1. Introduction
Mammalian telomeres are repetitive sequences of
TTAGGGs at the ends of linear chromosomes. They are associated with
a set of proteins, forming the shelterin complex, which protects
the chromosome ends from generating DNA repair responses. Telomere
length (TL) is gradually shortened after each cell division due to
the incomplete replication of the lagging strand from the DNA
polymerase. When telomeres reach a critical length, the cells are
introduced into a permanent growth arrest signaling process
(replicative senescence) (1).
Telomere attrition and senescence have traditionally been
considered a tumor suppressor pathway, preventing somatic cells
from indefinite replication.
Telomerase is an enzyme that adds nucleotides at the
ends of the chromosomes and counteracts their shortening. It
consists of two main components: Human telomerase reverse
transcriptase (hTERT), which is the catalytic subunit and also the
rate-limiting component of the protein's expression, and the human
telomerase RNA component (hTERC, also known as hTR), which serves
as a template for telomere replication. Human telomerase is
expressed during embryonic development; however, it is later
silenced in the majority of somatic cells upon differentiation and
its expression is restricted to germline and progenitor cells
(2).
Telomeres are critical contributors to genomic
stability and shorter telomeres have been associated with various
diseases, mostly involving premature aging phenotypes (1). Of note, shorter telomeres have been
observed in cancerous compared to healthy tissues (3). On the other hand, the activation of a
telomere maintenance mechanism represents a hallmark of cancer,
either through the activation of telomerase or, less frequently,
through alternative recombination-based mechanisms (alternative
lengthening of telomeres) (4).
Telomerase activation mechanisms may involve hTERT promoter
mutations, hTERT gene rearrangements, DNA copy
amplifications, or epigenetic alterations (3).
Therefore, it has been suggested that shorter
telomeres may be associated with genomic instability and the
development of pre-neoplastic lesions; on the other hand, there is
a critical point during oncogenesis when telomerase is activated,
enabling cancer cells to maintain replicative immortality (5). Although this general concept has been
proposed, a critical gap remains regarding the direct evidence and
the timing of telomere dysfunction in human pre-neoplastic lesions
and solid tumors. Furthermore, precursors and preinvasive lesions
represent heterogeneous entities with significant variations among
each human tissue. A better understanding of the natural history
and molecular characteristics of pre-invasive lesions will aid in
the resolution of diagnostic, prognostic and therapeutic challenges
associated with them and their corresponding invasive neoplasms. To
this end, the aim of the present narrative review was to summarize
and critically discuss the evidence regarding the role of telomeres
and telomerase across pre-neoplastic lesions.
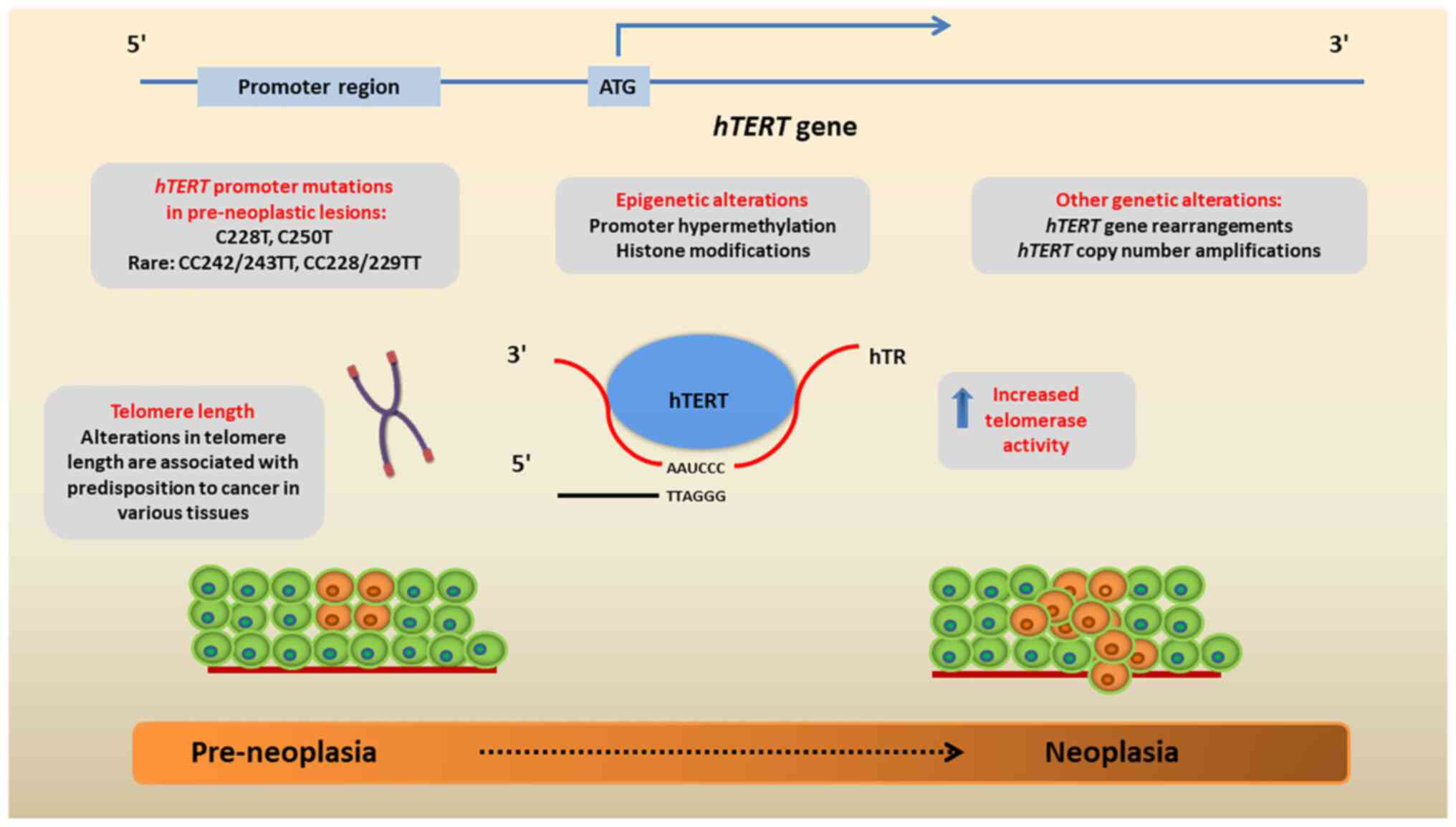
2. hTERT gene and telomerase
re-activation
The hTERT gene is located on chromosome
5p15.33. It is ~40 kb in length and is composed of 16 exons and 15
introns (6). Its promoter region
is the most critical regulatory element of telomerase expression,
and is located 330 bp upstream of the translational start site and
37 bp of exon 2(6). The functional
part for the transcriptional activation of hTERT in cancer
cells is however, located at the 181-bp fragment upstream of the
transcriptional start site. The hTERT promoter is a 5'
regulatory region, abundant with CpG nucleotides and specificity
protein 1 (Sp1) sites, and allows binding with either negative or
positive gene regulators (7).
Negative transcription factors include Mad1, p53, retinoblastoma
(Rb) and E2F, while the positive ones include c-myc, Sp1, the human
papillomavirus virus (HPV)16 protein E and steroid hormone
receptors (6).
As aforementioned, the main telomere maintenance
mechanism in cancer cells is the reactivation of telomerase due to
hTERT promoter mutations, gene rearrangements, DNA copy
amplifications or epigenetic alterations (8) (Fig.
1).
The most frequent hTERT promoter mutations
are found in the -124 (C228T) and -146 (C250T), which are C>T
transitions and can rarely co-exist; the exact location on the
chromosome is chr5, 1,295,228 and chr5, 1,295,250, respectively
(8,9). Both mutations upregulate hTERT
expression by elongating the promoter by 11 bases
5'-CCCCTTCCGGG-3', that include the binding section GGAA for E
twenty-six (ETS) transcriptional regulators in the complementary
strand. The overexpression of hTERT is induced possibly due
to the GA-binding protein alpha chain ETS factor, which is the only
one to form multimeric complexes when driving gene expression
(7-9).
Other rare genetic events leading to hTERT upregulation
include gene rearrangements and copy number amplifications
(8). More specifically, a variety
of structurally heterogeneous rearrangements of the hTERT
gene have been reported in high-risk neuroblastomas, which all
induce the massive transcriptional upregulation of the gene
(10). In a large genetic study on
several cancer types, hTERT was shown to be amplified in ~4%
of the cases, particularly in ovarian, lung (predominantly in
adenocarcinomas), esophageal and adrenocortical carcinomas
(3).
Additionally, epigenetic alterations, namely DNA
methylation, histone modifications and non-coding RNAs all play
roles in the regulation of hTERT expression in neoplasia
(11). As regards DNA methylation,
it is known that the hTERT promoter region includes several
GC motifs where methylation may take place and affect gene
expression (12). While the
promoter is largely hypomethylated in somatic cells, it is found
hypermethylated or partially methylated in numerous cancer cells
(13). From a mechanistic point of
view, the hypermethylation of the hTERT promoter reduces the
ability of the CCCTC-binding factor, that functions as a
transcriptional repressor for binding the CCCTC binding region,
thus preventing the inhibition of hTERT expression (14). Moreover, histone modifications in
the hTERT promoter may lead to the upregulation of the gene,
such as the H3K4me3 mark which is significantly enriched in cancer
cells (12). Finally, several
non-coding RNAs interact with hTERT by binding to
recognition sites, such as the 3' untranslated regions or the open
reading frames and regulate its activity in cancer cells (11).
3. Telomeres and telomerase across
pre-neoplastic lesions
Esophagus
A gradually increased telomerase activity, assessed
using the microdissection telomerase repeated amplification
protocol (TRAP) and the measurement of mRNA hTERT
expression, has been detected in the normal esophageal epithelium,
dysplastic tissue carcinoma in situ (CIS) and esophageal
squamous cell carcinoma (SCC) in two studies with clinical samples
(15,16). There was a statistically
significant difference between normal tissue and pre-neoplastic
lesions (P<0.01), whereas no marked difference was found between
pre-neoplasia and SCC (P>0.05) (15). Moreover, two studies investigated
telomerase activity in iodine-non-reactive esophageal tissues.
Those lesions, which remain unstained with Lugol's iodine staining,
were related to inflammation, dysplasia and cancer development
(17,18). By comparing telomerase activity
between Lugol-stained and unstained epithelia using TRAP assay, it
was concluded that the Lugol-unstained lesions presented a higher
mean telomerase activity compared to the stained ones from the same
patient. The unstained lesions included esophagitis, mild and
severe dysplasia, and intramucosal and advanced SCC. Additionally,
in the same study, the mRNA expression of hTERT was parallel
to the increase of the atypia and malignant transformation
(18).
Barret's esophagus (BE) is a pre-cancerous condition
associated with esophageal adenocarcinoma (EAC). In a previous
study, the methylation status of the promoters of the genes,
hTERT, adenomatous polyposis coli (APC), TIMP
metallopeptidase inhibitor 3 (TIMP3), cyclin-dependent
kinase inhibitor 2A and secreted frizzled related protein 1, was
compared between BE samples with EAC and those without EAC
(19). The methylation rates for
the first category were 92% for hTERT, 91% for TIMP3
and 100% for APC, while the other promoters reported no
methylation. In BE without EAC, the same promoters were methylated
in 17, 23 and 36% of the samples, respectively (P<0.0001).
Therefore, the promoter methylation of hTERT appears to be
involved in the carcinogenetic process of adenocarcinoma with
pre-existing BE (19).
Stomach
The mRNA expression of hTERT was assessed
using polymerase chain reaction (PCR) in 60 cases of chronic
gastritis, 15 of which presented intestinal metaplasia (IM)
(20). The results revealed that
hTERT mRNA expression was present in 23% of the cases, while the
frequency for IM and non-IM samples was 47 and 16%, respectively
(P=0.03) (20). Gastric carcinoma
(GC) presented a hTERT positivity rate of 89%, with rates
for well-differentiated and poorly differentiated tumors presenting
86 and 91%, respectively. hTERT expression was absent in the
normal gastric mucosa (20). Wang
et al (21) also found no
hTERT expression in normal tissue samples, while the
positivity rates for GC and pre-cancerous lesions were 87.5 and
47.4%, respectively (P<0.05). Finally, higher methylation rates
of the hTERT promoter were observed in cancerous samples
compared to the other groups (P<0.05), and there was no
association between hTERT mRNA levels and Helicobacter
pylori infection (20-22).
Of note, gastric ulcer specimens presented an hTERT protein
expression in 39% of the cases, while other studies reported no
enzyme activity. In this case, hTERT upregulation could
either contribute to the tissue's healing process or to its
malignant transformation (22).
Gastric carcinogenesis and hTERT
dysregulation are associated with the proto-oncogene MYC and
the dysregulation of the TP53 tumor suppressor gene. The
immunoreactivity of these genes was previously found to be higher
in IM compared to superficial and atrophic gastritis samples
(23). Additionally, the
expression of the telomeric proteins telomere repeat factor (TRF)1,
TRF2 and TERF1-interacting nuclear factor 2, which regulate TL,
were found to vary among normal mucosa, pre-neoplastic tissue and
GC specimens. Pre-cancerous lesions, GC, and GC with lymph node
metastasis presented significantly higher levels of these proteins
compared to normal tissue (P<0.01), while they were
significantly increased in GC samples compared to pre-cancerous
lesions (P<0.01). Finally, the mean TL was inversely related to
the expression levels of the studied proteins. It was significantly
shorter in the GC and GC samples with lymph node metastasis
compared to the pre-cancerous lesions and normal tissue (P<0.01)
(24).
Colon
It has been suggested that telomerase activation
occurs during the progression from low- to high-grade dysplasia in
adenomas, and increases progressively with the degree of dysplasia
and invasion during colorectal carcinogenesis (25). It has been found that hTERT
mRNA expression is a feature of the late-stage development of
colorectal cancer (25). In a
previous study, the expression of hTERT in normal colon mucosa from
patients with advanced colorectal adenoma was evaluated and
compared to that of the controls. The results did not reveal any
difference between the two groups of patients (26). In another study, the level of
hTERT mRNA expression in colorectal adenocarcinomas was
significantly higher than that in corresponding non-tumorous mucosa
tissues (P=0.009), and the expression level in the adenocarcinomas
was slightly higher than that of adenomas, although the difference
was not statistically significant (27). Of note, a higher level of
hTERT expression was often noted in the adenocarcinomas
arising from the left colon and rectum compared to those from the
right colon (P=0.029) (27).
Only a limited number of studies have evaluated the
association between colorectal cancer precursor lesions and TL. A
previous study suggested that individuals with a short leukocyte TL
had an increased risk of developing advanced adenomas (28). Roger et al (29) reported, in an experimental setting,
that extensive tissue telomere erosion could lead to chromosomal
instability and the initiation of colorectal cancer in polyps in
patients with familial adenomatous polyposis. A recent study
suggested that a short TL may be associated with an increased risk
of developing colorectal polyps in both the adenoma-carcinoma and
serrated pathways. That was the first study to report a
statistically significant association between TL and serrated
polyps, suggesting that telomeres may play an essential role along
the entire serrated pathway (30).
Liver
In the study by Nault et al (31), the occurrence of hTERT
promoter mutations during the malignant transformation of cirrhotic
nodules into hepatocellular carcinoma (HCC) was evaluated. Their
study included 58 patients with cirrhosis with HCC or pre-malignant
lesions, including low-grade dysplastic nodules (LGDNs), high-grade
dysplastic nodules (HGDNs), early HCC (eHCC), or small and
progressed HCC (31). hTERT
mutations were highly related to stepwise hepatocarcinogenesis,
since they were identified in 6% of LGDNs, 19% of HGDNs, 61% of
eHCCs, and 42% of small and progressed HCCs. There were 29
mutations which were detected in 96 nodules, including 25 cases
mutated in the first hotspot at 2,124 base pairs (bp) before the
ATG start (G>A substitution), and 4 cases mutated at the second
known hotspot at 2146 bp before the ATG start (G>A
substitution). These mutations were exclusive of each other. These
hTERT promoter mutations were not found in the
cirrhotic-matched tissues, indicating that they were somatic events
(31).
In another study by the same research group, 401
liver samples from HCC, HCC lines, cirrhotic tissues, cirrhotic
pre-neoplastic nodules and hepatocellular adenomas (HCAs) with or
without malignant transformation were analyzed for hTERT
promoter mutations (32).
Mutations in hotspot regions were found in 179 cases (58%) of HCC
and 15 cases (63%) of the HCC lines, indicating hTERT
promoter mutations as the most frequent somatic genetic alterations
in HCC. No mutation in the hTERT promoter was detected in
the cirrhotic tissues. Among the 60 typical HCAs, no mutation in
the hTERT promoter was detected. Of the 16 HCAs with
malignant transformations, seven were positive for hTERT
promoter mutations, indicating that hTERT promoter mutations
were involved in the final step of the malignant transformation of
HCA (32).
Pancreas
Matsuda et al (33) investigated TL between the normal
pancreatic duct epithelium, pancreatic intraepithelial neoplasia
(PanIN), and pancreatic cancer samples using in situ
hybridization. PanIN types 1, 2 and 3, as well as cancer cells,
exhibited weaker telomere signals in their nuclei and a decreased
telomere centromere ratio (TCR) compared to the normal epithelium
(P<0.05). Cancer cells also exhibited a lower TCR than the
PanINs (P<0.05); however, it was unrelated to tumor grade.
Atypical mitoses and anaphase bridges observed in PanIN and cancer
cells were negatively associated with TCR (33). Based on the aforementioned
findings, telomere shortening occurs early in pancreatic
carcinogenesis and progresses as the malignant transformation
develops (33).
Biliary tract and gallbladder
Pre-neoplastic conditions of the biliary tract
include hepatolithiasis, biliary epithelial hyperplasia and biliary
epithelial dysplasia. Dysplastic changes are related to cases of
chronic cholangitis, e.g., hepatolithiasis and primary sclerosing
cholangitis and are considered to be a progenitor of intrahepatic
cholangiocarcinoma (ICC). hTERT has been shown to be expressed in
dysplastic cells and ICC samples, but not in hyperplastic cells and
the normal bile duct epithelium (34). Furthermore, another study
demonstrated that samples of acute or chronic inflammation of the
gallbladder epithelium presented normal TLs (35). On the contrary, metaplastic lesions
with pyloric or intestinal metaplasia presented shorter telomeres
compared to normal cells (P<0.05) in 63% of the cases, whereas
dysplastic cells and CIS in 91% of the cases. Notably, two CIS
cases presented heterogeneity in TL, with both short and long
telomeres in the nuclei. Lastly, cholangiocarcinoma, infiltrating
adenocarcinoma of the gallbladder and extrahepatic bile ducts
presented shorter telomeres in 98% of the cases (35).
Congenital malformations of the biliary tree also
contribute to carcinogenesis through inflammation. Congenital
biliary dilation (CBD) causes the dilation of the extrahepatic bile
ducts and pancreaticobiliary maljunction (PBM), resulting in
chronic inflammation of the biliary tract and gallbladder
epithelium. Patients with CBD also have higher rates of cancer
development in these areas. Using the Q-FISH assay, Aoki et
al (36) calculated and
compared the normalized TCR between CBD, cholecystolithiasis and
normal tissue samples. All three categories exhibited significant
differences with each other (P<0.001), and the TCRs for each
tissue sample were 1.24, 1.96 and 1.77 for CBD, cholecystolithiasis
and normal tissue, respectively, indicating that CBD cells
presented shorter TLs (36).
Moreover, in another study, non-cancerous samples from PBM
gallbladders including chronic epithelial inflammation presented
telomerase activity, with a score of 3.06, 32.95 and 17.93 total
product generated (TPG) units in three different cases. On the
other hand, PBM gallbladder carcinoma samples scored 46.57, 85.18,
and 206.14 TPG units in three different cases. It is thus suggested
that telomerase is a catalytic factor in PBM carcinogenesis
(37).
Respiratory system
The characterization of pre-neoplastic lesions of
the lungs has been more comprehensively investigated in the case of
squamous carcinoma, and to a lesser extent in adenocarcinoma and
small-cell carcinoma. In the case of squamous carcinoma, it is
generally considered that a stepwise accumulation of precursor
phases occurs (38). The key
pre-neoplastic lesions of the bronchial epithelium are atypical
adenomatous hyperplasia (AAH), squamous dysplasia and CIS, as well
as diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.
These are lesions of the bronchial epithelium and the precursors of
lung adenocarcinoma, SCC and carcinoid tumors (39). Lantuejoul et al (40) performed an immunohistochemical and
in situ hybridization study in pre-invasive and invasive
bronchial lesions. They concluded that telomerase was increasingly
expressed from the normal epithelium to squamous metaplasia,
dysplasia and carcinoma in situ, and decreased in invasive
carcinoma (P<0.0001), with a direct correlation between protein
and mRNA levels of expression (P<0.0001). The expression of
hTERT was also associated with resistance to apoptosis
(40).
As regards TL, precancerous lung lesions present a
shorter relative TL (RTL) than normal bronchial or alveolar tissue
and invasive tumors. Thus, telomere shortening is considered an
early event in lung cancer development, which precedes p53/Rb
pathway inhibition and results in DNA damage responses (40,41).
This could be achieved by activating shelterin complex components,
such as the TERF1 and TERF2 proteins. These molecules stabilize the
telomeres; however, their mRNA expression is increased in
pre-neoplastic lesions, such as AAH. Lantuejoul et al
(41) investigated the RTL of
premalignant lung tissue using FISH and concluded that mild
dysplastic lesions presented a lower RTL (RTL=1.2; normal cells,
RTL=2), while this number increased in severe dysplasia, CIS and
SCC (RTL=2). However, these differences were not statistically
significant due to the low number of specimens studied. Similar
proportions were observed from AAH to advanced ADC; AHH presented
RTL=1.83, which increased in stage I-II ADC and stage III-IV ADC (2
and 1.88, respectively, P=0.047) (41). In addition, comparative genomic
hybridization studies in samples from early stages of non-small
cell lung cancer have shown that the genomic region that harbors
the hTERT gene, 5p15.33, is frequently amplified compared
with normal tissues (42).
Breast
An analysis of 56 pre-neoplastic breast tissues,
including atypical ductal or lobular hyperplasia and lobular in
situ carcinoma and comparison with healthy tissue and invasive
carcinomas revealed that the pre-neoplastic lesions were more
likely (60%) to have telomere shortening than normal breast tissue
(35%; P=0.0116) (43). As regards
invasive carcinomas, TLs were increased in 38.9% and markedly
decreased in 38.9% of breast carcinomas (P=0.0087 for comparisons
with pre-neoplastic lesions) (43). The telomere DNA content and the
number of sites of allelic imbalance were assessed in a set of
pre-invasive, invasive and healthy breast tissue samples. It was
observed that the level of genomic instability did not differ
between ductal carcinoma in situ and invasive carcinomas
(44). In another study,
telomerase activity was evaluated in 27 fibrocystic and dysplastic
tissue samples, and 28 fibroadenomas and phylloid tumors, and was
reported to be significantly increased in the dysplastic tissue and
fibroadenoma groups compared to normal tissues (45).
Cervix
A previous study with cervical samples from 100
patients revealed the overexpression of telomerase in 18.8% of the
normal cervical samples, 32.0% of cervical intraepithelial
neoplasia I (CIN I), 50.0% of CIN II, 60.0% of CIN III and 91.3% of
invasive cervical cancer (46).
Telomerase activity was significantly higher in patients with
invasive cancer compared to those with CIN or a normal cervix
(P<0.05), and its activity increased with the increasing CIN
stage (46-48).
Patients with benign lesions or CIN that exhibited TERC
amplification relapsed or progressed to CIN II and CIN III more
often than those without gene amplification (49-51).
In addition, the levels of telomerase activity increased in
parallel with the degree of CIN, with a significant increase during
the transition to CIN3(52).
Importantly, there is an association between HPV E6
proteins and the activation of hTERT, resulting in increased
risk of oncogenesis in the cervix (53). The human papillomavirus E6 protein
binds to and activates the hTERT promoter of polymerase,
promoting the precancerous transformation of the cervix. As
previously demonstrated, based on testing 29 types of the virus, it
was found that the oncogenic types specifically activate the
hTERT promoter, while the non-oncogenic types do not.
(53). The amplification of
hTERT has been used in combination with HPV testing and
cMYC amplification in order to optimize screening for
malignancy in cytological samples (54).
Endometrium
The normal human endometrium expresses significant
telomerase activity in a menstrual phase-dependent manner (55). In a previous study, a total of 32
normal endometrial tissues at various stages of menstruation or
postmenopausal conditions were tested for telomerase expression
(56). The expression of
hTERT mRNA was characteristic in the normal endometrium and
was dependent on the menstrual cycle phase. In the intrauterine
proliferative phase, there was a rapid increase in the expression
of hTERT, although not in the secretory phase. In additional
to its expression in the normal endometrium, hTERT,
hTR and TP1 were also found to be involved in the
development of precancerous and cancerous lesions in the
endometrium (56). Another study
using a telomere-FISH assay to measure TLs compared chromosomal arm
loss or gain in premalignant endometrial lesions with normal
endometrium and reported TLs to be stable with the pathological
transformation in endometrial hyperplasia and in endometrial
carcinoma (57). A significantly
increased number of telomere aggregates has been observed in
atypical hyperplastic cells in mouse endometrial cancer models. It
has been shown that alterations in the nuclear 3D telomere
architecture are present in early proliferative lesions of mouse
uterine tissues, indicative of endometrial cancer development
(58).
Ovaries
In comparison with normal ovary/cystadenoma (32%), a
previous study found a markedly higher frequency of moderate
activity in low-malignant-potential tumors (67%) or invasive
carcinomas (57%), suggesting a close association between the latter
two categories (59). That study
demonstrated a high prevalence of telomerase activity in
low-malignant-potential tumors or invasive carcinomas, with the
high telomerase activity associated exclusively with invasive
ovarian carcinomas (59). In
another study, telomerase activity assessed using TRAP assay was
markedly increased in malignant compared to borderline tumors,
benign tumors and normal ovaries (P<0.05) (60). The allelic discrimination analysis
of primary and recurrent adult granulosa cell tumors has indicated
that hTERT C228T promoter mutations are already present in
some primary tumors; however, they may be late events that occur
during adult granulosa cell tumor progression (61).
Urothelium
It has been suggested that hTERT promoter
mutations represent the earlier onset of a clonal molecular process
from which urothelial tumorigenesis may occur (62). hTERT promoter mutations were
previously investigated among urothelial papilloma (UP) tumors and
papillary urothelial neoplasms of low malignant potential
(PUNLMPs). The results revealed that 46% of UPs and 43% of PUNLMPs
carried a mutated hTERT promoter, and the activating
hTERT promoter mutation C228T was detected in all mutant
cases (63). These findings
suggest that these low-malignancy entities share a transformation
path similar to aggressive urothelial carcinomas (63). Another study investigated the
significance of the hTERT promoter mutation pathway in the
pathogenesis of inverted UP (IUP), an entity whose neoplastic
nature is debated (64). The
results demonstrated that 15% of inverted papillomas, 58% of
urothelial carcinomas with inverted growth, 63% of conventional
urothelial carcinomas and none of the cystic glandular specimens
harbored a hTERT promoter mutation. It was suggested that a
subset of inverted papilloma shares a developmental pathway similar
to the carcinogenesis pathway of urothelial carcinoma. It has to be
mentioned that a female predominance was noted in
hTERT-mutated inverted papillomas, taking into account the
strong male predilection of inverted papillomas in the general
population. All hTERT promoter mutations were C228T, apart
from two C250T mutations observed in two invasive urothelial
carcinoma cases (64). However,
another study conducted a comprehensive genetic analysis for
multiple oncogenic genes in a sample size of 11 UPs and 11 IUPs. No
IUP tumors had a mutated hTERT promoter. One UP tumor
harbored a hTERT promoter mutation and was found in a
patient with recurrent non-invasive papillary urothelial carcinomas
(65). The results of that study
are in contrast to those of previous studies on activating
hTERT promoter mutations in IUP and UP, which may be
attributed to different methodologies (63,64).
In another study, hTERT promoter mutations in
cases of de novo PUNLMP were associated with a risk of
recurrence (66). Recurrence with
or without progression was encountered in 13 of 30 (43%) cases of
PUNLMP, which were included. More specifically, 31% of the cases
recurred as PUNLMP, 69% exhibited progression (54% progressed to
non-invasive low-grade papillary urothelial carcinoma, 8% to
non-invasive high grade papillary urothelial carcinoma and 8%
developed stage progression to invasive high-grade urothelial
carcinoma) (66). Among the
recurrent tumors, 80% harbored a hTERT promoter mutation,
including C250T and C228T, in contrast to 53% among the cases that
did not recur (66).
Moreover, the presence of hTERT promoter
mutations was previously assessed in a morphological spectrum of
microdissected urothelia from urinary bladder specimens with and
without keratinizing squamous metaplasia (KSM) and non-KSM (NKSM),
including cases of neurogenic lower urinary tract dysfunction
(NLUTD), and urothelial and squamous carcinomas (67). The results demonstrated that 94% of
cancer foci, 68% of KSM and 70% of NKSM foci were positive for
hTERT promoter mutations. The authors of that study
suggested an association between conditions with chronic urinary
bladder injury (such as NLUTD) and a higher risk of developing
bladder cancer (67). In a recent
study, hTERT promoter mutations were examined in whole-organ
bladder samples, including cancerous tissue and samples of the
tumor-associated normal urothelium, non-invasive urothelial
lesions, carcinoma in situ and muscle-invasive bladder
cancers (68). That study
demonstrated that hTERT mutations were detected in
tumor-associated normal urothelium and non-invasive urothelial
lesions. Therefore, mutated hTERT promoter regions within
non-invasive urothelial lesions are insufficient to establish
cancerous growth, indicating the contribution of other gene
mutations as a requirement for tumor development (68).
Prostate gland
Pre-neoplastic lesions of the prostate gland include
prostate intraepithelial neoplasia (PIN) and possibly atypical AAH,
while benign conditions include benign prostate hyperplasia (BPH),
which presents no risk for malignant transformation. Previously,
when comparing BPH, PIN and prostate cancer telomeric fusion
frequencies, the rates for each lesion were found to be similar and
comparable: 65, 55 and 62%, respectively. The majority of normal
prostatic epithelial tissue samples did not harbor telomeric
fusions. As regards hTERT, all tissue samples presented
detectable levels of its mRNA, with the rates being 69% in BPH, 60%
in PIN and 94% in cancerous tissues. The normal adjacent epithelium
also presented hTERT mRNA expression in 86% of the samples
(69).
Variations are also evident in TL, as it appears
that cancer telomeres are significantly shortened in comparison to
normal tissue (P<0.05) (69).
This characteristic is also shared by PIN, but not by BPH, as
Southern blot analysis has revealed that normal epithelium and BPH
have similar average TLs (6.6 and 6.4 kb, respectively) (70). Using Q-FISH assay, Cheng et
al (71) compared TLs between
normal epithelium, AAH, high-grade PIN and prostatic adenocarcinoma
(PCA). Shortened telomeres were present in 20% of AAH, 68% of
high-grade PIN and 83% of PCA samples. The reduction percentages
for each lesion when compared with the normal epithelium were 86%
(P<0.001), 72% (P<0.001) and 68% (P<0.01), respectively.
TLs of these lesions differed significantly when compared with one
another and the normal tissue (P<0.001). These findings, along
with AMACR expression, suggest the premalignant nature of AAH and
its role in prostate cancer development (71). Lastly, TL in BPH is associated with
race, as African American males have been shown to exhibit
significantly shorter telomeres compared to Caucasian males; longer
telomeres have also been found to be associated with an increased
risk of cancer (72).
Central nervous system (CNS)
hTERT promoter mutations (C250T and C228T)
are a key event during the carcinogenesis of CNS tumors (73). The frequency of these mutations
varies among different tumors, such as primary glioma (80%),
medulloblastoma (19.8%) and meningioma (7.4%). The WHO grade of
primary gliomas is associated with the frequency of hTERT
mutations. Similarly, it has been reported that glioblastomas
(grade IV), oligodendrogliomas (grade II-III) and astrocytomas
(grade I) present these mutations in 80, 60-70 and 30-40% of cases,
respectively. The multi-sector sequencing of glioblastomas has
revealed the clonal nature of the hTERT mutations in these
tumors, indicating their essential role in the transformation from
precancerous lesions to malignant tumors (73). As a result, increased TL and
telomerase activation are significant risk factors for glioma and
glioblastoma development. In addition, inherited mutations near
hTERT and other telomere-related genes, namely hTERC,
RTEL1 and POT1, could increase the susceptibility of
neural cells to oncogenesis (74).
Skin
Actinic keratosis (AK) and Bowen's disease (BD) are
pre-invasive, in situ forms of cutaneous SCC (cSCC). Both AK
and BD harbor hTERT promoter mutations: -146C>T or C250T,
-124C>T or C228T, -138/-139CC>TT or CC242/243TT (genome
location: chr.5.1295242_1295243CC>TT) and -124/-125CC>TT or
CC228/229TT (genome location: chr.5.1295228_1295229CC>TT), which
gradually decrease following treatment (75). Consequently, telomerase activity in
those lesions has been found to be increased. Moreover, TL has been
shown to be associated with a greater tumor invasiveness, as the
telomere centromere ratio values in cases of cSCC are lower than
those in BD and AK. It is therefore understood that telomere
shortening plays a crucial role in the invasive progression of cSCC
from its precursors, as it precedes UV-induced p53 mutations
(76).
Benign, precancerous and malignant melanocytic
proliferations exhibit different telomerase activity levels, which
increase with tumor invasiveness. These findings have been
confirmed by immunohistochemistry, as well as by the PCR-based TRAP
assay. As expected, benign nevi, such as Spitz and acquired nevi
exhibited lower enzyme activity levels than dysplastic nevi, which
had similar scores with stage I melanoma (77,78).
It should be noted that hTERT promoter mutations were
initially described in familial melanoma and subsequently, in
sporadic melanoma (79). Notably,
a previous study identified hTERT promoter mutations in the
early stages of melanoma. A total of 77% of areas of intermediate
lesions and melanomas in situ harbored hTERT promoter
mutations. This finding indicates that these mutations are selected
at an unexpectedly early stage of the neoplastic progression
(80).
Of note, telomerase activity has been reported to
be higher in other non-malignant conditions, for instance,
psoriatic lesioned skin, UV-damaged skin and poison ivy dermatitis
(81).
Head and neck
Among head and neck squamous carcinomas, hTERT
promoter mutations are frequent in cases which are derived from the
oral cavity (82). As regards
pre-invasive lesions, immunohistochemical analyses have
demonstrated that oral epithelial dysplasia exhibit an increased
hTERT expression compared to normal mucosa cells, while in oral
SCC, the immunohistochemical expression of the protein has been
found to be higher than in the dysplastic and normal tissue
(83). Additionally, a sub-type of
oral leucoplakia featuring ortho-keratotic dysplasia has been shown
to exhibit a shorter TL than SCC in situ and the normal
epithelium (84). Oral submucous
fibrosis (OSMF) is a potential precursor of oral SCC. A study
comparing telomerase activation (hTERT expression) between normal
mucosa, OSMF and oral SCC found an increased enzyme activity in the
latter two conditions. Finally, hTERT levels increased with the
histological grading of the SCCs, which indicates that telomerase
reactivation is critical during the malignant transformation of
OSMF to SCC (85).
Thyroid gland
The presence of hotspot hTERT mutations in
malignant thyroid tumors has been found to be associated with a
worse prognosis and a poor response to treatment (86-89).
Apart from hTERT promoter mutations, epigenetic alterations,
hTERT gene copy number variations and alternative splicing
are implicated in the pathogenesis of thyroid malignancies
(90). Several studies have
investigated premalignant and benign thyroid nodules as controls
for identifying hTERT promoter mutations. The vast majority
of the samples have not been found to harbor hTERT mutations
(91-97).
A hotspot hTERT promoter C228T mutation was
described in a case report of a 68-year-old female with a thyroid
follicular adenoma (98). It was
considered that hTERT promoter mutations comprised a
potential early genetic event in the pathogenesis of follicular
thyroid carcinoma (98). Moreover,
a study including primary tumors from 58 patients with follicular
adenoma, 18 with atypical follicular adenoma with uncertain
malignant potential, 52 with follicular carcinoma and 20 negative
controls from non-tumorous thyroids lesions revealed hTERT
promoter hotspot mutations in one follicular adenoma (C228T), three
atypical follicular adenomas (all C228T), nine follicular
carcinomas (8 C228T and 1 C250T) and in none of the negative
controls (99). The lesions that
presented the mutations also tested positive for hTERT mRNA
and telomerase activity. The C228T mutation was associated with
NRAS gene mutations (P=0.16), the most common mutations in
thyroid nodules. The TL was also examined in the follicular adenoma
and atypical follicular adenoma specimens; however, no significant
difference between the hTERT promoter mutation positive and
negative was found (99). Another
study examined the frequency of hTERT promoter mutations in
34 well-differentiated thyroid carcinomas, 29 follicular adenomas
and 33 sporadic adenomas. hTERT promoter mutations were
found in 6 patients with adenoma, although no hTERT promoter
mutations were detected in the sporadic adenoma group (100).
As regards hTERT expression, a study found that 12
out of 33 follicular adenomas and 4 out of 31 multinodular goiters
were positive for hTERT expression. The difference between them was
significant (P=0.03); however, no significant difference was found
between follicular adenomas and carcinomas (101). Of note, in another study, a
positive hTERT mRNA expression among adenomas was associated
with lymphocytic infiltration and thyroiditis rather than a worse
prognosis, and it was suggested that since lymphocytes express
hTERT, lymphocytic infiltration of the examined tissue may
influence hTERT expression analysis (102).
A comprehensive investigation of
hTERT-related divergence, namely mRNA expression, promoter
mutations, promoter hypermethylation and gene copy number
alterations, was explored in a study including 43 follicular
adenomas and 33 follicular tumors of uncertain malignant potential
(FT-UMP). hTERT mRNA was expressed in 6/43 (14%) adenomas
and 9/23 (39%) of FT-UMPs (P=0.020). No hTERT promoter
mutations were found in Fas, while 6/32 (19%) FT-UMPs were positive
(P=0.005). No difference in median mutation frequency was observed
between the FT-UMPs and follicular carcinomas (P=0.858). The
promoter methylation intensity was higher in follicular carcinomas
(13%) and FT-UMPs (11%) compared to FA (8%) (P<0.001 and
P=0.045). hTERT gene copy number exhibited variations (gain
or loss) in 5/19 FT-UMPs, similar to 11/77 follicular carcinomas
(103).
4. Overview
Numerous studies have investigated the role of
telomeres and telomerase during the development of pre-neoplasia
and progression to cancer across a variety of tissues. The
literature review revealed that the pre-neoplastic lesions and
pre-invasive neoplasms generally express higher levels of hTERT
compared to healthy tissues, as indicated by immunohistochemical
and PCR-based studies of clinical samples. Certain studies have
reported an association between an increased hTERT expression with
the histopathological progression of the pre-neoplastic lesion to
cancer (47,52); however, this finding is not
consistent in all studies; other studies have reported similar
expression levels between pre-neoplastic lesions and corresponding
carcinomas (26,27). As regards the occurrence of
hTERT promoter mutations, which is a critical mechanism of
telomerase reactivation in cancer cells, it appears that their role
is crucial in certain pre-neoplastic lesions (and corresponding
cancers), e.g., in liver tissue, CNS, urothelium and thyroid gland.
TL is another parameter that appears to be tissue-dependent; in
certain cases, the pre-neoplastic lesions are associated with
shorter telomeres, e.g. in colorectal, pancreatic and biliary tract
pre-invasive lesions (29,33,35),
although in a few cases, such as in CNS lesions, longer telomeres
have been observed (3). It should
be noted that mutations and common variants that lead to longer
telomeres have been associated with increased risk of melanoma and
lung adenocarcinoma (104,105).
Therefore, it appears that whether short or long telomeres
predispose to cancer remains ambiguous and requires further
investigation. Other alterations have been reported in
pre-neoplastic lesions, including those affecting the additional
proteins that comprise the telomerase holoenzyme complex.
Notably, the role of hTERT during carcinogenesis
appears to be tissue-dependent and may reflect the differential
dependence of tissue homeostasis to progenitor cell capacity
(106). In particular,
hTERT promoter mutations are frequently detected in specific
types of cancers and not in other (107). It has been suggested that the
occurrence of hTERT promoter mutations is essential in the
early steps of cancer development in tissues with limited
replicative potential, which do not continually self-renew, such as
the nervous system and liver (107). Another explanation may be that
these mutations can also result from environmental factors, such as
ultraviolet radiation and chemical carcinogens, as suggested by
their high frequency in melanoma and bladder cancer (97). The literature review revealed that
this observation is also relevant in the case of pre-neoplastic
lesions of the corresponding neoplasms.
Finally, although hTERT is the rate-limiting
component of telomerase expression, whether hTERT expression
translates directly to active telomerase activity remains unclear.
Given that a large amount of research on this topic, mainly in
older studies, is based on the investigation of hTERT expression,
the results need to be interpreted with caution (2). Future research is required to
incorporate novel fields, such as computational investigation, to
recapitulate telomerase's function in different settings.
It has been proposed that the existence of
hTERT promoter mutations in an intermediate pre-invasive
phenotype, at least in some tumors, may translate into the
development of a potentially useful diagnostic biomarker (8). A recent study reported that
hTERT promoter mutations could be detected in urine samples
up to 10 years prior to the diagnosis of bladder cancer, while they
were not present among matched controls that did not develop cancer
(108). Such findings indicate a
slow tumorigenic process in certain cases, which could provide a
window of opportunity for early molecular detection and
intervention (108).
Nevertheless, it should be noted that the timing and method of
intervention in the pre-invasive phenotypes remain to be determined
and require carefully designed randomized trials.
5. Conclusions
Pre-neoplastic lesions across different tissues are
associated with the increased expression of hTERT, abnormal
TL and the occurrence of hTERT mutations, indicating the
critical involvement of telomerase reactivation during
carcinogenesis. The timing and relevant importance of telomerase
reactivation appear to be tissue-dependent and may be associated
with the differential self-renewing features of the homeostasis of
each tissue. Future research is required to focus on elucidating
the role of telomerase activation in pre-neoplasia in order to
address its diagnostic and therapeutic potential.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
EKa, AK and AA performed the literature review and
wrote the original manuscript. EKa, AK, AA, EKo and GG wrote the
revised manuscript and prepared the figure. EKo and GG supervised
the work. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shay JW and Wright WE: Telomeres and
telomerase: Three decades of progress. Nat Rev Genet. 20:299–309.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roake CM and Artandi SE: Regulation of
human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol.
21:384–397. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barthel FP, Wei W, Tang M,
Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang
Q, et al: Systematic analysis of telomere length and somatic
alterations in 31 cancer types. Nat Genet. 49:349–357.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumour suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cukusić A, Skrobot Vidacek N, Sopta M and
Rubelj I: Telomerase regulation at the crossroads of cell fate.
Cytogenet Genome Res. 122:263–272. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pestana A, Vinagre J, Sobrinho-Simões M
and Soares P: TERT biology and function in cancer: Beyond
immortalisation. J Mol Endocrinol. 58:R129–R146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colebatch AJ, Dobrovic A and Cooper WA:
TERT gene: Its function and dysregulation in cancer. J Clin Pathol.
72:281–284. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin
L and Garraway LA: Highly recurrent TERT promoter mutations in
human melanoma. Science. 339:957–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peifer M, Hertwig F, Roels F, Dreidax D,
Gartlgruber M, Menon R, Krämer A, Roncaioli JL, Sand F, Heuckmann
JM, et al: Telomerase activation by genomic rearrangements in
high-risk neuroblastoma. Nature. 526:700–704. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lewis KA and Tollefsbol TO: Regulation of
the telomerase reverse transcriptase subunit through epigenetic
mechanisms. Front Genet. 7(83)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dogan F and Forsyth NR: Telomerase
regulation: A role for epigenetics. Cancers (Basel).
13(1213)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee DD, Leão R, Komosa M, Gallo M, Zhang
CH, Lipman T, Remke M, Heidari A, Nunes NM, Apolónio JD, et al: DNA
hypermethylation within TERT promoter upregulates TERT expression
in cancer. J Clin Invest. 129:223–229. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Renaud S, Loukinov D, Bosman FT,
Lobanenkov V and Benhattar J: CTCF binds the proximal exonic region
of hTERT and inhibits its transcription. Nucleic Acids Res.
33:6850–6860. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li C, Liang Y, Wu M, Xu L and Cai W:
Telomerase activity analysis of esophageal carcinoma using
microdissection-TRAP assay. Chin Med J (Engl). 115:1405–1408.
2002.PubMed/NCBI
|
|
16
|
Yu HP, Xu SQ, Lu WH, Li YY, Li F, Wang XL
and Su YH: Telomerase activity and expression of telomerase genes
in squamous dysplasia and squamous cell carcinoma of the esophagus.
J Surg Oncol. 86:99–104. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koyanagi K, Ozawa S, Ando N, Mukai M,
Kitagawa Y, Ueda M and Kitajima M: Telomerase activity as an
indicator of malignant potential in iodine-nonreactive lesions of
the esophagus. Cancer. 88:1524–1529. 2000.PubMed/NCBI
|
|
18
|
Inai M, Kano M, Shimada Y, Sakurai T,
Chiba T and Imamura M: Telomerase activity of the Lugol-stained and
-unstained squamous epithelia in the process of oesophageal
carcinogenesis. Br J Cancer. 85:1006–1013. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Clément G, Braunschweig R, Pasquier N,
Bosman FT and Benhattar J: Methylation of APC, TIMP3, and TERT: A
new predictive marker to distinguish Barrett's oesophagus patients
at risk for malignant transformation. J Pathol. 208:100–107.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Suzuki K, Kashimura H, Ohkawa J, Itabashi
M, Watanabe T, Sawahata T, Nakahara A, Muto H and Tanaka N:
Expression of human telomerase catalytic subunit gene in cancerous
and precancerous gastric conditions. J Gastroenterol Hepatol.
15:744–751. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Z, Xu J, Geng X and Zhang W: Analysis
of DNA methylation status of the promoter of human telomerase
reverse transcriptase in gastric carcinogenesis. Arch Med Res.
41:1–6. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Duarte MC, Babeto E, Leite KR, Miyazaki K,
Borim AA, Rahal P and Silva AE: Expression of TERT in precancerous
gastric lesions compared to gastric cancer. Braz J Med Biol Res.
44:100–104. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Silva TC, Leal MF, Calcagno DQ, de Souza
CR, Khayat AS, dos Santos NP, Montenegro RC, Rabenhorst SH,
Nascimento MQ, Assumpção PP, et al: hTERT, MYC and TP53
deregulation in gastric preneoplastic lesions. BMC Gastroenterol.
12(85)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu H, Zhang Y, Zou M, Yang S and Liang XQ:
Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is
associated with telomere shortening and may contribute to
multistage carcinogenesis of gastric cancer. J Cancer Res Clin
Oncol. 136:1407–1414. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kanamaru T, Tanaka K, Kotani J, Ueno K,
Yamamoto M, Idei Y, Hisatomi H and Takeyama Y: Telomerase activity
and hTERT mRNA in development and progression of adenoma to
colorectal cancer. Int J Mol Med. 10:205–210. 2002.PubMed/NCBI
|
|
26
|
Choi JY, Yoon H, Na G, Choi YJ, Shin CM,
Park YS, Kim N and Lee DH: Evaluation of the expression of the
inhibitor of apoptosis protein family and human telomerase reverse
transcriptase in patients with advanced colorectal adenoma. J
Cancer Prev. 22:98–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Saleh S, Lam AK and Ho YH: Real-time PCR
quantification of human telomerase reverse transcriptase (hTERT) in
colorectal cancer. Pathology. 40:25–30. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Riegert-Johnson DL, Boardman LA, Crook JE,
Thomas CS, Johnson RA and Roberts ME: Shorter peripheral blood
telomeres are a potential biomarker for patients with advanced
colorectal adenomas. Int J Biol Markers. 27:e375–e380.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Roger L, Jones RE, Heppel NH, Williams GT,
Sampson JR and Baird DM: Extensive telomere erosion in the
initiation of colorectal adenomas and its association with
chromosomal instability. J Natl Cancer Inst. 105:1202–1211.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hardikar S, Burnett-Hartman AN, Phipps AI,
Upton MP, Zhu LC and Newcomb PA: Telomere length differences
between colorectal polyp subtypes: A colonoscopy-based case-control
study. BMC Cancer. 18(513)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nault JC, Calderaro J, Di Tommaso L,
Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M and Zucman-Rossi
J: Telomerase reverse transcriptase promoter mutation is an early
somatic genetic alteration in the transformation of premalignant
nodules in hepatocellular carcinoma on cirrhosis. Hepatology.
60:1983–1992. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nault JC, Mallet M, Pilati C, Calderaro J,
Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C and
Zucman-Rossi J: High frequency of telomerase reverse-transcriptase
promoter somatic mutations in hepatocellular carcinoma and
preneoplastic lesions. Nat Commun. 4(2218)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Matsuda Y, Ishiwata T, Izumiyama-Shimomura
N, Hamayasu H, Fujiwara M, Tomita K, Hiraishi N, Nakamura K,
Ishikawa N, Aida J, et al: Gradual telomere shortening and
increasing chromosomal instability among PanIN grades and normal
ductal epithelia with and without cancer in the pancreas. PLoS One.
10(e0117575)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shimonishi T, Sasaki M and Nakanuma Y:
Precancerous lesions of intrahepatic cholangiocarcinoma. J
Hepatobiliary Pancreat Surg. 7:542–550. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hansel DE, Meeker AK, Hicks J, De Marzo
AM, Lillemoe KD, Schulick R, Hruban RH, Maitra A and Argani P:
Telomere length variation in biliary tract metaplasia, dysplasia,
and carcinoma. Mod Pathol. 19:772–779. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aoki Y, Aida J, Kawano Y, Nakamura KI,
Izumiyama-Shimomura N, Ishikawa N, Arai T, Nakamura Y, Taniai N,
Uchida E, et al: Telomere length of gallbladder epithelium is
shortened in patients with congenital biliary dilatation:
Measurement by quantitative fluorescence in situ hybridization. J
Gastroenterol. 53:291–301. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ichikawa Y, Kamiyama M, Sekido H, Ishikawa
T, Miura Y, Kamiya N, Morita T and Shimada H: Telomerase activity
and Bcl-2 expression in gallbladders of pancreaticobiliary
maljunction patients: A preliminary study. J Hepatobiliary Pancreat
Surg. 11:34–39. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ishizumi T, McWilliams A, MacAulay C,
Gazdar A and Lam S: Natural history of bronchial preinvasive
lesions. Cancer Metastasis Rev. 29:5–14. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Greenberg AK, Yee H and Rom WN:
Preneoplastic lesions of the lung. Respir Res. 3(20)2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lantuejoul S, Soria JC, Morat L, Lorimier
P, Moro-Sibilot D, Sabatier L, Brambilla C and Brambilla E:
Telomere shortening and telomerase reverse transcriptase expression
in preinvasive bronchial lesions. Clin Cancer Res. 11:2074–2082.
2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lantuejoul S, Raynaud C, Salameire D,
Gazzeri S, Moro-Sibilot D, Soria JC, Brambilla C and Brambilla E:
Telomere maintenance and DNA damage responses during lung
carcinogenesis. Clin Cancer Res. 16:2979–2988. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kang JU, Koo SH, Kwon KC, Park JW and Kim
JM: Gain at chromosomal region 5p15.33, containing TERT, is the
most frequent genetic event in early stages of non-small cell lung
cancer. Cancer Genet Cytogenet. 182:1–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Raynaud CM, Hernandez J, Llorca FP,
Nuciforo P, Mathieu MC, Commo F, Delaloge S, Sabatier L, André F
and Soria JC: DNA damage repair and telomere length in normal
breast, preneoplastic lesions, and invasive cancer. Am J Clin
Oncol. 33:341–345. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Heaphy CM, Bisoffi M, Joste NE,
Baumgartner KB, Baumgartner RN and Griffith JK: Genomic instability
demonstrates similarity between DCIS and invasive carcinomas.
Breast Cancer Res Treat. 117:17–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Simícková M, Nekulová M, Pecen L, Cernoch
M, Vagundová M and Pacovský Z: Quantitative determination of
telomerase activity in breast cancer and benign breast diseases.
Neoplasma. 48:267–273. 2001.PubMed/NCBI
|
|
46
|
Nagai N, Oshita T, Murakami J and Ohama K:
Semiquantitative analysis of telomerase activity in cervical cancer
and precancerous lesions. Oncol Rep. 6:325–328. 1999.PubMed/NCBI
|
|
47
|
Liu Y, Fan P, Yang Y, Xu C, Huang Y, Li D,
Qing Q, Sun C and Zhou H: Human papillomavirus and human telomerase
RNA component gene in cervical cancer progression. Sci Rep.
9(15926)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhu Y, Han Y, Tian T, Su P, Jin G, Chen J
and Cao Y: MiR-21-5p, miR-34a, and human telomerase RNA component
as surrogate markers for cervical cancer progression. Pathol Res
Pract. 214:374–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ravaioli S, Tumedei MM, Amadori A,
Puccetti M, Chiadini E and Bravaccini S: Role of telomerase in
cervical lesions as prognostic marker: A comparison between
immunohistochemistry and fluorescence in situ hybridization. J Low
Genit Tract Dis. 21:42–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao XY, Cui Y, Jiang SF, Liu KJ, Han HQ,
Liu XS and Li Y: Human telomerase gene and high-risk human
papillomavirus infection are related to cervical intraepithelial
neoplasia. Asian Pac J Cancer Prev. 16:693–697. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
He H, Pan Q, Pan J, Chen Y and Cao L:
Study on the correlation between hTREC and HPV load and cervical
CINI/II/III lesions and cervical cancer. J Clin Lab Anal.
34(e23257)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang SZ, Sun JH, Zhang W, Jin SQ, Wang HP,
Jin YS, Qu P, Liu Y and Li M: Telomerase activity in cervical
intraepithelial neoplasia. Chin Med J (Engl). 117:202–206.
2004.PubMed/NCBI
|
|
53
|
Van Doorslaer K and Burk RD: Association
between hTERT activation by HPV E6 proteins and oncogenic risk.
Virology. 433:216–219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ji W, Lou W, Hong Z, Qiu L and Di W:
Genomic amplification of HPV, h-TERC and c-MYC in liquid-based
cytological specimens for screening of cervical intraepithelial
neoplasia and cancer. Oncol Lett. 17:2099–2106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Alnafakh RAA, Adishesh M, Button L,
Saretzki G and Hapangama DK: Telomerase and telomeres in
endometrial cancer. Front Oncol. 9(344)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kyo S, Kanaya T, Takakura M, Tanaka M and
Inoue M: Human telomerase reverse transcriptase as a critical
determinant of telomerase activity in normal and malignant
endometrial tissues. Int J Cancer. 80:60–63. 1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Maida Y, Kyo S, Forsyth NR, Takakura M,
Sakaguchi J, Mizumoto Y, Hashimoto M, Nakamura M, Nakao S and Inoue
M: Distinct telomere length regulation in premalignant cervical and
endometrial lesions: Implications for the roles of telomeres in
uterine carcinogenesis. J Pathol. 210:214–223. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Danescu A, Herrero Gonzalez S, Di
Cristofano A, Mai S and Hombach-Klonisch S: Three-dimensional
nuclear telomere architecture changes during endometrial carcinoma
development. Genes Chromosomes Cancer. 52:716–732. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Datar RH, Naritoku WY, Li P, Tsao-Wei D,
Groshen S, Taylor CR and Imam SA: Analysis of telomerase activity
in ovarian cystadenomas, low-malignant-potential tumors, and
invasive carcinomas. Gynecol Oncol. 74:338–345. 1999.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sun PM, Wei LH, Luo MY, Liu G, Wang JL,
Mustea A, Könsgen D, Lichtenegger W and Sehouli J: The telomerase
activity and expression of hTERT gene can serve as indicators in
the anti-cancer treatment of human ovarian cancer. Eur J Obstet
Gynecol Reprod Biol. 130:249–257. 2007.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pilsworth JA, Cochrane DR, Xia Z, Aubert
G, Färkkilä AEM, Horlings HM, Yanagida S, Yang W, Lim JLP, Wang YK,
et al: TERT promoter mutation in adult granulosa cell tumor of the
ovary. Mod Pathol. 31:1107–1115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gunes C, Wezel F, Southgate J and Bolenz
C: Implications of TERT promoter mutations and telomerase activity
in urothelial carcinogenesis. Nat Rev Urol. 15:386–393.
2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cheng L, Montironi R and Lopez-Beltran A:
TERT promoter mutations occur frequently in urothelial papilloma
and papillary urothelial neoplasm of low malignant potential. Eur
Urol. 71:497–498. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cheng L, Davidson DD, Wang M,
Lopez-Beltran A, Montironi R, Wang L, Tan PH, MacLennan GT,
Williamson SR and Zhang S: Telomerase reverse transcriptase (TERT)
promoter mutation analysis of benign, malignant and reactive
urothelial lesions reveals a subpopulation of inverted papilloma
with immortalizing genetic change. Histopathology. 69:107–113.
2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Isharwal S, Hu W, Sarungbam J, Chen YB,
Gopalan A, Fine SW, Tickoo SK, Sirintrapun SJ, Jadallah S, Loo FL,
et al: Genomic landscape of inverted urothelial papilloma and
urothelial papilloma of the bladder. J Pathol. 248:260–265.
2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rodriguez Pena MDC, Tregnago AC, Eich ML,
Springer S, Wang Y, Taheri D, Ertoy D, Fujita K, Bezerra SM, Cunha
IW, et al: Spectrum of genetic mutations in de novo PUNLMP of the
urinary bladder. Virchows Arch. 471:761–767. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Taylor AS, Newell B, Chinnaiyan AM, Hafez
KS, Weizer AZ, Spratt DE, Cameron AP, Al-Ahmadie HA, Gupta S,
Montgomery JS, et al: TERT promoter mutations in keratinizing and
nonkeratinizing squamous metaplasia of the urinary tract. Eur Urol
Open Sci. 35:74–78. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Weyerer V, Eckstein M, Strissel PL,
Wullweber A, Lange F, Tögel L, Geppert CI, Sikic D, Taubert H, Wach
S, et al: TERT promoter mutation analysis of whole-organ mapping
bladder cancers. Genes. 12(230)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Tu L, Huda N, Grimes BR, Slee RB, Bates
AM, Cheng L and Gilley D: Widespread telomere instability in
prostatic lesions. Mol Carcinog. 55:842–852. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Graham MK and Meeker A: Telomeres and
telomerase in prostate cancer development and therapy. Nat Rev
Urol. 14:607–619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cheng L, Montironi R, Davidson DD, Wang M,
Lopez-Beltran A and Zhang S: Molecular evidence supporting the
precursor nature of atypical adenomatous hyperplasia of the
prostate. Mol Carcinog. 58:1272–1278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rybicki BA, Sadasivan SM, Chen Y, Loveless
I, Gupta NS, Chitale DA, Williamson SR, Rundle AG and Tang DL: Race
differences in telomere length in benign prostate biopsies and
subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers
Prev. 31:991–998. 2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Patel B, Taiwo R, Kim AH and Dunn GP:
TERT, a promoter of CNS malignancies. Neurooncol Adv.
2(vdaa025)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Walsh KM, Wiencke JK, Lachance DH, Wiemels
JL, Molinaro AM, Eckel-Passow JE, Jenkins RB and Wrensch MR:
Telomere maintenance and the etiology of adult glioma. Neuro Oncol.
17:1445–1452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Srinivas N, Neittaanmäki N, Heidenreich B,
Rachakonda S, Karppinen TT, Grönroos M, Tani TT, Salmivuori M,
Snellman E, Hemminki K and Kumar R: TERT promoter mutations in
actinic keratosis before and after treatment. Int J Cancer.
146:2932–2934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ventura A, Pellegrini C, Cardelli L, Rocco
T, Ciciarelli V, Peris K and Fargnoli MC: Telomeres and telomerase
in cutaneous squamous cell carcinoma. Int J Mol Sci.
20(1333)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Fullen DR, Zhu W, Thomas D and Su LD:
hTERT expression in melanocytic lesions: An immunohistochemical
study on paraffin-embedded tissue. J Cutan Pathol. 32:680–684.
2005.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Miracco C, Pacenti L, Santopietro R,
Laurini L, Biagioli M and Luzi P: Evaluation of telomerase activity
in cutaneous melanocytic proliferations. Hum Pathol. 31:1018–1021.
2000.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Shain AH, Yeh I, Kovalyshyn I, Sriharan A,
Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, et al:
The genetic evolution of melanoma from precursor lesions. N Engl J
Med. 373:1926–1936. 2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Taylor RS, Ramirez RD, Ogoshi M, Chaffins
M, Piatyszek MA and Shay JW: Detection of telomerase activity in
malignant and nonmalignant skin conditions. J Invest Dermatol.
106:759–765. 1996.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Chang KP, Wang CI, Pickering CR, Huang Y,
Tsai CN, Tsang NM, Kao HK, Cheng MH and Myers JN: Prevalence of
promoter mutations in the TERT gene in oral cavity squamous cell
carcinoma. Head Neck. 39:1131–1137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Raghunandan BN, Sanjai K, Kumaraswamy J,
Papaiah L, Pandey B and Jyothi BM: Expression of human telomerase
reverse transcriptase protein in oral epithelial dysplasia and oral
squamous cell carcinoma: An immunohistochemical study. J Oral
Maxillofac Pathol. 20:96–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Aida J, Kobayashi T, Saku T, Yamaguchi M,
Shimomura N, Nakamura K, Ishikawa N, Maruyama S, Cheng J, Poon SS,
et al: Short telomeres in an oral precancerous lesion: Q-FISH
analysis of leukoplakia. J Oral Pathol Med. 41:372–378.
2012.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Raju KL, Haragannavar VC, Patil S, Rao RS,
Nagaraj T, Augustine D, Venkatesiah SS and Nambiar S: Expression of
hTERT in oral submucous fibrosis and oral squamous cell
carcinoma-an immunohistochemical analysis. Pathol Oncol Res.
26:1573–1582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
George JR, Henderson YC, Williams MD,
Roberts DB, Hei H, Lai SY and Clayman GL: Association of TERT
promoter mutation, but not BRAF mutation, with increased mortality
in PTC. J Clin Endocrinol Metab. 100:E1550–E1559. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Landa I, Ganly I, Chan TA, Mitsutake N,
Matsuse M, Ibrahimpasic T, Ghossein RA and Fagin JA: Frequent
somatic TERT promoter mutations in thyroid cancer: Higher
prevalence in advanced forms of the disease. J Clin Endocrinol
Metab. 98:E1562–E1566. 2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Liu R and Xing M: Diagnostic and
prognostic TERT promoter mutations in thyroid fine-needle
aspiration biopsy. Endocr Relat Cancer. 21:825–830. 2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Liu T, Wang N, Cao J, Sofiadis A, Dinets
A, Zedenius J, Larsson C and Xu D: The age- and shorter
telomere-dependent TERT promoter mutation in follicular thyroid
cell-derived carcinomas. Oncogene. 33:4978–4984. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
McKelvey BA, Umbricht CB and Zeiger MA:
Telomerase reverse transcriptase (TERT) regulation in thyroid
cancer: A Review. Front Endocrinol (Lausanne).
11(485)2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G,
Murugan AK, Guan H, Yu H, Wang Y, et al: TERT promoter mutations
and their association with BRAF V600E mutation and aggressive
clinicopathological characteristics of thyroid cancer. J Clin
Endocrinol Metab. 99:E1130–E1136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Nikiforov YE, Carty SE, Chiosea SI, Coyne
C, Duvvuri U, Ferris RL, Gooding WE, Hodak SP, LeBeau SO, Ohori NP,
et al: Highly accurate diagnosis of cancer in thyroid nodules with
follicular neoplasm/suspicious for a follicular neoplasm cytology
by ThyroSeq v2 next-generation sequencing assay. Cancer.
120:3627–3634. 2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Qasem E, Murugan AK, Al-Hindi H, Xing M,
Almohanna M, Alswailem M and Alzahrani AS: TERT promoter mutations
in thyroid cancer: A report from a Middle Eastern population.
Endocr Relat Cancer. 22:901–908. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Su JJ, Hui LZ, Xi CJ and Su GQ:
Correlation analysis of ultrasonic characteristics, pathological
type, and molecular markers of thyroid nodules. Genet Mol Res.
14:9–20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Suh YJ, Kwon MJ, Noh HM, Lee HK, Ra YJ and
Kim NY: Limited clinical and diagnostic utility of circulating
tumor DNA detection in patients with early-stage
well-differentiated thyroid cancer: Comparison with benign thyroid
nodules and healthy individuals. Healthcare (Basel).
9(386)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kachko VA, Vanushko VE, Platonova NM,
Abrosimov AY and Mel'nichenko GA: Somatic mutations in the BRAF,
KRAS, NRAS, EIF1AX, and TERT genes: Diagnostic value in thyroid
neoplasms. Bull Exp Biol Med. 169:669–672. 2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4(2185)2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Topf MC, Wang ZX, Tuluc M and Pribitkin
EA: TERT, HRAS, and EIF1AX mutations in a patient with follicular
adenoma. Thyroid. 28:815–817. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wang N, Liu T, Sofiadis A, Juhlin CC,
Zedenius J, Höög A, Larsson C and Xu D: TERT promoter mutation as
an early genetic event activating telomerase in follicular thyroid
adenoma (FTA) and atypical FTA. Cancer. 120:2965–2979.
2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Boaventura P, Batista R, Pestana A, Reis
M, Mendes A, Eloy C, Sobrinho-Simões M and Soares P: TERT promoter
mutations: A genetic signature of benign and malignant thyroid
tumours occurring in the context of tinea capitis irradiation. Eur
J Endocrinol. 176:49–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Sayiner A and Suren D: Expression of human
telomerase reverse transcriptase (hTERT) in thyroid neoplasms. J
BUON. 23:229–233. 2018.PubMed/NCBI
|
|
102
|
Pestana A, Batista R, Celestino R, Canberk
S, Sobrinho-Simões M and Soares P: Comprehensive assessment of TERT
mRNA expression across a large cohort of benign and malignant
thyroid tumours. Cancers (Basel). 12(1846)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Paulsson JO, Mu N, Shabo I, Wang N,
Zedenius J, Larsson C and Juhlin CC: TERT aberrancies: A screening
tool for malignancy in follicular thyroid tumours. Endocr Relat
Cancer. 25:723–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Seow WJ, Cawthon RM, Purdue MP, Hu W, Gao
YT, Huang WY, Weinstein SJ, Ji BT, Virtamo J, Hosgood HD III, et
al: Telomere length in white blood cell DNA and lung cancer: A
pooled analysis of three prospective cohorts. Cancer Res.
74:4090–4098. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
McNally EJ, Luncsford PJ and Armanios M:
Long telomeres and cancer risk: The price of cellular immortality.
J Clin Invest. 129:3474–3481. 2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Gomatou G, Masaoutis C, Vamvakaris I,
Kotteas E, Bouros E, Tzilas V and Bouros D: Differential
immunohistochemical expression of hTERT in lung cancer patients
with and without idiopathic pulmonary fibrosis. Pulmonology:
S2531-0437(22)00002-2, 2022 (Epub ahead of print).
|
|
107
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026.
2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Hosen MI, Sheikh M, Zvereva M, Scelo G,
Forey N, Durand G, Voegele C, Poustchi H, Khoshnia M, Roshandel G,
et al: Urinary TERT promoter mutations are detectable up to 10
years prior to clinical diagnosis of bladder cancer: Evidence from
the Golestan cohort study. EBioMedicine. 53(102643)2020.PubMed/NCBI View Article : Google Scholar
|