Introduction
Liver cancer is one of the most common types of
cancer worldwide, with the highest incidence rates reported in Asia
and Africa. Hepatocellular carcinoma (HCC) mainly includes
hepatocellular liver cancer, cholangiocarcinoma and mixed cell
carcinoma (1). Of these, HCC
accounts for 75-85% of all liver cancers (2). The annual worldwide incidence of HCC
is increasing by 3-9% annually (3). Liver cancer has a poor prognosis and
is the second leading cause of cancer-related deaths (4), with a 5-year overall survival (OS)
rate of <10% (5). Treatment
options for early stage liver cancer include liver resection, radio
frequency ablation (RFA) and liver transplantation. However, due to
its insidious onset, >50% of patients are diagnosed during the
middle or late disease stages of the disease, missing the
opportunity for curative treatment. Consequently, transcatheter
arterial chemoembolization (TACE) has become the first-line
treatment for intermediate and advanced-stage liver cancer
(6).
Survival times among patients receiving TACE exhibit
significant differences due to the heterogeneity of the disease.
This is a challenging task for the duplication or cessation of
space therapy (7-9).
To provide the best individualized treatment to patients with
cancer, it is necessary to identify biomarkers that can effectively
predict the survival outcomes. Currently, alpha-fetoprotein (AFP)
is the most commonly used marker for predicting the onset and
recurrence of liver cancer. However, ~1/3 of patients with liver
cancer are AFP-negative (10).
Various studies have attempted to develop risk prediction models to
predict treatment outcomes, including specially designed nomograms
and post-TACE prognostic scoring systems (11-13).
These models revealed the influence of liver function and the
baseline tumor characteristics on the survival of TACE-treated
patients with liver cancer. Tumor characteristics such as
pathological type, differentiation, tumor size, number of tumors
and vascular invasion are the main indicators for predicting
prognosis (14-16).
Biochemical indicators such as liver function, serum gamma-glutamyl
transferase, serum vascular endothelial growth factor, C-reactive
protein and novel metabolism-related gene have also attracted
significant attention and research (17-19).
However, these biomarkers are insufficient for accurately
predicting prognosis, with reported inaccuracies in 45% of cases
(12,20,21).
Furthermore, the scoring system is complex, and its clinical
application is difficult to promote. Therefore, such biomarkers are
not widely used in clinical practice at present (22).
Serum ferritin (SF) is an iron storage protein
composed of 24 subunits and was first discovered by the French
scientist Laufberger in 1937(23).
SF is the oldest known protein involved in iron metabolism and
plays essential roles in cell proliferation, angiogenesis,
immunosuppression and iron transport (24). Abnormal SF levels have been shown
to be closely associated with tumor progression and poor prognosis
(25). Some studies have suggested
that SF levels may reflect the extent of liver inflammation and
fibrosis, and SF may be a poor prognostic risk factor for survival
and recurrence after percutaneous RFA in patients with HCC
(26). Furthermore, preoperative
SF is an independent prognostic factor for liver cancer after liver
resection (27). Despite this,
there is limited research on the impact of ferritin levels on the
prognosis of patients with HCC, and the current results are
conflicting. In addition, the prognostic value of SF in HCC
patients undergoing TACE is unclear. Therefore, the present study
aimed to investigate the impact of preoperative SF levels on the
survival outcomes of patients with HCC undergoing TACE treatment,
and to determine whether preoperative SF levels can serve as an
independent prognostic biomarker for these patients.
Patients and methods
Patients and study design
Clinical data of 223 patients were collected and
reviewed from the case database of the Mianyang City Center
Hospital (Mianyang, China) between February 2006 and March 2022.
The follow-up time was limited to 50 months. These patients were
diagnosed with unresectable or inoperable liver cancer and
underwent TACE. The inclusion criterion was a diagnosis of
unresectable HCC, which was based on clinical imaging, AFP levels,
medical history, or confirmed histology. Only patients who
underwent TACE as their first-line treatment were included.
Patients with combined HCC with and other tumors, recurrent HCC,
resectable primary HCC, and those with incomplete data were
excluded. The present study was conducted in accordance with the
principles of the Declaration of Helsinki revised in 2013, and was
approved (approval no. S20230320-02) by the Medical Ethics
Committee of Mianyang Central Hospital (Mianyang, China). The data
were analyzed anonymously; thus, informed consent was not obtained
from the participants.
Demographic and clinicopathological characteristics
and biochemical indicators of the included patients were
investigated. Demographic and clinicopathological characteristics
included sex, age, tumor size, number of tumors, cirrhotic
Child-Pugh stage, presence of extrahepatic metastases, tumor
necrosis, vascular invasion and previous treatment history.
Laboratory tests included preoperative SF, preoperative AFP,
alanine aminotransferase, aspartate aminotransferase (AST),
gamma-glutamyl transpeptidase (GGT), albumin, as well as total
bilirubin levels, and biochemical indicators such as the presence
of hepatitis B or hepatitis C viral infection. Clinical staging was
performed using the BCLC system. The frequency of TACE treatment
performed during the follow-up period until the last follow-up date
was also recorded.
OS
OS was defined as the time from the first day of
initial treatment to death. Where a patient was lost to follow-up
or death records was unavailable, the patient was censored.
Survival time in censored patients was defined as the duration from
the commencement of treatment to the last day of follow-up or the
date when their survival status was last confirmed.
Statistical analysis
Categorical variables are presented as counts and
percentages, and comparisons were performed using Pearson's
chi-square or Fisher's exact test. Continuous data are expressed as
the median and range, and were compared using the Mann-Whitney U
test. If the survival time was incomplete, right censoring was used
in the survival analysis. Survival curves were plotted using the
Kaplan-Meier method, and were compared using the log-rank test.
Single- and multi-factor analyses of independent prognostic factors
for OS were performed using the Cox proportional hazards model.
Statistical analysis was performed using the SPSS 26.0 software
(IBM Corp.), and P<0.05 based on a two-tailed test was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The pathological characteristics and laboratory
indicators of the included patients are summarized in Table I. Among the 223 patients, 183
(82.1%) were male, and 162 (72.6%) were aged >50 years. Most
patients (54.7%) underwent a single treatment. The vast majority of
patients either had a solitary tumor (81.6%) or a tumor diameter
>5 cm (65%). A total of 134 patients (60.1%) had cirrhosis, 172
(77.1%) had tumor necrosis, 85 (38.1%) had pathological vascular
invasion and 33 (14.8%) had extrahepatic metastases. Among them,
162 patients (72.6%) belonged to Child-Pugh class A, 151 (67.7%)
were classified as BCLC stage B, and 55 (24.7%) were designated as
BCLC stage C.
 | Table IBaseline demographic and
clinicopathological characteristics of patients. |
Table I
Baseline demographic and
clinicopathological characteristics of patients.
| Clinicopathological
characteristics | Number (total
n=223) | Percentage (%) |
|---|
| Age, years | | |
|
≤50 | 61 | 27.4 |
|
>50 | 162 | 72.6 |
| Sex | | |
|
Male | 183 | 82.1 |
|
Female | 40 | 17.9 |
| TACE frequency | | |
|
1/2/3/4/5/6/7/8/9 |
122/39/27/16/4/5/6/1/2 |
54.7/17.5/12.1/7.2/1.8/2.2/2.7/0.4/0.9 |
| Serum ferritin
(ng/ml) | | |
|
≤274 | 120 | 53.8 |
|
>274 | 103 | 46.2 |
| HBV infection | | |
|
Absent | 65 | 29.1 |
|
Present | 158 | 70.9 |
| HCV infection | | |
|
Absent | 206 | 92.4 |
|
Present | 17 | 7.6 |
| ALT (IU/l) | | |
|
≤50 | 119 | 53.4 |
|
>50 | 104 | 46.6 |
| AST (IU/l) | | |
|
≤40 | 45 | 20.2 |
|
>40 | 180 | 79.8 |
| GGT (IU/l) | | |
|
≤60 | 43 | 19.3 |
|
>60 | 180 | 80.7 |
| AFP (ng/ml) | | |
|
≤400 | 148 | 66.4 |
|
>400 | 75 | 33.6 |
| Total bilirubin
(µmol/l) | | |
|
≤26 | 16 | 72.2 |
|
>26 | 62 | 27.8 |
| Albumin (g/l) | | |
|
≤35 | 64 | 28.7 |
|
>35 | 159 | 71.3 |
| Cirrhosis | | |
|
Absent | 89 | 39.9 |
|
Present | 134 | 60.1 |
| Tumor size
(cm) | | |
|
≤5 | 78 | 35 |
|
>5 | 145 | 65 |
| Tumor necrosis | | |
|
Absent | 51 | 22.9 |
|
Present | 172 | 77.1 |
| Tumor number | | |
|
Single | 182 | 81.6 |
|
Multiple | 41 | 18.4 |
| Extrahepatic
metastases | | |
|
Absent | 190 | 85.2 |
|
Present | 33 | 14.8 |
| Vascular
invasion | | |
|
Absent | 138 | 61.9 |
|
Present | 85 | 38.1 |
| Child-Pugh
class | | |
|
A | 162 | 72.6 |
|
B | 60 | 26.9 |
|
C | 1 | 0.4 |
| BCLC stage | | |
|
0 | 4 | 1.8 |
|
A | 12 | 5.4 |
|
B | 151 | 67.7 |
|
C | 55 | 24.7 |
|
D | 1 | 0.4 |
Correlation between SF and
clinicopathological variables
According to the upper limit of the normal reference
value for SF, the 223 patients were divided into the low (SF ≤274
ng/ml) and high SF (SF >274 ng/ml) groups. Next, the
relationship between preoperative SF levels and clinicopathological
parameters was studied. As demonstrated in Table II, some factors were associated
with SF. Specifically, HBV infection, AST, ALT and GGT were
significantly correlated with preoperative SF levels, while other
laboratory indicators were not. Additionally, there was a
discernible correlation between preoperative SF levels and sex,
cirrhosis and tumor number. Details of the relationship between
clinicopathological variables and preoperative SF levels are
summarized in Table II.
 | Table IIAssociation of preoperative SF level
with clinicopathological parameters. |
Table II
Association of preoperative SF level
with clinicopathological parameters.
| | Level of
preoperative SF, number (percentage %) | |
|---|
| Clinicopathological
characteristics | Low SF Group (≤274
ng/ml) (n=120) | High SF Group
(>274 ng/ml) (n=103) | P-value |
|---|
| Age, years | | | 1.000 |
|
≤50 | 91 (75.8%) | 78 (75.7%) | |
|
>50 | 35 (24.1%) | 29 (24.3%) | |
| Sex | | | 0.001 |
|
Male | 89 (74.2%) | 94 (90.4%) | |
|
Female | 31 (25.8%) | 9 (9.6%) | |
| TACE frequency | | | 0.574 |
|
1/2/3/4/5/6/7/8/9 | 64 (53.3%)/20
(16.6%)/16 (13.3%)/7 (5.8%)/32 (26.6%) | 58 (56.3%)/19
(18.4%)/11 (10.7%)/9 (8.7%)/2 (1.9%)/1 (0.9%)/1 (0.9%)/1 (0.9%)/1
(0.9%) | |
| HBV infection | | | 0.039 |
|
Absent | 28 (23.3%) | 37 (36%) | |
|
Present | 92 (76.7%) | 66 (64%) | |
| HCV infection | | | 0.349 |
|
Absent | 109 (90.8%) | 97 (94.2%) | |
|
Present | 11 (9.2%) | 6 (5.8%) | |
| AST (IU/l) | | | 0.009 |
|
≤40 | 32 (26.7%) | 13 (12.7%) | |
|
>40 | 88 (73.3%) | 90 (87.3%) | |
| ALT (IU/l) | | | 0.016 |
|
≤50 | 73 (60.8%) | 46 (44.7%) | |
|
>50 | 47 (39.2%) | 57 (55.3%) | |
| GGT (IU/l) | | | |
|
≤60 | 36 (30.0%) | 7 (6.8%) | <0.0001 |
|
>60 | 84 (70.0%) | 96 (93.2%) | |
| AFP (ng/ml) | | | 0.215 |
|
≤400 | 84 (70.0%) | 64 (62.1%) | |
|
>400 | 36 (30.0%) | 39 (37.9%) | |
| Total bilirubin
(µmol/l) | | | 0.276 |
|
≤26 | 83 (69.2%) | 78 (75.7%) | |
|
>26 | 37 (30.8%) | 25 (24.3%) | |
| Albumin (g/l) | | | 0.643 |
|
≤35 | 36 (30.0%) | 28 (27.2%) | |
|
>35 | 84 (70.0%) | 75 (72.8%) | |
| Cirrhosis | | | 0.07 |
|
Absent | 38 (31.7%) | 51 (49.5%) | |
|
Present | 82 (68.3%) | 52 (50.5%) | |
| Tumor size
(cm) | | | 0.772 |
|
≤5 | 43 (35.9%) | 35 (34%) | |
|
>5 | 77 (64.1%) | 68 (66%) | |
| Tumor necrosis | | | 0.076 |
|
Absent | 33 (27.5%) | 18 (17.4%) | |
|
Present | 87 (72.5%) | 85 (82.5%) | |
| Tumor number | | | 0.005 |
|
Single | 106 (88.3%) | 76 (73.8%) | |
|
Multiple | 14 (11.7%) | 27 (26.2%) | |
| Extrahepatic
metastases | | | 0.774 |
|
Absent | 103 (85.9%) | 87 (84.5%) | |
|
Present | 17 (14.1%) | 16 (15.5%) | |
| Vascular
invasion | | | 0.943 |
|
Absent | 74 (61.7%) | 64 (62.1%) | |
|
Present | 46 (38.3%) | 39 (37.9%) | |
| Child-Pugh
class | | | 0.549 |
|
A | 87 (72.5%) | 75 (72.8%) | |
|
B | 33 (27.5%) | 27 (26.2%) | |
|
C | 0 | 1 (0.9%) | |
| BCLC stage | | | 0.676 |
|
0 | 2 (1.6%) | 2 (1.9%) | |
|
A | 5 (4.2%) | 7 (6.7%) | |
|
B | 81 (67.5%) | 70 (68%) | |
|
C | 32 (26.7%) | 32 (31.1%) | |
|
D | 0 | 1 (0.9%) | |
Determination of prognostic factors
for OS
Single-factor analysis was used to determine the
predictive factors for proportional hazards regression multivariate
analysis. Regarding clinicopathological factors, the presence of
extrahepatic metastasis, vascular invasion and cirrhosis were
significantly associated with poor survival outcomes. In terms of
laboratory factors, increased AST (AST >40 IU/l), elevated GGT
(GGT >60 IU/l), high AFP (AFP >400 ng/ml) and elevated total
bilirubin (bilirubin >26 µmol/l) were significantly associated
with poor survival outcomes. There was no significant correlation
between preoperative SF levels and patient survival (P=0.309)
(Table III).
 | Table IIIUnivariate analysis of potential
prognostic factors for survival. |
Table III
Univariate analysis of potential
prognostic factors for survival.
| Clinicopathological
characteristics | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Sex | 1.137
(0.684-1.891) | 0.620 |
|
Male | | |
|
Female | | |
| Age, years | 1.184
(0.745-1.883) | 0.474 |
|
≤50 | | |
|
>50 | | |
| Serum ferritin
(ng/ml) | 0.810
(0.539-1.216) | 0.309 |
|
≤274 | | |
|
>274 | | |
| HBV infection | 1.342
(0.885-2.036) | 0.166 |
|
Absent | | |
|
Present | | |
| HCV infection | 0.810
(0.421-1.560) | 0.529 |
|
Absent | | |
|
Present | | |
| ALT (IU/l) | 0.764
(0.512-1.139) | 0.186 |
|
≤50 | | |
|
>50 | | |
| AST (IU/l) | 0.520
(0.303-0.893) | 0.018 |
|
≤40 | | |
|
>40 | | |
| GGT (IU/l) | 0.527
(0.304-0.915) | 0.023 |
|
≤60 | | |
|
>60 | | |
| AFP (ng/ml) | 0.559
(0.398-0.901) | 0.014 |
|
≤400 | | |
|
>400 | | |
| Total bilirubin
(µmol/l) | 0.678
(0.442-1.039) | 0.045 |
|
≤26 | | |
|
>26 | | |
| Albumin (g/l) | 1.465
(0.959-0-2.238) | 0.078 |
|
≤35 | | |
|
>35 | | |
| Cirrhosis | 0.631
(0.406-0.982) | 0.041 |
|
Absent | | |
|
Present | | |
| Tumor size
(cm) | 0.717
(0.469-1.095) | 0.124 |
|
≤5 | | |
|
>5 | | |
| Tumor necrosis | 0.850
(0.532-1.357) | 0.495 |
|
Absent | | |
|
Present | | |
| Tumor number | 1.045
(0.633-1.726) | 0.863 |
|
Single | | |
|
Multiple | | |
| Extrahepatic
metastases | 0.456
(0.273-0.764) | 0.003 |
|
Absent | | |
|
Present | | |
| Vascular
invasion | 0.376
(0.249-0.568) | <0.0001 |
|
Absent | | |
|
Present | | |
| Child-Pugh
class | 0.703
(0.703-1.603) | 0.776 |
|
A | | |
|
B | | |
|
C | | |
| BCLC stage | 1.214
(0.864-1.705) | 0.264 |
|
0 | | |
|
A | | |
|
B | | |
|
C | | |
|
D | | |
For multivariate analysis, a Cox proportional
hazards model that included all significant factors from the
univariate analysis was used to determine the independent
predictive factors for OS. In this model, the significant
independent prognostic factors affecting survival model included
the presence of extrahepatic metastasis and vascular invasion.
Specifically, extrahepatic metastasis (HR=0.490; 95%
CI=0.282-0.843; P=0.010) and vascular invasion (HR=0.373; 95%
CI=0.225-0.619; P<0.0001) emerged as independent prognostic
factors for OS (Table IV).
 | Table IVMultivariate analysis of potential
prognostic factors for overall survival. |
Table IV
Multivariate analysis of potential
prognostic factors for overall survival.
| Clinicopathological
characteristics | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Extrahepatic
metastases | 0.490
(0.282-0.843) | 0.010 |
|
Absent | | |
|
Present | | |
| Vascular
invasion | 0.373
(0.225-0.619) | <0.0001 |
|
Absent | | |
|
Present | | |
Survival analysis
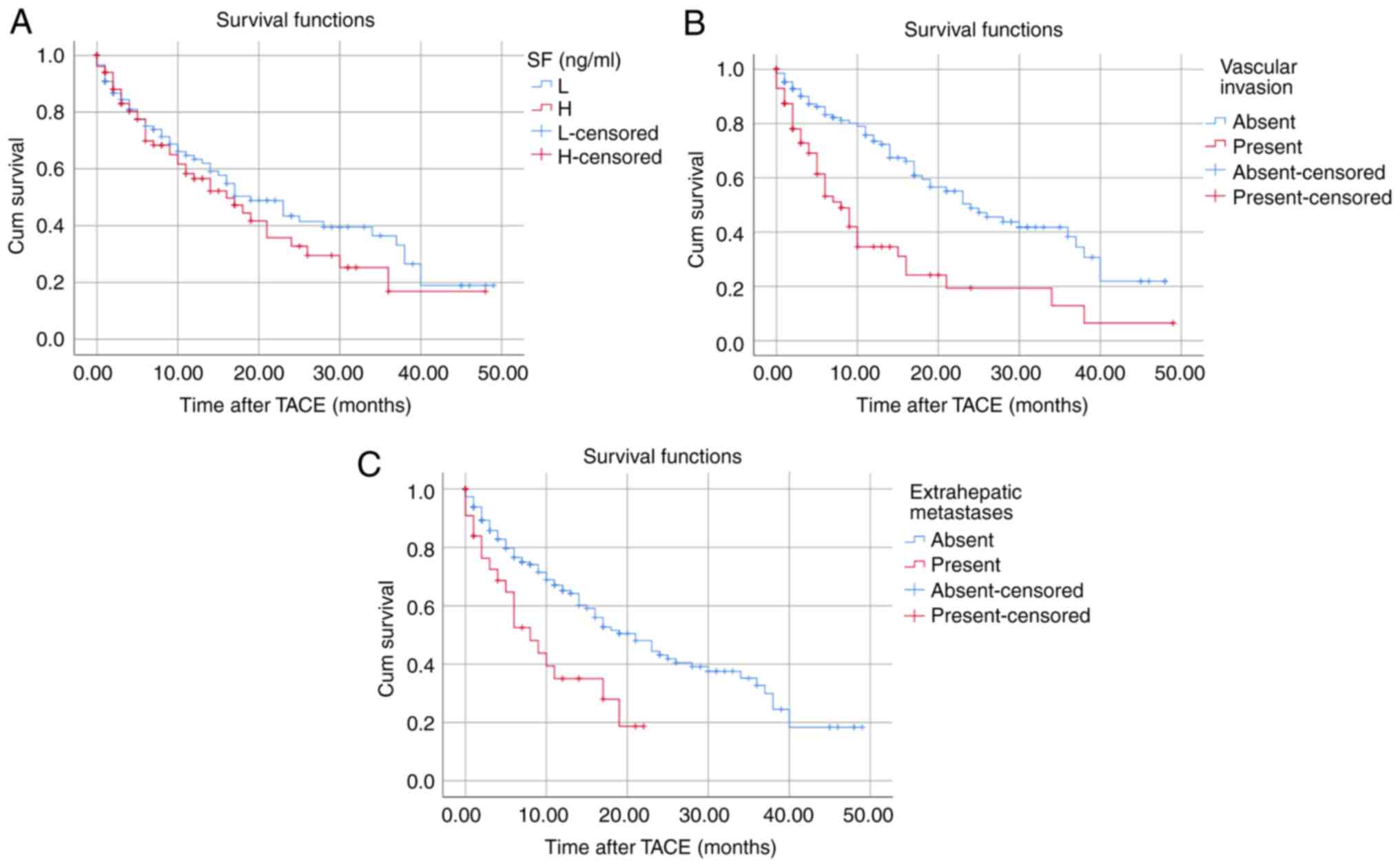
A total of 98 patients died during the follow-up
period. The median OS was 17 months. The 1-, 3-, and 5-year OS
rates were 92, 83 and 77%, respectively. The median OS of patients
did not significantly differ between the low (≤274 ng/ml) and high
(>274 ng/ml) SF groups (Fig.
1A). The presence of vascular invasion (P<0.0001) and
extrahepatic metastasis (P=0.010) significantly shortened the
survival time of patients (Fig. 1B
and C).
Discussion
Liver cancer is the second leading cause of
cancer-related deaths in China. For most patients with unresectable
or inoperable HCC, TACE is considered the first-line treatment
option. TACE is considered to cause tumor necrosis by creating a
hypoxic environment and producing cytotoxic effects on tumor cells
by concentrating high doses of chemotherapy drugs locally on the
tumor (28). TACE can improve the
quality of life and extend the survival of patients in intermediate
or advanced HCC stages (29,30).
SF is a group of proteins that play an important
role in iron storage, and is primarily found in the liver, spleen
and bone marrow. Under normal physiological conditions SF is mainly
composed of light (L) chains; however, in numerous malignant tumors
the ratio of heavy (H) ferritin and H/L ferritin increases
(31). The reasons for the
increase in SF in liver cancer are as follows (32): i) Liver cancer cells can synthesize
and secrete ferritin or hetero-ferritin; ii) the uptake and
clearance of ferritin in liver cancer tissue are affected; and iii)
hepatocyte damage and necrosis cause the release of stored ferritin
in the hepatocyte cytoplasm into the bloodstream. Elevated SF
levels have also been reported in malignant tumors of the blood
system (33), as well as non-tumor
diseases, including hemochromatosis, chronic kidney disease,
diabetes (34-36),
rheumatoid arthritis and adult Still's disease (37). Multiple pathological factors
influence the levels of SF, and its instability leads to a lack of
specificity. Therefore, predicting prognosis based on SF is
challenging.
In the present study, it was determined that 274
ng/ml was the cut-off point for SF. By contrast, Wu et al
(27) used 267 ng/ml as the
optimal SF cut-off point. The cut-off point for SF in a Korean
study cohort was 150 ng/ml, whereas an Italian study reported that
the optimal prognostic threshold for SF was 244 ng/ml (26,38).
These variations suggested that the normal range of SF may be
influenced by factors such as differences in laboratory equipment,
region and ethnicity. Thus, the currently published data on SF as a
prognostic tool for liver cancer lack generalizability and
applicability. Furthermore, SF levels can also be affected by the
batch of experimental reagents and equipment. Thus, the accuracy of
preoperative SF levels in predicting the prognosis of liver cancer
may be compromised.
The correlation between SF levels and
clinicopathological variables was then studied, and it was found
that HBV infection, AST, ALT and GGT were significantly correlated
with preoperative SF levels, while being unrelated to other
laboratory parameters. Increased preoperative SF levels were also
positively correlated with sex and cirrhosis. AST, ALT and GGT are
indicators of liver cell injury. The presence of HBV infection and
cirrhosis suggests the impairment of liver function. The
destruction of normal liver cells and the presence of liver cancer
cells can both lead to the release of ferritin into the
bloodstream, resulting in increased SF levels. The correlation
between SF levels and clinicopathological variables was also
investigated. Wu et al (27) reported that in HCC patients
undergoing liver resection TNM and BCLC stages closely correlated
with preoperative SF levels while remaining unrelated to other
clinicopathological variables. By contrast, Facciorusso et
al (26) reported that in HCC
patients undergoing RFA treatment, no significant correlation was
found between SF levels and other prognostic factors. It was
inferred that the different treatments employed may explain this
inconsistency in the results.
In the present study, univariate analysis revealed
that the presence of extrahepatic metastasis, vascular invasion,
cirrhosis, and AST, GGT, AFP and total bilirubin levels were
predictors of OS. However, the multivariate Cox analysis refined
the number of predictors for OS, focusing on the presence of
extrahepatic metastasis and vascular invasion. The presence of
extrahepatic metastasis and vascular invasion both indicate tumor
progression and are associated with increased mortality. However,
the present findings indicated that preoperative SF levels are not
an independent predictor of mortality in patients with HCC
undergoing TACE. A recent study found limited prognostic value for
SF in patients with decompensated cirrhosis, suggesting it may not
be an independent predictor of mortality (39). Consequently, the value of SF for
liver disease prognosis remains controversial.
The present study had certain limitations that
should be acknowledged. First, the analysis did not include changes
in SF levels after TACE. Second, no distinction was made between
various interventional embolization methods, although research has
revealed that drug-eluting bead-transarterial chemoembolization has
no advantage over conventional transarterial chemoembolization in
patients with unresectable HCC (40). Furthermore, the extended time span
of the study means there are no mature guideline for earlier cases
as a reference. According to BCLC, the study might not have been
suitable candidates for TACE. Fourth, the present study was
designed as a retrospective single-group analysis, with a
relatively small sample size, which could introduce bias;
therefore, its conclusions may require further validation through
randomized controlled trials or large-scale prospective cohort
studies. Finally, imaging follow-up data were unavailable for
numerous patients, resulting in cases where only OS data were
available without corresponding disease-free survival data.
In conclusion, this single-center study demonstrated
that preoperative SF levels in patients with HCC undergoing TACE
was not significantly correlated with prognosis. The present
findings indicated that SF has limited utility as a prognostic
indicator for patients with HCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and BT conceived and designed the study. MF, TN,
BL and FG collected and analyzed the data. MF and TM drafted the
manuscript. XD, BT, MF, TN, BL and FG contributed to the data
interpretation and discussion. XD and BT confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
S20230320-01) by the Medical Ethics Committee of Mianyang Central
Hospital (Mianyang, China). The data were analyzed anonymously;
thus, informed consent was not obtained from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao YX, Yang TW, Yin JM, Yang PX, Kou BX,
Chai MY, Liu XN and Chen DX: Progress and prospects of biomarkers
in primary liver cancer (Review). Int J Oncol. 57:54–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Chiang CL and Dawson LA: Efficacy
and safety of radiotherapy for primary liver cancer. Chin Clin
Oncol. 10(9)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Velázquez RF, Rodríguez M, Navascués CA,
Linares A, Pérez R, Sotorríos NG, Martínez I and Rodrigo L:
Prospective analysis of risk factors for hepatocellular carcinoma
in patients with liver cirrhosis. Hepatology. 37:520–527.
2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide:Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alisi A and Balsano C: Enhancing the
efficacy of hepatocellular carcinoma chemotherapeutics with natural
anticancer agents. Nutr Rev. 65 (12 Pt 1):550–553. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han K and Kim JH: Transarterial
chemoembolization in hepatocellular carcinoma treatment: Barcelona
clinic liver cancer staging system. World J Gastroenterol.
21:10327–10335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Raoul JL, Forner A, Bolondi L, Cheung TT,
Kloeckner R and de Baere T: Updated use of TACE for hepatocellular
carcinoma treatment: How and when to use it based on clinical
evidence. Cancer Treat Rev. 72:28–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee SW, Peng YC, Lien HC, Ko CW, Tung CF
and Chang CS: Clinical values of Barcelona Clinic Liver Cancer
subgroup and up-to-7 criteria in intermediate stage hepatocellular
carcinoma with transcatheter arterial chemoembolization. World J
Clin Cases. 10:7275–7284. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kotsifa E, Vergadis C, Vailas M, Machairas
N, Kykalos S, Damaskos C, Garmpis N, Lianos GD and Schizas D:
Transarterial chemoembolization for hepatocellular carcinoma: Why,
when, how? J Pers Med. 12(436)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Samman BS, Hussein A, Samman RS and
Alharbi AS: Common sensitive diagnostic and prognostic markers in
hepatocellular carcinoma and their clinical significance: A review.
Cureus. 14(e23952)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinato DJ, Arizumi T, Jang JW, Allara E,
Suppiah PI, Smirne C, Tait P, Pai M, Grossi G, Kim YW, et al:
Combined sequential use of HAP and ART scores to predict survival
outcome and treatment failure following chemoembolization in
hepatocellular carcinoma: A multi-center comparative study.
Oncotarget. 7:44705–44718. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Op den Winkel M, Nagel D, Op den Winkel P,
Trojan J, Paprottka PM, Steib CJ, Schmidt L, Göller M, Stieber P,
Göhring P, et al: Transarterial chemoembolization for
hepatocellular carcinoma: development and external validation of
the Munich-TACE score. Eur J Gastroenterol Hepatol. 30:44–53.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ruf A, Dirchwolf M and Freeman RB: From
Child-Pugh to MELD score and beyond: Taking a walk down memory
lane. Ann Hepatol. 27(100535)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ,
Guo RP, Lin XJ and Lau WY: Prognostic nomogram for patients with
unresectable hepatocellular carcinoma after transcatheter arterial
chemoembolization. J Hepatol. 63:122–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mähringer-Kunz A, Weinmann A, Schmidtmann
I, Koch S, Schotten S, Pinto Dos Santos D, Pitton MB, Dueber C,
Galle PR and Kloeckner R: Validation of the SNACOR clinical scoring
system after transarterial chemoembolisation in patients with
hepatocellular carcinoma. BMC Cancer. 18(489)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kadalayil L, Benini R, Pallan L, O'Beirne
J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, et
al: A simple prognostic scoring system for patients receiving
transarterial embolisation for hepatocellular cancer. Ann Oncol.
24:2565–2570. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang JB, Chen Y, Zhang B, Xie X, Zhang L,
Ge N, Ren Z and Ye SL: Prognostic significance of serum
gamma-glutamyl transferase in patients with intermediate
hepatocellular carcinoma treated with transcatheter arterial
chemoembolization. Eur J Gastroenterol Hepatol. 23:787–793.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xuan ZD, Zhou L, Wang Y and Zheng X:
Prognostic value of the combination of serum levels of vascular
endothelial growth factor, C-reactive protein and contrast-enhanced
ultrasound in patients with primary liver cancer who underwent
transcatheter arterial chemoembolization. Expert Rev Anticancer
Ther. 17:1169–1178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan C, Yuan M, Chen M, Ouyang J, Tan W,
Dai F, Yang D, Liu S, Zheng Y, Zhou C and Cheng Y: Prognostic
implication of a novel metabolism-related gene signature in
hepatocellular carcinoma. Front Oncol. 11(666199)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rahimi-Dehkordi N, Nourijelyani K,
Nasiri-Tousi M, Ghodssi-Ghassemabadi R, Azmoudeh-Ardalan F and
Nedjat S: Model for End stage Liver Disease (MELD) and
Child-Turcotte-Pugh (CTP) scores: Ability to predict mortality and
removal from liver transplantation waiting list due to poor medical
conditions. Arch Iran Med. 17:118–121. 2014.PubMed/NCBI
|
|
21
|
Peng Y, Qi X and Guo X: Child-Pugh versus
MELD score for the assessment of prognosis in liver cirrhosis:A
systematic review and meta-analysis of observational studies.
Medicine (Baltimore). 95(e2877)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang W, Knovich MA, Coffman LG, Torti FM
and Torti SV: Serum ferritin: Past, present and future. Biochim
Biophys Acta. 1800:760–769. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Plays M, Müller S and Rodriguez R:
Chemistry and biology of ferritin. Metallomics.
13(mfab021)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo Q, Li L, Hou S, Yuan Z, Li C, Zhang W,
Zheng L and Li X: The role of iron in cancer progression. Front
Oncol. 11(778492)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Facciorusso A, Del Prete V, Antonino M,
Neve V, Crucinio N, Di Leo A, Carr BI and Barone M: Serum ferritin
as a new prognostic factor in hepatocellular carcinoma patients
treated with radiofrequency ablation. J Gastroenterol Hepatol.
29:1905–1910. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu SJ, Zhang ZZ, Cheng NS, Xiong XZ and
Yang L: Preoperative serum ferritin is an independent prognostic
factor for liver cancer after hepatectomy. Surg Oncol. 29:159–167.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ghanaati H and Mohammadifard M and
Mohammadifard M: A review of applying transarterial
chemoembolization (TACE) method for management of hepatocellular
carcinoma. J Family Med Prim Care. 10:3553–3560. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Manjunatha N, Ganduri V, Rajasekaran K,
Duraiyarasan S and Adefuye M: Transarterial chemoembolization and
unresectable hepatocellular carcinoma: A narrative review. Cureus.
14(e28439)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chung SW, Park MK, Cho YY, Park Y, Lee CH,
Oh H, Jang H, Kim MA, Kim SW, Nam JY, et al: Effectiveness of
transarterial chemoembolization-first treatment for advanced
hepatocellular carcinoma: A propensity score matching analysis. J
Hepatocell Carcinoma. 8:587–598. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alkhateeb AA and Connor JR: The
significance of ferritin in cancer: Anti-oxidation, inflammation
and tumorigenesis. Biochim Biophys Acta. 1836:245–254.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nielsen P, Günther U, Dürken M, Fischer R
and Düllmann J: Serum ferritin iron in iron overload and liver
damage: Correlation to body iron stores and diagnostic relevance. J
Lab Clin Med. 135:413–418. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Henter JI, Horne A, Aricó M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: diagnostic and therapeutic guidelines for
hemophagocytic lymphohisti-ocytosis. Pediatr Blood Cancer.
48:124–131. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kalantar-Zadeh K and Lee GH: The
fascinating but deceptive ferritin: To measure it or not to measure
it in chronic kidney disease? Clin J Am Soc Nephrol. 1 (Suppl
1):S9–S18. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Fang X, Zheng W, Zhou J, Song Z,
Xu M, Min J and Wang F: Genetic support of a causal relationship
between iron status and type 2 diabetes: A mendelian randomization
study. J Clin Endocrinol Metab. 106:e4641–e4651. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Suárez-Ortegón MF, Ensaldo-Carrasco E, Shi
T, McLachlan S, Fernández-Real JM and Wild SH: Ferritin, metabolic
syndrome and its components: A systematic review and meta-analysis.
Atherosclerosis. 275:97–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jia J, Wang M, Meng J, Ma Y, Wang Y, Miao
N, Teng J, Zhu D, Shi H, Sun Y, et al: Ferritin triggers neutrophil
extracellular trap-mediated cytokine storm through Msr1
contributing to adult-onset Still's disease pathogenesis. Nat
Commun. 13(6804)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee S, Song A and Eo W: Serum ferritin as
a prognostic biomarker for survival in relapsed or refractory
metastatic colorectal cancer. J Cancer. 7:957–964. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo G, Sun M, Li Y, Yang W, Wang X, Yu Z,
Li C, Hui Y, Fan X, Jiang K and Sun C: Serum ferritin has limited
prognostic value on mortality risk in patients with decompensated
cirrhosis: A propensity score matching analysis. Lab Med. 54:47–55.
2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Facciorusso A, Di Maso M and Muscatiello
N: Drug-eluting beads versus conventional chemoembolization for the
treatment of unresectable hepatocellular carcinoma: A
meta-analysis. Dig Liver Dis. 48:571–577. 2016.PubMed/NCBI View Article : Google Scholar
|