0 at presentation, and 367 patients (58.1%)
had stage III disease at presentation. A tracheostomy was performed
for 148 (23.5%) patients. Out of the total 630 patients, 451
(71.5%) received chemotherapy. Most patients (46.8%) received CCRT.
The most common IC agents used were PF and the majority of patients
(96.2%) had cisplatin as the concurrent chemotherapy. Conventional
2D RT was delivered to 606 patients (96.2%). Only 54 patients
(8.6%) had treatment interruption exceeding 7 days (reasons
including machine failure, toxicity and poor compliance), whereas
the remaining patients completed the planned treatment without
interruptions.
Treatment outcomes are detailed in Table II. After the planned radical
treatment, 549 patients (87.1%) had complete response at 3-4 months
post-treatment. Out of the total 630 patients, 75 (11.9%) had
residual disease, of which 35 patients had residual disease in the
primary site, 37 in the nodal site and three in both sites. A total
of 11 patients with residual disease underwent salvage surgery.
 | Table IIResults of treatment outcome. |
Table II
Results of treatment outcome.
| Treatment
outcome | Patients,
n=630 | % |
|---|
| Status at 3-4 month
follow-up | | |
|
Complete
response | 549 | 87.0 |
|
Partial
response | 75 | 12.0 |
|
Unknown | 6 | 0.9 |
| Residual disease
(partial/no response) | 75 | 12.0 |
|
Primary
site | 35 | 47.0 |
|
Nodal
site | 37 | 49.0 |
|
Primary and
nodal | 3 | 4.0 |
| Salvage surgery for
residual disease | 11 | 14.7 |
|
Primary | 6 | 54.5 |
|
Nodal | 3 | 27.3 |
|
Both primary
and nodal | 2 | 18.2 |
| Pattern of
relapse | 134 | 21.3 |
|
Local | 65 | 48.5 |
|
Regional | 32 | 23.9 |
|
Locoregional | 5 | 3.7 |
|
Distant | 32 | 23.9 |
| Salvage surgery for
relapse | 31 | 4.9 |
|
Local | 21 | 67.7 |
|
Regional | 9 | 29.0 |
|
Locoregional | 1 | 3.2 |
| Second
malignancy | | |
|
No | 602 | 95.6 |
|
Yes | 21 | 3.3 |
|
Unknown | 7 | 1.1 |
The median follow-up period for the entire group of
630 patients was 59 months (range, 2-175 months). The 5-year
follow-up information was available for 84% of patients. At the
median follow-up of 59 months, 134 patients (21.2%) relapsed and
the median time to relapse was 16 months (range, 6-87 months). Of
those patients that relapsed, 65 (11.48%) relapsed locally, 32
(5.6%) relapsed in the nodal site, 5 (0.8%) relapsed locoregionally
and 32 (5.6%) had distant recurrence. In the patients who relapsed,
salvage surgery was performed for 21 patients with local
recurrence, nine patients with nodal recurrence and one patient
with locoregional recurrence. The remaining 103 patients that
relapsed were treated with palliative chemotherapy or best
supportive care. During the follow-up period, 21 patients (3%)
developed a second malignancy, with the most common being lung
cancer.
Survival outcomes
The 5-year OS rate was 48.7% and the 5-year DFS rate
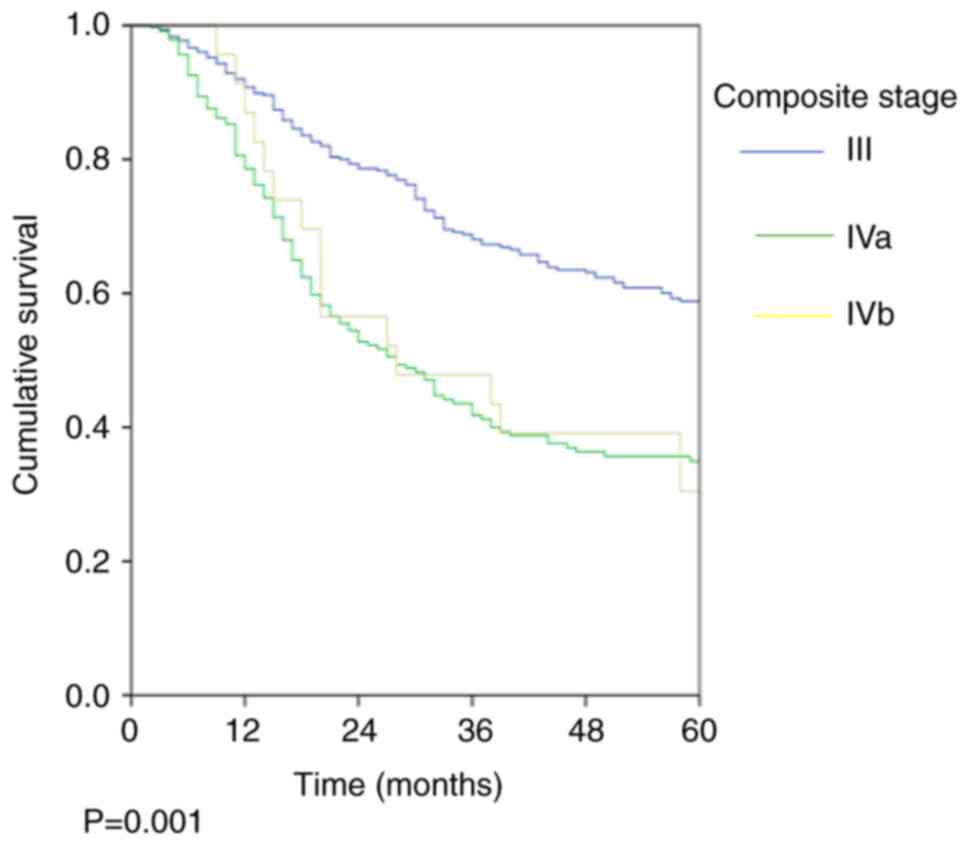
was 45.7%. The stage-wise OS rates were 58.9, 34.9 and 30.4%
(P=0.001; Fig. 1; Table III) and the stage-wise DFS rates
were 56.3, 32 and 21.7% (P=0.001; Fig.
2; Table III) for stage III,
Iva and Ivb, respectively.
 | Table IIIStage-wise OS and DFS rates. |
Table III
Stage-wise OS and DFS rates.
| Stage | OS, % (SEM, %) | P-value | DFS % (SEM, %) | P-value |
|---|
| All patients | 48.7 (2.2) | | 45.7 (2.2) | |
| Stage III
(n=367) | 58.9 (2.9) | 0.001 | 56.3 (2.9) | 0.001 |
| Stage IVa
(n=240) | 34.9 (3.5) | | 32.0 (3.4) | |
| Stage IVb
(n=23) | 30.4 (9.6) | | 21.7 (8.6) | |
The outcome measures of univariate analysis for OS
and DFS were associated with various patient factors and
treatment-related factors, and were tested for significance
(Table IV). With respect to age,
patients <50 years old had better 5-year DFS (P=0.050), but
there was no statistically significant difference in 5-year OS
(P=0.147). The 5-year OS (P=0.003) and DFS (P=0.002) were higher in
non-smokers when compared with smokers. Patients with hypertension
had significantly lower OS (P=0.001) and DFS (P=0.002), and those
with diabetes mellitus had lower DFS (P=0.043). Heart disease was
associated with slightly lower OS (P=0.247) and DFS (P=0.077) but
it was not statistically significant. With respect to disease
stage, the DFS and OS were significantly lower with advanced T4,
N2+3 and Stage IVb disease (all P=0.001). Sex, alcohol consumption,
heart disease and sequencing of chemotherapy did not show any
significant association with OS or DFS.
 | Table IVUnivariate analysis of OS and
DFS. |
Table IV
Univariate analysis of OS and
DFS.
| Clinicopathological
characteristic | Patients,
n=630 | % | OS, % (SEM %) | P-value | DFS, % (SEM,
%) | P-value |
|---|
| Age, years | | | | | | |
|
<50 | 67 | 10.6 | 60.3 (6.8) | 0.147 | 60.2 (6.8) | 0.05 |
|
50-70 | 477 | 75.7 | 47.2 (2.6) | | 43.2 (2.5) | |
|
>70 | 86 | 13.7 | 48.3 (6.3) | | 48.0 (6.3) | |
| Sex | | | | | | |
|
Male | 601 | 95.4 | 48.0 (2.3) | 0.199 | 45.1 (2.3) | 0.218 |
|
Female | 29 | 4.6 | 65.9 (10.7) | | 60.9 (11.0) | |
| Habit | | | | | | |
|
Smoking | | | | | | |
|
Yes | 384 | 61.0 | 42.9 (2.9) | 0.003 | 39.8 (2.8) | 0.002 |
|
No | 246 | 39.0 | 57.1 (3.5) | | 54.4 (3.5) | |
|
Alcohol
consumption | | | | | | |
|
Yes | 263 | 41.0 | 43.9 (2.7) | 0.261 | 42.1 (2.6) | 0.071 |
|
No | 367 | 59.0 | 57.6 (4.1) | | 57.4 (4.1) | |
| Comorbidity | | | | | | |
|
Diabetes | | | | | | |
|
Yes | 274 | 43.5 | 45.7 (2.5) | 0.075 | 42.5 (2.5) | 0.043 |
|
No | 356 | 56.5 | 59.5 (4.6) | | 57.3 (4.7) | |
|
Hypertension | | | | | | |
|
Yes | 156 | 24.8 | 44.3 (2.6) | 0.001 | 41.9 (2.6) | 0.002 |
|
No | 474 | 75.2 | 61.1 (4.3) | | 56.9 (4.3) | |
|
Heart
disease | | | | | | |
|
Yes | 112 | 17.8 | 47.9 (2.4) | 0.247 | 44.6 (2.4) | 0.077 |
|
No | 518 | 82.2 | 55.1 (6.6) | | 55.1 (6.6) | |
| Disease
characteristics | | | | | | |
|
T stage | | | | | | |
|
T1
+ T2 | 116 | 18.5 | 44.5 (5.2) | 0.001 | 42.8 (5.1) | 0.001 |
|
T3 | 413 | 65.6 | 54.0 (2.8) | | 51.4 (2.8) | |
|
T4 | 101 | 16.1 | 32.1 (5.2) | | 26.2 (4.9) | |
|
N stage | | | | | | |
|
N0 | 257 | 40.8 | 59.1 (3.5) | 0.001 | 56.2 (3.5) | 0.001 |
|
N1 | 162 | 25.7 | 55.8 (4.3) | | 51.4 (4.3) | |
|
N2
+ N3 | 211 | 33.5 | 30.2 (3.6) | | 28.5 (3.5) | |
|
Stage | | | | | | |
|
III | 367 | 58.1 | 58.9 (2.9) | 0.001 | 56.3 (2.9) | 0.001 |
|
IVa | 240 | 38.3 | 34.9 (3.5) | | 32.0 (3.4) | |
|
IVb | 23 | 3.6 | 30.4 (9.6) | | 21.7 (8.6) | |
| Sequencing of
chemotherapy | | | | | | |
|
CCRT | 295 | 46.8 | 48.6 (3.3) | 0.785 | 45.7 (3.2) | 0.729 |
|
IC +
CCRT | 139 | 22.1 | 48.9 (4.8) | | 43.9 (4.7) | |
|
IC alone
followed by RT | 17 | 2.6 | 62.5 (12.1) | | 50.0 (12.5) | |
|
No
chemotherapy | 177 | 28.1 | 47.6 (4.3) | | 46.8 (4.3) | |
|
Unknown | 2 | 0.4 | n/a | | | n/a |
The factors found significant on univariate analysis
were subjected to multivariate analysis. On multivariate analysis,
T stage (T3 vs. T4; P=0.001), N stage (N2 + N3 vs. N0; P=0.001) and
smoking status (P=0.012) were shown to have significant association
with OS (Table V), whereas smoking
status (P=0.005) and composite stage (IVa and IVb; both P=0.001)
had significant association with respect to DFS (Table VI).
 | Table VMultivariate analysis of overall
survival. |
Table V
Multivariate analysis of overall
survival.
| | 95.0% CI for
HR | |
|---|
| Factor
assessed | HR | Lower | Upper | P-value |
|---|
| T stage (T1 + T2
vs. T3) | 1.05 | 0.75 | 1.47 | 0.796 |
| T stage (T4 vs.
T3) | 1.69 | 1.24 | 2.31 | 0.001 |
| N stage (N1 vs.
N0) | 1.04 | 0.73 | 1.48 | 0.832 |
| N stage (N2 + N3
vs. N0) | 2.21 | 1.63 | 3 | 0.001 |
| Smoking status (yes
vs. no) | 1.39 | 1.07 | 1.79 | 0.012 |
 | Table VIMultivariate analysis of disease-free
survival. |
Table VI
Multivariate analysis of disease-free
survival.
| | 95.0% CI for
HR | |
|---|
| Factor
assessed | HR | Lower | Upper | P-value |
|---|
| Composite stage
(IVa vs. III) | 1.98 | 1.55 | 2.53 | 0.001 |
| Composite stage
(IVb vs. III) | 2.58 | 1.58 | 4.22 | 0.001 |
| Smoking status (yes
vs. no) | 1.43 | 1.12 | 1.82 | 0.005 |
Discussion
Until the early 1990s, the standard treatment for
locally advanced laryngeal carcinoma was total laryngectomy
followed by adjuvant RT. A fundamental change in the management of
laryngeal cancer began in 1991 when the Veteran Affairs laryngeal
cancer study was published (18).
This trial included 332 patients who were randomized to receive
either three cycles of IC (PF) followed by RT or undergo primary
surgery followed by postoperative RT. The 2-year OS was 68% for
both arms and 64% of patients receiving PF + RT had successfully
preserved larynx without compromising survival. This study
demonstrated that IC followed by RT is a reasonable alternative to
laryngectomy for patients with locally advanced laryngeal cancer.
Another phase 2 trial for patients with stage III and IV laryngeal
cancer reported that one cycle of IC (PF) followed by CCRT in
responders resulted in excellent larynx preservation and improved
OS rates compared with historical results (19). A voice-related quality of life
analysis was conducted in the patients of the aforementioned trial,
and quality of life was found to be better in those who received
chemoradiation therapy compared with salvage laryngectomy (20).
A meta-analysis of chemotherapy in head and neck
cancer and its subsequent updates established the role of CCRT
along with RT in squamous cell cancer of the head and neck region,
with an absolute 5-year OS benefit of 5.4% for laryngeal cancer in
the subset analysis (21-25).
The role of CCRT as an organ preservation approach for laryngeal
cancer was studied in the Radiation Therapy Oncology Group (RTOG)
91-11 trial and its update (26,27).
This study showed that the 10-year laryngeal preservation rate was
significantly higher in the CCRT arm compared with the IC followed
by RT or RT-alone arms. Thus, the standard treatment for patients
with stage III and IV laryngeal cancer who have intact cartilage
and a functional larynx is CCRT. Those with cartilage destruction
or dysfunctional larynx are not ideal candidates for organ
preservation (28).
The superiority of the three-drug IC (TPF) in
locally advanced head and neck cancer in terms of OS and DFS was
established by TAX 323(30) and
TAX 324(31) trials. The GORTEC
2000-01 trial evaluated the role of TPF in organ preservation in
laryngeal and hypopharyngeal cancer in which patients were
randomized to receive IC with either TPF or PF regimens (32). The responders to IC were given
radical RT, whereas non-responders underwent total laryngectomy
followed by adjuvant RT. The overall response was higher in the TPF
arm (80%) compared with the PF arm (59.2%) (P=0.002). The study had
a median follow-up of 105 months, and the long-term efficacy and
safety of the trial reported significant differences in the 5-year
(74.0 vs. 58.1%) and the 10-year (70.3 vs. 46.5%) larynx
preservation rates in the TPF and PF arms (both P=0.01) (33). A number of studies have shown that
TPF IC is not superior to CCRT alone in head and neck squamous cell
carcinoma (HNSCC) in terms of survival (34-36).
The ongoing phase 3 French trial (GORTEC 2014-2103-SALTORL) is
continuing to compare the role of TPF IC followed by RT with CCRT
in patients with laryngeal and hypopharyngeal cancer (37).
Based on these previous reports, the present study
analyzed the profiles, the main modalities of treatment used for
locally advanced laryngeal cancer, the outcome of various
modalities of treatment with regard to survival, as well as
patient- and treatment-related factors predicting the outcome for
patients admitted to Regional Cancer Centre, Thiruvananthapuram.
The significance of various prognostic factors in the present study
are detailed below.
In the present study, the patients were stratified
into three age groups (<50, 50-70 and >70 years), with the
majority belonging to the 50-70 years group. No significant
difference in OS was identified between the three groups; however,
there was a significant difference in terms of DFS favoring the
younger group. It may be that the younger patients tolerated
aggressive chemoradiation better than the elderly patients.
Previous studies have also shown that age is an important predictor
of survival outcome. Lacy et al (38) found that younger patients (≤40
years) had a significantly better 5-year OS rate compared with
middle-aged or older patients. In a large retrospective study from
Norway, Brandstorp-Boesen et al (39) reported that the OS was better in
patients aged <60 years.
With respect to smoking, the present study showed
that the OS rate was significantly higher in smokers compared with
non-smokers. Similarly, Browman et al (40) demonstrated a better 2-year OS rate
for non-smokers (66% for abstainers vs. 39% for active smokers;
P=0.005) with a risk difference of 27%. Similarly, Fortin et
al (41) revealed the
following a 5-year OS rates for 1,871 patients with locally
advanced HNSCC: 68% for patients that never smoked, 55% for former
smokers and 50% for active smokers (P=0.001).
In the present study, comorbidities such as diabetes
mellitus, heart disease and hypertension were present; however, a
statistically significant reduction in OS was determined only for
patients with hypertension, and a lower DFS was indicated for those
with hypertension and diabetes mellitus. Previous studies have
shown an association between coexisting comorbidities (diabetes
mellitus, hypertension, heart disease, pulmonary diseases and
neurological disease) and low OS in patients, although there are
limited data on comorbidities and DFS in patients with laryngeal
cancer. Fong et al (42)
showed incidence in comorbidity was associated with inferior OS
(HR=1.24; P<0.001) and inferior progression-free survival
(HR=1.14; P=0.007). Bøje et al (43) studied the impact of comorbidity on
treatment outcome in a series of 12,623 patients in a Danish head
and neck cancer study and found that comorbidities, such as heart
disease and diabetes mellitus, significantly decreased the 5-year
OS (P<0.001).
The present study showed that high T and N stages
were associated with poor outcome. Fong et al (42) also showed that advanced N stage was
associated with worse OS (HR, 3.52; P<0.001) and DFS (HR, 3.23;
P<0.001), and a higher T stage was associated with inferior OS
and (HR 1.61; P=0.02). The majority of patients in the present
study had stage III (58.1%) at presentation, followed by stage IVa
(38.3%) and stage IVb (3.6%). Analysis of different disease stages
in the present study revealed a significant difference in survival
probability with advanced stages in both univariate and
multivariate analyses.
In the present study organ preservation strategies
used were radical RT alone, IC followed by radical RT, CCRT and IC
followed by CCRT. No significant difference was observed for OS or
DFS between any of the treatment groups. Although IC followed by RT
showed a non-significant improved outcome compared with other
chemotherapy sequence groups with regard to OS and DFS, the number
of patients in this group was too small to identify the
significance. In the RTOG 91-11 study, even though there was no
statistically significant difference in OS in any of the three
treatment arms, locoregional control and laryngeal preservation
were significantly higher in the CCRT-alone arm compared with the
other two arms (IC followed by RT or RT-alone) (26,27).
In the present study, the 5-year OS and DFS rates
for all patients combined were 48.7 and 45.7%, respectively, and a
stage-wise decrease in OS was observed from 58.9 to 30.4%. These
results were similar to other studies that have shown 5-year
survival rates of 40-50% in stage III and 30-35% in stage IV
locally advanced laryngeal carcinoma (44,45).
A total of 134 patients (23.6%) had recurrence in the present
study, the most common being local recurrence. In the RTOG 91-11
study (26), the proportion of
patients in the IC, CCRT and RT-alone groups with recurrence were:
Local, 33.3, 22.3 and 35.8%; regional, 7.6, 3.3 and 11.5%; and
distant, 10.4, 11.2 and 14.9%, respectively.
The best sequence of chemotherapy and radiation to
achieve optimum results could not be determined from the present
study results, as no difference in OS was determined. It must be
noted that treatment comparison based on non-randomized data are
generally not recommended as they are prone to bias, and hence no
conclusion could be reached on the outcomes with different organ
preservation approaches in laryngeal cancer from the present
study.
Weber et al (46) studied the outcome of salvage
surgery in patients following organ preservation and concluded that
salvage surgery was associated with acceptable morbidity with
excellent locoregional control In the present study, only 31
patients with recurrence and 11 patients with residual disease
underwent salvage surgery. Others were offered either palliative
chemotherapy or best supportive care in view of poor general
condition and/or advanced disease. This is likely the main reason
that the OS and DFS closely correspond with each other in the
present study.
In the present study, univariate analysis showed
that the factors associated with OS were smoking, hypertension, T
stage, N stage and composite stage, and those associated with DFS
were age, smoking, diabetes, hypertension, T stage, N stage and
composite stage. On multivariate analysis, T stage, N stage and
smoking habit were associated with OS, whereas composite stage and
smoking habit were associated with DFS. In a study by Daneshi et
al (47), multivariate Cox
regression analysis suggested that age at diagnosis, cancer stage,
type of treatment, N stage and tumor grade affected the survival of
patients with locally advanced laryngeal carcinoma.
The retrospective nature of the present study, the
small number of patients in various treatment groups and
non-uniform treatment decisions for the entire population were the
major limitations of the present study. The heterogenous treatment
received by the study group made it unfeasible to derive the best
treatment modality for the patients. However, in this
single-institution study, the total number of patients in the
cohort was high, and the majority of the patients completed the
planned course of treatment without interruptions.
Anti-EGFR therapy has not shown any added benefit in
locally advanced head and neck cancer in addition to standard CCRT
(48-50);
however, it is a reasonable option in patients who cannot tolerate
platinum-based chemotherapy (51,52).
The role of immune checkpoint inhibitors has shown promising
results in the first-line and second-line treatments for recurrent
or metastatic HNSCC (53-55),
but they have not shown any effect on locally advanced head and
neck cancer (56).
In the present study, a major concern was the high
relapse rate (21.3%), even in patients who had completed the
planned course of treatment. Newer approaches to detect the various
biomarkers in patients with advanced laryngeal cancer, and thus
offer a better personalized treatment approach, may help to
overcome the relapse challenges. For example, Jun et al
(57) showed that low expression
of ERCC1 was an independent predictor for prolonged survival in
HNSCC, and ERCC1 expression may be a useful biomarker for these
tumors in patients treated with cisplatin-based CCRT. Hence, the
evaluation of ERCC1 is recommended for future correlative biomarker
studies. A consensus panel summary on laryngeal preservation
suggested a new endpoint called laryngo-esophageal dysfunction-free
survival, and also suggested that correlative biomarker studies for
near-term trials should include EGFR, ERCC-1, E-cadherin and
β-catenin, epiregulin and amphiregulin, as well as TP53 mutation
(58).
In conclusion, the present retrospective study
evaluated the outcomes of patients with locally advanced laryngeal
carcinoma who received chemoradiation/radiation. Chemoradiation is
the standard of care in locally advanced laryngeal carcinoma. The
aim of the present study was to demonstrate the feasibility of
delivering chemoradiation protocols in developing countries with
poor resources, and it has shown good results with a 5-year OS rate
of 48.7% and DFS rate of 45.7% in locally advanced laryngeal
cancer. The salvage rates were poor for those with recurrence
(4.9%) and/or residual disease (14.7%). Ideal sequencing of
chemotherapy with RT is an ongoing area of research.
Acknowledgements
The abstract was presented during the European
Society for Medical Oncology meeting, 2021 and was published as
abstract no. 878P in Annals of Oncology, 2021.
Funding
Funding: No funding was received.
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
KCT made substantial contributions to the conception
and design of the study. AF performed acquisition of the data. Data
analysis was mainly done by PG and the interpretation of data was
performed by AF, RRK, MR, FN, AMP, NK and KR. KCT and AF confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The retrospective study protocol was approved by the
scientific review committee institutional review board of Regional
Cancer Centre, Thiruvananthapuram. Data were retrieved from case
files using a structured proforma.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bobdey S, Jain A and Balasubramanium G:
Epidemiological review of laryngeal cancer: An Indian perspective.
Indian J Med Paediatr Oncol. 36:154–160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
(India) NCRP. India population based
cancer registries 2009-2011 Bangalore: Indian Council of Medical
Research (ICMR); 2013. Available from: https://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2009_2011/ALL_CONTENT/Printed_Version.htm.
|
|
4
|
https://www.cancer.org/cancer/laryngeal-and-hypopharyngealcancer/detection-diagnosis-staging/survival-rates.html.
|
|
5
|
Brandstorp-Boesen J, Sorum Falk R,
Folkvard Evensen J, Boysen M and Brondbo K: Risk of recurrence in
laryngeal cancer. PLoS One. 11(e0164068)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johansen LV, Grau C and Overgaard J:
Laryngeal carcinoma-multivariate analysis of prognostic factors in
1252 consecutive patients treated with primary radiotherapy. Acta
Oncol. 42:771–778. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rothman KJ, Cann CI, Flanders D and Fried
MP: Epidemiology of laryngeal cancer. Epidemiol Rev. 2:195–209.
1980.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Clemente CD: Anatomy; A regional atlas of
human body. Philadelphia PA; lea and febiger, 1975.
|
|
9
|
Muscat JE and Wynder EL: Tobacco, alcohol,
asbestos, and occupational risk factors for laryngeal cancer.
Cancer. 69:2244–2251. 1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Graham S: Diet and cancer. Am J Epidemiol.
112:247–252. 1980.
|
|
11
|
Ward PH and Hanson DG: Reflux as an
etiological factor of carcinoma of the laryngopharynx.
Laryngoscope. 98:1195–1199. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bacciu A, Mercante G, Ingegnoli A, Ferri
T, Muzzetto P, Leandro G, Di Mario F and Bacciu S: Effects of
gastroesophageal reflux disease in laryngeal carcinoma. Clin
Otolaryngol Allied Sci. 29:545–548. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mao L, Hong WK and Papadimitrakopoulou VA:
Focus on head and neck cancer. Cancer Cell. 5:311–316.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Almadori G, Bussu F, Cadoni G, Galli J,
Paludetti G and Maurizi M: Molecular markers in laryngeal squamous
cell carcinoma: Towards an integrated clinicobiological approach.
Eur J Cancer. 41:683–693. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kapral M, Strzalka B, Kowalczyk M, Jurzak
M, Mazurek U, Gierek T, Paluch J, Markowski J, Swiatkowska L and
Weglarz L: Transforming growth factor beta isoforms (TGF-beta1,
TGF-beta2, TGF-beta3) messenger RNA expression in laryngeal cancer.
Am J Otolaryngol. 29:233–237. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Loyo M and Pai SL: The molecular genetics
of laryngeal cancer. Otolaryngol Clin North Am. 41:657–672, v.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoo SS, Carter D, Turner BC, Sasaki CT,
Son YH, Wilson LD, Glazer PM and Haffty BG: Prognostic significance
of cyclin D1 protein levels in early-stage larynx cancer treated
with primary radiation. Int J Cancer. 90:22–28. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Department of Veterans Affairs Laryngeal
Cancer Study Group. Wolf GT, Fisher SG, Hong WK, Hillman R,
Spaulding M, Laramore GE, Endicott JW, McClatchey K and Henderson
WG: Induction chemotherapy plus radiation compared with surgery
plus radiation in patients with advanced laryngeal cancer. N Engl J
Med. 324:1685–1690. 1991.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Urba S, Wolf G, Eisbruch A, Worden F, Lee
J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N and Taylor
J: Single-cycle induction chemotherapy selects patients with
advanced laryngeal cancer for combined chemoradiation: A new
treatment paradigm. J Clin Oncol. 24:593–598. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fung K, Lyden TH, Lee J, Urba SG, Worden
F, Eisbruch A, Tsien C, Bradford CR, Chepeha DB, Hogikyan ND, et
al: Voice and swallowing outcomes of an organ-preservation trial
for advanced laryngeal cancer. Int J Radiat Oncol Biol Phys.
63:1395–1399. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lacas B, Carmel A, Landais C, Wong SJ,
Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, et al:
Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An
update on 107 randomized trials and 19,805 patients, on behalf of
MACH-NC group. Radiother Oncol. 156:281–293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. Meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955.
2000.PubMed/NCBI
|
|
23
|
Bourhis J, Amand C and Pignon JP: Update
of MACH-NC (meta-analysis of chemotherapy in head & neck
cancer) database focused on concomitant chemoradiotherapy. J Clin
Oncol. 22 (14 Suppl)(S5505)2004.
|
|
24
|
Pignon JP, le Maître A, Maillard E and
Bourhis J: MACH-NC Collaborative Group. Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Blanchard P, Baujat B, Holostenco V,
Bourredjem A, Baey C, Bourhis J and Pignon JP: MACH-CH
Collaborative group. Meta-analysis of chemotherapy in head and neck
cancer (MACH-NC): A comprehensive analysis by tumour site.
Radiother Oncol. 100:33–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098.
2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91-11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bhattacharyya T and Kainickal CT: Current
Status of organ preservation in carcinoma larynx. World J Oncol.
9:39–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
http://www.cancerstaging.org/.
|
|
30
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lorch JH, Goloubeva O, Haddad RI, Cullen
K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE and Posner
MR: TAX 324 Study Group. Induction chemotherapy with cisplatin and
fluorouracil alone or in combination with docetaxel in locally
advanced squamous-cell cancer of the head and neck: Long-term
results of the TAX 324 randomised phase 3 trial. Lancet Oncol.
12:153–159. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pointreau Y, Garaud P, Chapet S, Sire C,
Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M and Calais
G: Randomized trial of induction chemotherapy with cisplatin and
5-fluorouracil with or without docetaxel for larynx preservation. J
Natl Cancer Inst. 101:498–506. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Budach W, Bölke E, Kammers K, Gerber PA,
Orth K, Gripp S and Matuschek C: Induction chemotherapy followed by
concurrent radio-chemotherapy versus concurrent radio-chemotherapy
alone as treatment of locally advanced squamous cell carcinoma of
the head and neck (HNSCC): A meta-analysis of randomized trials.
Radiother Oncol. 118:238–243. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Trial of laryngeal preservation comparing
induced CT followed by RT vs CT concomitant to RT
(SALTORL)-NCT03340896. ClinicalTrials.gov, 2022.
|
|
38
|
Lacy PD, Piccirillo JF, Merritt MG and
Zequeira MR: Head and neck squamous cell carcinoma: Better to be
young. Otolaryngol Head Neck Surg. 122:253–258. 2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brandstorp-Boesen J, Falk RS, Boysen M and
Brøndbo K: Long-term trends in gender, T-stage, subsite and
treatment for laryngeal cancer at a single center. Eur Arch
Otorhinolaryngol. 271:3233–3239. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Browman GP, Wong G, Hodson I, Sathya J,
Russell R, McAlpine L, Skingley P and Levine MN: Influence of
cigarette smoking on the efficacy of radiation therapy in head and
neck cancer. N Engl J Med. 328:159–163. 1993.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fortin A, Wang CS and Vigneault E:
Influence of smoking and alcohol drinking behaviors on treatment
outcomes of patients with squamous cell carcinomas of the head and
neck. Int J Radiat Oncol Biol Phys. 74:1062–1069. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fong PY, Tan SH, Lim DWT, Tan EH, Ng QS,
Sommat K, Tan DSW and Ang MK: Association of clinical factors with
survival outcomes in laryngeal squamous cell carcinoma (LSCC). PLoS
One. 14(e0224665)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bøje CR, Dalton SO, Grønborg TK, Primdahl
H, Kristensen CA, Andersen E, Johansen J, Andersen LJ and Overgaard
J: The impact of comorbidity on outcome in 12 623 Danish head and
neck cancer patients: A population based study from the DAHANCA
database. Acta Oncol. 52:285–293. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Karlsson TR, Al-Azzawe M, Aziz L, Hurman D
and Finizia C: Survival outcome depending on different treatment
strategies in advanced stages III and IV laryngeal cancers: An
audit of data from two European centres. Eur Arch Otorhinolaryngol.
271:547–554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gourin CG, Conger BT, Sheils WC, Bilodeau
PA, Coleman TA and Porubsky ES: The effect of treatment on survival
in patients with advanced laryngeal carcinoma. Laryngoscope.
119:1312–1317. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weber RS, Berkey BA, Forastiere A, Cooper
J, Maor M, Goepfert H, Morrison W, Glisson B, Trotti A, Ridge JA,
et al: Outcome of salvage total laryngectomy following organ
preservation therapy: The radiation therapy oncology group trial
91-11. Arch Otolaryngol Head Neck Surg. 129:44–49. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Daneshi N, Fararouei M, Mohammadianpanah
M, Zare-Bandamiri M, Parvin S and Dianatinasab M: Effects of
different treatment strategies and tumor stage on survival of
patients with advanced laryngeal carcinoma: A 15-year cohort study.
J Cancer Epidemiol. 2018(9678097)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Patil VM, Noronha V, Joshi A, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, Mahimkar M, Juvekar
S, et al: A randomized phase 3 trial comparing nimotuzumab plus
cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy
alone in locally advanced head and neck cancer. Cancer.
125:3184–3197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Eriksen JG, Maare C, Johansen J, Primdahl
H, Evensen JF, Kristensen CA, Andersen LJ and Overgaard J:
Evaluation of the EGFR-inhibitor zalutumumab given with primary
curative (Chemo) radiation therapy to patients with squamous cell
carcinoma of the head and neck: Results of the DAHANCA 19
randomized phase 3 trial: Definitive management of head-and-neck
squamous. Int J Radiat Oncol Biol Phys. 88(P465)2014.
|
|
51
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-Year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chow LQM, Haddad R, Gupta S, Mahipal A,
Mehra R, Tahara M, Berger R, Eder JP, Burtness B, Lee SH, et al:
Antitumor activity of pembrolizumab in biomarker-unselected
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion
cohort. J Clin Oncol. 34:3838–3845. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yu Y and Lee NY: JAVELIN head and neck
100: A phase III trial of avelumab and chemoradiation for locally
advanced head and neck cancer. Future Oncol. 15:687–694.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Jun HJ, Ahn MJ, Kim HS, Yi SY, Han J, Lee
SK, Ahn YC, Jeong HS, Son YI, Baek JH and Park K: ERCC1 expression
as a predictive marker of squamous cell carcinoma of the head and
neck treated with cisplatin-based concurrent chemoradiation. Br J
Cancer. 99:167–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lefebvre JL and Ang KK: Larynx
Preservation Consensus Panel. Larynx preservation clinical trial
design: Key issues and recommendations-a consensus panel summary.
Head Neck. 31:429–441. 2009.PubMed/NCBI View Article : Google Scholar
|