Introduction
Breast cancer is the most common cancer in women and
the second leading cause of cancer-related mortality worldwide
(1). There are four primary
subtypes of breast cancer: luminal A, luminal B, human epidermal
growth factor receptor 2 (HER2)-positive, and triple-negative
breast cancer (TNBC). These classifications are based on molecular
markers, such as estrogen receptors, progesterone receptors and
HER2. TNBC is the most malignant subtype of breast cancer,
accounting for ~15-20% of all breast cancers (2,3).
Since patients with TNBC lack relevant receptor markers, they do
not benefit from established endocrine- or HER2-targeted agents.
Although TNBC is the subtype that responds best to standard
chemotherapy regimens, such as paclitaxel or anthracyclines, some
patients with TNBC have poor outcomes. This could be due to the
high heterogeneity of TNBC and the presence of primary and
secondary resistance (3).
Therefore, the search for effective therapeutic targets for TNBC
and the mechanisms underlying its resistance to chemotherapy are of
great interest.
Leptin is a hormone predominantly secreted by
adipocytes, which plays a crucial role in regulating energy balance
by transmitting signals from adipose tissue to the hypothalamus.
The synthesis and concentration of leptin in the bloodstream are
closely associated with the mass of adipose tissue (4). Leptin has been extensively studied
both in vivo and in vitro for its impact on different
aspects of breast cancer biology. Apart from adipose tissue, leptin
is also secreted by cancer cells and its receptors are often
overexpressed in these cells (4).
Leptin levels have been linked to various characteristics of breast
cancer, including its type, grade, stage, lymph node involvement,
hormone receptor status and recurrence (5). A meta-analysis conducted by Gu et
al (5) comprising 43 studies
indicated that serum leptin might have a significant impact on the
development and metastasis of breast cancer. Another meta-analysis,
involving 23 studies, demonstrated that circulating leptin levels
were lower in healthy individuals than in those with benign breast
disease, breast cancer, or lymph node metastases, implying that
leptin levels could serve as a potential diagnostic tool for tumor
formation (6). Pan et al
(7) conducted a meta-analysis
involving 35 studies, suggesting that leptin could serve as a
potential biomarker for breast cancer risk, particularly in
overweight/obese or postmenopausal women. It may also be a valuable
biomarker for identifying individuals at high risk for breast
cancer, aiding in preventive therapy (7). Furthermore, elevated serum leptin
levels, coupled with increased expression of leptin receptor mRNA
in breast cancer tissues, are associated with poor prognosis
(8). Leptin promotes mitochondrial
fusion and contributes to drug resistance in gall bladder cancer
(9). Leptin also interferes with
the action of tamoxifen in MCF-7 cells by inducing an increase in
the nuclear expression of ERα. Thus, leptin may favor tamoxifen
resistance, and inhibition of leptin expression may be a novel
approach to circumvent resistance to anti-estrogen therapy
(10). A previous study reported
that leptin-induced microRNA-342-3p enhances gemcitabine resistance
in pancreatic ductal adenocarcinoma (11). These findings underscore the
possible advantage of targeting leptin signaling as a strategy to
inhibit breast cancer malignancy.
Leptin has been found to influence the behavior of
tumor-associated macrophages (TAMs), which are a major source of
inflammatory cytokines and a key component of the tumor
microenvironment (12).
Stimulation of macrophages by leptin induces the release of
pro-inflammatory cytokines, which can result in a pro-inflammatory
reaction. Clinical evidence suggests that the presence of
macrophages within the tumor microenvironment is associated with
unfavorable outcomes in individuals with cancer (13). Macrophages are a highly
heterogeneous group of immune cells with different functions and
phenotypes that participate in both innate and adaptive immunity in
the body (14). Macrophages
exhibit both antitumor and pro-tumor effects. Under conditions of
tumorigenesis, macrophages have an antitumor effect, whereas once a
tumor has formed, they have a pro-tumor effect (15). Under the influence of the complex
tumor microenvironment, macrophages can be recruited to the tumor
area and polarized into either a tumor growth-inhibiting M1 state
or a tumor growth-promoting M2 state (14,16).
In the present study, TAMs represent M2 type-associated tumor
cells. Cao et al (17)
found that leptin promotes tumor progression and metastasis by
triggering the production of the M2 macrophage-associated cytokine
IL-18. These findings further establish the connection between the
tumor microenvironment and breast cancer cells.
In summary, leptin plays a role in every stage of
breast cancer progression and can stimulate the secretion of
inflammatory factors by M2-type TAMs to influence the development
of malignant tumors. However, whether it affects the docetaxel
sensitivity of MDA-MB-231 TNBC cells has not been reported.
Therefore, the present study was conducted to explore whether this
is relevant and to initially explore the mechanism of
resistance.
Materials and methods
Cell culture
MDA-MB-231 cells (cat. no. AW-CCH048; http://abiowell.com/) were cultured in DMEM/F12 medium
containing 10% FBS (cat. no. D8437; Sigma-Aldrich; Merck KGaA) + 1%
dual antibody. THP-1 cells (cat. no. AW-CCH098; http://abiowell.com/) were cultured in RPMI-1640
medium containing 10% FBS + 1% dual antibody + 0.05 mM
β-mercaptoethanol in RPMI-1640 medium (cat. no. R8758;
Sigma-Aldrich; Merck KGaA).
Western blotting (WB)
Cells were washed with ice-cold PBS and 200 µl RIPA
lysate was added. Afterwards, cells were scraped with a scraper,
and the suspension was collected and sonicated for 1.5 min on ice.
The lysate remained for 10 min on ice. The centrifuge was
pre-cooled at 4˚C, and the lysate was centrifuged at 13780 x g for
15 min. The supernatant was transferred to a 1.5 ml tube. Protein
concentration was determined with a BCA protein quantification kit.
TEMED was mixed with 10 or 15% separating gel, and gel was sealed
with isopropyl alcohol. Gel was allowed to set until stable line
formed. The top layer of isopropyl alcohol was poured off, and add
gelatin concentrate was added to solidify the gel. A total of 160
µl protein supernatant was mixed with 40 µl loading buffer, boiled
for 5 min and left to cool on ice. The first well was spotted with
2 µl of marker, and the other wells were sampled with 10-20 µl of
denatured protein. The marker and denatured protein were injected
into wells, and electrophoresis was run at 75 V for 130 min. The
electrophoresis was terminated when the dye reached the gel bottom.
The gel was cut by molecular weight, and transferred to a
nitrocellulose membrane which was subsequently washed with 1X PBST.
5% skim milk powder was prepared with 1X PBST (cat. no. AWI0130;
http://abiowell.com/), and the membranes were
immersed and left at room temperature for 90 min. The primary
antibody (IL-8; 1:1,000; cat. no. MA5-23697; Thermo Fisher
Scientific, Inc.) was diluted with 1X PBST, and the membrane was
incubated at room temperature for 90 min. Subsequently, the
membrane was washed three times with 1X PBST for 10 min each.
HRP-labeled secondary antibodies [Goat anti-Mouse IgG (H+L)
(1:5,000; cat. no. AWS0001; http://abiowell.com/) and Goat anti-Rabbit IgG (H+L)
(1:5,000; cat. no. AWS0002; http://abiowell.com)] was diluted with 1X PBST, and
incubated with membrane at room temperature for 90 min. The
membrane was then washed three times with 1X PBST for 15 min each.
The membrane was incubated with ECL solution (AWB0005; http://abiowell.com/) for 1 min. The membrane was
wrapped with plastic wrap, and images were captured with gel
imaging system. β-actin (1:5,000; cat. no. 66009-1-Ig; Proteintech
Group, Inc.) was used as an internal control.
Cell Counting Kit-8 (CCK-8) assay
The cells were seeded into 96-well plates at a
density of 5x103 cells/well with 100 µl of medium per
well. A total of three replicate wells were prepared for each
group. After the cells were attached to the plate, they were
treated as aforementioned for a specific period. Following the
treatment, 10 µl of CCK-8 solution (cat. no. NU679; Dojindo
Laboratories, Inc.) was added to each well. The CCK-8 solution was
prepared in the complete medium by replacing the drug-containing
medium, and 100 µl of medium containing CCK-8 was added to each
well. The plates were incubated at 37˚C with 5% CO2 for
4 h, and then the absorbance at 450 nm was measured using a HUISON
zymography reader.
ELISA assay (IL-8; cat. no. KE00006;
Proteintech Group, Inc.)
Reagents were equilibrated at room temperature for
30 min. Standard and sample wells were set up; a total of 100 µl of
standard/sample was added to each well, mixed well and incubated
covered at 37˚C for 2 h. Then, liquid was discarded and the plate
was washed 4 times with 200 µl wash solution each, shake dry after
each wash. A total of 100 µl detection antibody working solution
was added to each well and incubated covered at 37˚C for 1 h. The
liquid was discarded, and the plate was washed 4 times as
aforementioned. HRP-labeled affinity protein working solution (100
µl) was added to each well and incubated covered at 37˚C for 40
min. The liquid was discarded, and the plate was washed 4 times as
aforementioned. Substrate solution (100 µl) was added to each well
and incubated at 37˚C for 15-20 min, avoiding light. The reaction
was terminated by adding 100 µl termination solution to each well.
OD was measured at 450 nm within 5 min. Data was analyzed using
Curve Expert software (Curve Expert1.4) to create a standard curve.
Sample concentration was calculated using regression equation
derived from the standard curve and the sample's OD value.
Transwell assay
One day before the experiment, sterile lance tips,
EP tubes, Matrigel and Transwell chambers were pre-cooled overnight
at 4˚C. Matrigel was diluted with 100 µl of ice-cold, serum-free
DMEM/F12 medium per well to a final concentration of 200 µg
Matrigel per well. Matrigel was incubated for 30 min at 37˚C and
the supernatant was removed. A total of 500 µl of 10% FBS Complete
Medium was added to the lower chamber of Transwell. Treated cells
were digested with trypsin to obtain single cells, resuspended in
serum-free medium at a concentration of 2x106 cells/ml,
and 100 µl of cells was added to each well. Cells were incubated at
37˚C for 48 h. The upper chamber was removed, washed with PBS three
times, and the cells were wiped off the upper chamber with a cotton
ball. Subsequently, cells were fixed with 4% paraformaldehyde for
20 min at room temperature, and then the membrane was removed. The
membranes were stained with 0.1% crystal violet for 5 min at room
temperature, rinsed 5 times with water, placed on slides, and
microscopic images were captured. Using an inverted light
microscope, three randomly selected fields of view were used to
observe the cells on the outer surface of the upper chamber. The
chamber was removed and immersed in 500 µl of 10% acetic acid for
decolorization. The absorbance (OD) value was measured at 550 nm
using an enzyme standard instrument, repeating the process three
times to obtain consistent results.
Wound healing assay
Horizontal lines were drawn evenly in a 6-well
plate, and ~5x105 cells were added in each well after
being digested with trypsin. After the cells spread all over the
plate, the tip of the pipette was used to compare with the ruler
and draw the horizontal line perpendicular to the horizontal line.
The cells were washed 3 times with sterile PBS to remove the
scratched cells and serum-free DMEM/F12 medium was added. Images of
the scratch were captured at 0 h, using 3 fields of view at each
time point. After incubation at 37˚C with 5% CO2 for 24
and 48 h, images were again captured to record.
Flow cytometric assay
Cells from different treatments were collected by
digestion with EDTA-free trypsin, washed twice with PBS, each time
centrifuged (4˚C) at (713 x g for 5 min. Cells were collected and
500 µl of binding buffer was added to suspend the cells. A total of
5 µl of Annexin V-APC (cat. no. KGA1030; Nanjing KeyGen Biotech
Co., Ltd.) and 5 µl of propidium iodide were added and mixed well
at room temperature. The reaction was carried out at room
temperature, protected from light for 10 min, and then observed and
detected by flow cytometry [Flow cytometer (cat. no. A00-1-1102);
analysis software: CytExpert_Setup-2.5.0.77; both from Beckman
Coulter, Inc.)].
Induction of M2 TAMs
THP-1 cells were cultured in RPMI-1640 medium
containing 10% FBS + 1% dual antibody + 0.05 mM β-mercaptoethanol
and placed in a saturated humidity incubator at 37˚C with 5%
CO2. THP-1 cells in logarithmic growth were stimulated
and induced to adherence using phorbol ester (PMA). Interleukin 4
(IL-4) was then added to induce monocyte differentiation into TAMs.
The cells with completed induction were collected, 5 µl of CD206
(cat. no. 12-2069-42; eBioscience; Thermo Fisher Scientific, Inc.)
antibody was added, mixed and incubated for 30 min at room
temperature away from light. The expression of CD206 was analyzed
by flow cytometry.
Neutralization of IL-8
The levels of IL-8 were detected by the ELLSA method
after 0, 24 and 48 h of leptin action on TAMs. The culture medium
was collected and IL-8 was neutralized with IL-8 antibody (Cell
Line IL-8 antibody).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 9.0 (Dotmatics) and SPSS 22.0 (IBM Corp.), and all values
were the result of at least three independent measurements.
Statistical analyses were performed using unpaired t-test or
one-way ANOVA with Tukey's or Dunnett's as post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Enhancement of MDA-MB-231 docetaxel
resistance in TNBC cells after leptin action on TAMs
The culture media of leptin + TAMs, leptin, TAMs and
blank groups were collected following 48 h of incubation to culture
the MDA-MB-231 cell line. The appropriate concentration of
docetaxel was then added and culture continued for 0, 24 and 48 h,
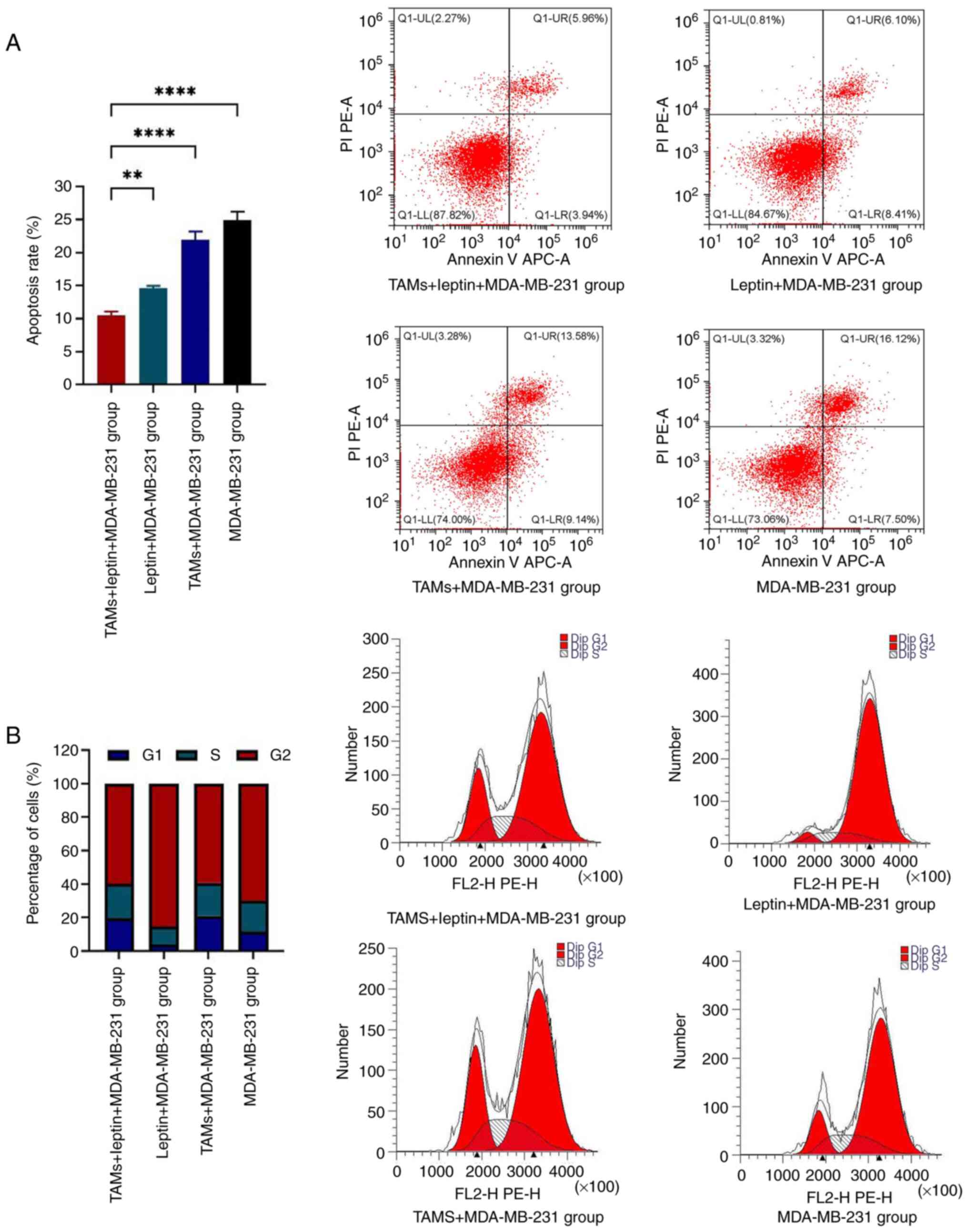
followed by the detection of apoptosis (Fig. 1A) and cell-cycle distributions of
the MDA-MB-231 cells in all the groups (Fig. 1B) using flow cytometry. Compared
with the other three groups, the leptin + TAMs + MDA-MB-231 group
displayed a significantly lower apoptotic rate. The flow cytometric
results revealed that after a combination of TAMs and docetaxel
acted on MDA-MB-231 cells, there was a significant increase in
G1-phase cells and a significant decrease in G2-phase cells.
However, after a combination of leptin and docetaxel acted on
MDA-MB-231 cells, there was a significant decrease in G1-phase
cells and a significant increase in G2-phase cells (Fig. 1B).
Leptin affects IL-8 secretion by
TAMs
Numerous studies have demonstrated the significant
involvement of IL-8 in the development of tumor resistance to drugs
(18). To investigate the possible
mechanism by which leptin combined with TAMs affects the resistance
of TNBC cell lines to docetaxel, the supernatants of the four
groups of the aforementioned culture media were collected to detect
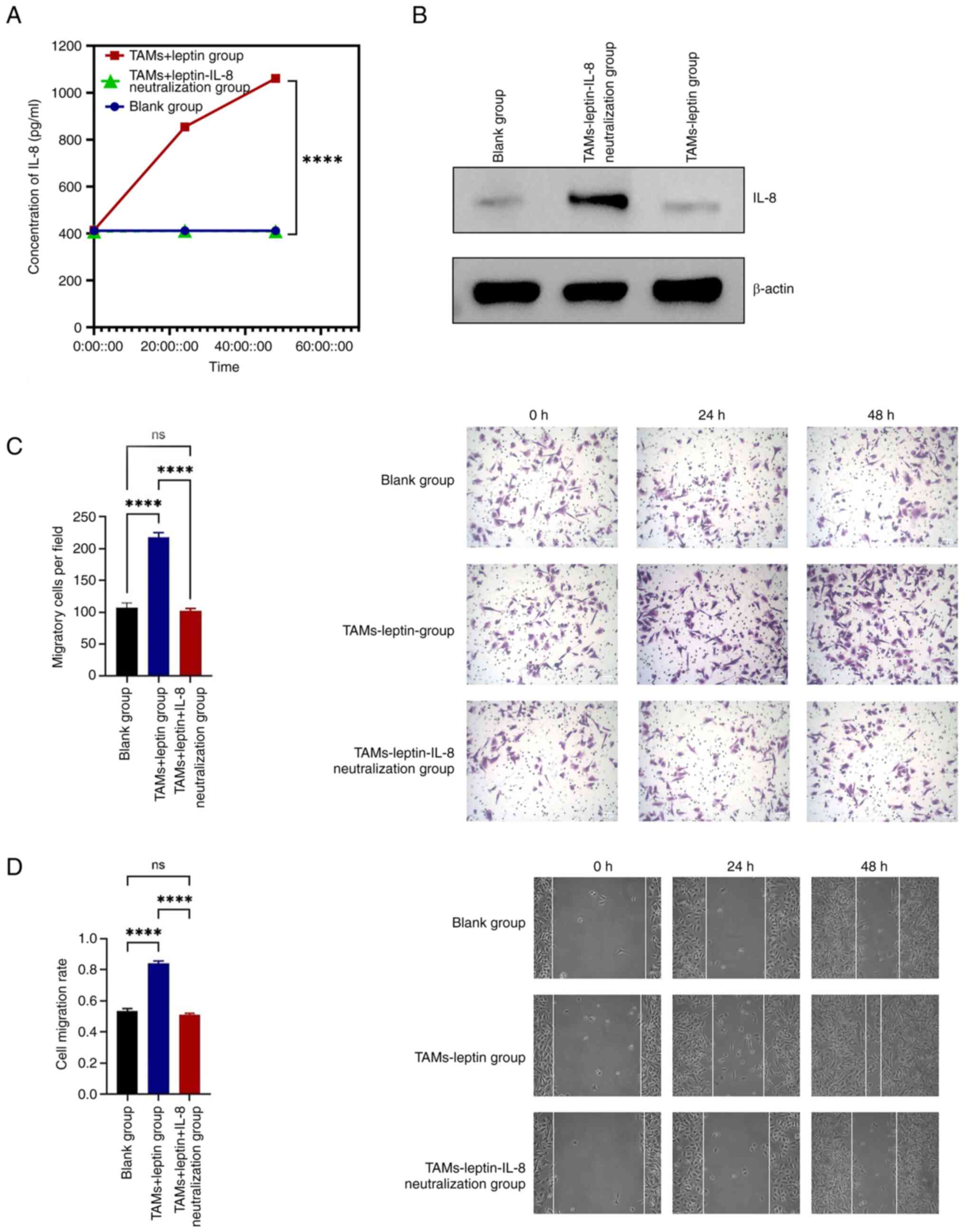
the expression of IL-8. The ELISA results demonstrated that IL-8
expression in the leptin + TAMs group was significantly higher than
that in the remaining three groups, with a statistically
significant difference (Fig. 2A).
WB was also used to detect the expression of IL-8, and the results
supported a significant increase in IL-8 expression in the leptin +
TAMs group (Fig. 2B).
Leptin-stimulated IL-8 secretion from
TAMs promotes MDA-MB-231 invasion and migration
To further investigate the effect of
leptin-stimulated IL-8 secreted by TAMs on TNBC cell lines, ELISA
and WB were used to detect the expression of IL-8 in the leptin +
TAMs group, leptin + TAMs-neutralized and IL-8 groups, and blank
group. ELISA results identified that the expression of IL-8 in the
leptin + TAMs group was significantly higher than that in the other
two groups. The difference in IL-8 expression between the leptin +
TAMs neutralized IL-8 group and the blank group was not
statistically significant, indicating that neutralization was
effective (Fig. 3A). WB results
were consistent with ELISA results (Fig. 3B).
The Transwell assay showed that the number of
migratory cells was significantly lower in the leptin + TAMs
neutralized IL-8 group than in the leptin + TAMs group (P<0.01)
(Fig. 3C).
The experimental results from the cell scratch assay
were consistent with those from the Transwell assay. The migration
distance and number of invasive cells in the leptin + TAMs group
were significantly greater than those in the leptin + TAMs
neutralized IL-8 group at 24 and 48 h after scratching (P<0.01)
(Fig. 3D).
Discussion
According to the latest data released by the
International Agency for Research on Cancer of the World Health
Organization, cancer is the leading cause of death worldwide, with
nearly 10 million deaths in 2020(1). Breast cancer accounts for the highest
number of new cancer cases, at 2.26 million cases yearly,
surpassing that of lung cancer. Breast cancer has now replaced lung
cancer as the world's most predominant cancer, and it is one of the
four leading causes of cancer-related deaths (19). Despite considerable progress and
advances in the diagnosis and treatment of breast cancer,
chemoresistance-induced metastatic recurrence remains a challenge
for basic and clinical researchers. Leptin expression is
significantly higher than normal in patients with breast cancer.
Leptin and leptin receptors are expressed in normal breast
epithelial cells; however, their overexpression is associated with
breast cancer progression. Tumors present in the mesenchyme may
include cells, such as fibroblasts, epithelial cells, macrophages,
T lymphocytes, dendritic cells, neutrophils and adipocytes;
structural components such as lymphatic and blood vessels; and
soluble factors, including growth factors, cytokines and
chemokines, in cell-tumor interactions (20). The mesenchyme normally acts as an
antitumor barrier; however, it can be transformed into a
tumor-promoting state, either as an intrinsic change, as in the
case of an inefficient vascular system, or as an acquired change,
as in the case of responsiveness to chemotherapy and radiotherapy
mediated by fibroblasts and immunosuppressive cells (21). Macrophages, an important component
of the tumor microenvironment, account for up to 50% of the tumor
mass in some cases and are associated with poor prognosis in most
cancers (22). Alterations in
macrophage phenotype can occur at all stages of tumor formation,
including initiation, progression and metastasis. In the present
study, the effect of leptin stimulation of M2 TAMs was investigated
in the tumor microenvironment on the sensitivity of TNBCs to
chemotherapy and the possible mechanisms that may guide future
clinical research and treatment were preliminarily explored.
A link between leptin levels, macrophage function
and cancer progression is plausible. Numerous studies have
highlighted the role of leptin signaling in the progression,
metastasis, stemness induction, angiogenesis and therapeutic
efficacy of breast, colorectal, melanoma, ovarian and other types
of cancer (23). Leptin
significantly promotes breast tumor growth and development of lung
metastases from breast cancer; application of macrophage removers
has been demonstrated to attenuate these effects of leptin
(12). In the present study,
leptin stimulation of M2 TAMs followed by co-culture with
MDA-MB-231 triple-negative breast adenocarcinoma cells reduced
sensitivity to docetaxel compared with that in other groups,
suggesting that the interaction between leptin and M2 TAMs enhances
chemoresistance in TNBC cell lines, which is consistent with the
findings of most previous studies.
Leptin induces the secretion of vascular endothelial
growth factors and proinflammatory cytokines by macrophages. IL-8,
acting as chemotactic factor, promotes autocrine and/or paracrine
tumors and has the potential to serve as a prognostic and/or
predictive cancer biomarker. It is mainly produced by monocytes,
but other cells, such as fibroblasts, epithelial cells, endothelial
cells and hepatocytes, can also produce IL-8 under appropriate
stimulatory conditions (24).
Evidence points to a functional crossover between the leptin and
estrogen signaling pathways; leptin promotes breast cancer tumor
cell development and progression through activation of the
JAK2/STAT3 pathway. Furthermore, leptin induces expression of the
cell cycle protein D1 through STAT3 activation, which regulates the
cell cycle and promotes breast cancer cell growth (25). Leptin-activated STAT3 also promotes
cancer cell stemness and drug resistance through the expression of
key enzymes of the acidic β-oxidation pathway. The growth-promoting
effect of leptin through the ERK pathway has been demonstrated in
breast cancer models (26). The
PI3K/Akt signaling pathway has been implicated in regulating the
leptin-induced epithelial-mesenchymal transition in breast cancer.
This pathway is also considered to contribute to the upregulation
of IL-8 and pyruvate kinase M2 in response to leptin (27). Collectively, these findings
suggested that leptin indirectly promotes the progression of breast
cancer by triggering the release of oncogenic factors from M2-type
macrophages.
The present results suggested that leptin stimulates
IL-8 secretion from TAMs to influence resistance to docetaxel in
TNBC cell lines. Leptin-stimulated IL-8 secretion from TAMs
promotes MDA-MB-231 invasion and migration. These findings provided
further confirmation that the secretion of IL-8 from M2-type TAMs
induced by leptin is linked to the progression of breast cancer and
its resistance to drugs. It was found that IL-8 expression in the
leptin-activated M2-type TAM group was significantly higher than
that in the other groups.
The present findings also suggested that
leptin-stimulated production of IL-8 by TAMs significantly improved
the invasive ability of MDA-MB-231 TNBC cells. Upon treatment with
an IL-8 neutralizing agent before culturing MDA-MB-231 TNBC cells,
the invasive metastatic ability of the cell lines in the
neutralized group was found to be significantly lower than that of
the experimental group, with no significant difference from that of
the blank control group. In summary, the interaction between leptin
and M2-type TAMs may promote IL-8 production by M2-type TAMs,
influencing the development of breast cancer cells. The current
study suggested that there is at least one source of IL-8, which
promotes the interaction of leptin with M2-type TAMs in the tumor
microenvironment. Furthermore, the ability of leptin to stimulate
the production of IL-8 in TAMs contributes to the progression of
breast cancer. Leptin reduces the sensitivity of TNBC cell lines to
docetaxel after its action on M2-type tumor macrophages. Therefore,
the involvement of the leptin-TAMs-IL-8 axis in the interaction
between the tumor microenvironment and breast cancer adds an
additional level of intricacy that contributes to cancer
progression. This interaction may also potentially correlate with
chemoresistance. Therefore, the leptin-TAMs-IL-8 axis may be an
important target for addressing chemoresistance in breast
cancer.
Leptin is primarily produced by secretion from white
adipocytes (28). It has been
previously reported that obesity is not only related to metabolic
diseases but is also closely associated with the development of
breast cancer (29). Leptin levels
are higher in more malignant breast cancers (30). Adipose tissue occupies 90% of the
volume of the mammary gland and can secrete a large number of
lipotropic factors, such as leptin, which can promote breast cancer
invasion, metastasis, neoangiogenesis and epithelial mesenchymal
transition through paracrine secretion (31). Moreover, leptin can accelerate
breast cancer progression by recruiting macrophages in the tumor
microenvironment (32). The effect
of leptin on breast cancer also requires numerous signaling
pathways to achieve. An in-depth study of the leptin signaling
pathway to explore its intrinsic connection with breast cancer
development will lay the foundation for the next step in finding
effective targeted drugs to cut off the leptin-mediated signaling
pathway.
The present study has certain strengths and
weaknesses. Free doxorubicin levels may be reduced due to the
higher binding of doxorubicin to proteins. The proteins are able to
bind doxorubicin and form complexes that limit the availability of
doxorubicin. This process may affect the action of doxorubicin on
tumor cells. Therefore, to avoid the direct effect of leptin on
tumor cell lines as well as docetaxel, the supernatant was
collected after the action of leptin on TAMs before completing
subsequent experiments. This experimental protocol minimizes the
direct effects of leptin on docetaxel. It was partly proved that
the action of leptin on TAM would increase the drug resistance of
tumor cells, and this process might work by regulating IL-8
secretion. However, the process is not rigorous enough to directly
determine the source and destination of IL-8, and further in
vivo experiments and immunolabeling are needed for subsequent
verification.
In conclusion, leptin reduces the sensitivity of
TNBC cell lines to docetaxel after action on M2 tumor macrophages.
The leptin-TAMs-IL-8 axis plays a role in this, potentially
correlating with chemoresistance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG and SD performed the experiments and collected
the primary data. SG, SD and ZT wrote the main manuscript and
prepared figures. All authors reviewed the manuscript. All authors
read and approved the final manuscript. All authors confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Zhang H, Merkher Y, Chen L, Liu N,
Leonov S and Chen Y: Recent advances in therapeutic strategies for
triple-negative breast cancer. J Hematol Oncol.
15(121)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maroni P: Leptin, Adiponectin, and Sam68
in bone metastasis from breast cancer. Int J Mol Sci.
21(1051)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gu L, Wang CD, Cao C, Cai LR, Li DH and
Zheng YZ: Association of serum leptin with breast cancer: A
meta-analysis. Medicine (Baltimore). 98(e14094)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Niu J, Jiang L, Guo W, Shao L, Liu Y and
Wang L: The association between leptin level and breast cancer: A
meta-analysis. PLoS One. 8(e67349)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pan H, Deng LL, Cui JQ, Shi L, Yang YC,
Luo JH, Qin D and Wang L: Association between serum leptin levels
and breast cancer risk: An updated systematic review and
meta-analysis. Medicine (Baltimore). 97(e11345)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miyoshi Y, Funahashi T, Tanaka S, Taguchi
T, Tamaki Y, Shimomura I and Noguchi S: High expression of leptin
receptor mRNA in breast cancer tissue predicts poor prognosis for
patients with high, but not low, serum leptin levels. Int J Cancer.
118:1414–1419. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang WJ, Lai HY, Zhang F, Shen WJ, Chu PY,
Liang HY, Liu YB and Wang JM: MCL1 participates in leptin-promoted
mitochondrial fusion and contributes to drug resistance in
gallbladder cancer. JCI Insight. 6(e135438)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen X, Zha X, Chen W, Zhu T, Qiu J, Røe
OD, Li J, Wang Z and Yin Y: Leptin attenuates the anti-estrogen
effect of tamoxifen in breast cancer. Biomed Pharmacother.
67:22–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma L, Fan Z, Du G and Wang H:
Leptin-elicited miRNA-342-3p potentiates gemcitabine resistance in
pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun.
509:845–853. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li K, Wei L, Huang Y, Wu Y, Su M, Pang X,
Wang N, Ji F, Zhong C and Chen T: Leptin promotes breast cancer
cell migration and invasion via IL-18 expression and secretion. Int
J Oncol. 48:2479–2487. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ojalvo LS, King W, Cox D and Pollard JW:
High-density gene expression analysis of tumor-associated
macrophages from mouse mammary tumors. Am J Pathol. 174:1048–1064.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nadella V, Garg M, Kapoor S, Barwal TS,
Jain A and Prakash H: Emerging neo adjuvants for harnessing
therapeutic potential of M1 tumor associated macrophages (TAM)
against solid tumors: Enusage of plasticity. Ann Transl Med.
8(1029)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li C, Xu X, Wei S, Jiang P, Xue L, Wang J
and Senior Correspondence: Tumor-associated macrophages: Potential
therapeutic strategies and future prospects in cancer. J Immunother
Cancer. 9(e001341)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cao H, Huang Y, Wang L, Wang H, Pang X, Li
K, Dang W, Tang H, Wei L, Su M, et al: Leptin promotes migration
and invasion of breast cancer cells by stimulating IL-8 production
in M2 macrophages. Oncotarget. 7:65441–65453. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fousek K, Horn LA and Palena C:
Interleukin-8: A chemokine at the intersection of cancer
plasticity, angiogenesis, and immune suppression. Pharmacol Ther.
219(107692)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Seferbekova Z, Lomakin A, Yates LR and
Gerstung M: Spatial biology of cancer evolution. Nat Rev Genet.
24:295–313. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bejarano L, Jordāo MJC and Joyce JA:
Therapeutic targeting of the tumor microenvironment. Cancer Discov.
11:933–959. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Smrekar K, Belyakov A and Jin K: Crosstalk
between triple negative breast cancer and microenvironment.
Oncotarget. 14:284–293. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singh A, Mayengbam SS, Yaduvanshi H, Wani
MR and Bhat MK: Obesity programs macrophages to support cancer
progression. Cancer Res. 82:4303–4312. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Habanjar O, Bingula R, Decombat C,
Diab-Assaf M, Caldefie-Chezet F and Delort L: Crosstalk of
inflammatory cytokines within the breast tumor microenvironment.
Int J Mol Sci. 24(4002)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saxena NK, Vertino PM, Anania FA and
Sharma D: Leptin-induced growth stimulation of breast cancer cells
involves recruitment of histone acetyltransferases and mediator
complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem.
282:13316–13325. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yuan HJ, Sun KW and Yu K: Leptin promotes
the proliferation and migration of human breast cancer through the
extracellular-signal regulated kinase pathway. Mol Med Rep.
9:350–354. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, Tang C, Cao H, Li K, Pang X, Zhong
L, Dang W, Tang H, Huang Y, Wei L, et al: Activation of IL-8 via
PI3K/Akt-dependent pathway is involved in leptin-mediated
epithelial-mesenchymal transition in human breast cancer cells.
Cancer Biol Ther. 16:1220–1230. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grossmann ME, Ray A, Nkhata KJ, Malakhov
DA, Rogozina OP, Dogan S and Cleary MP: Obesity and breast cancer:
Status of leptin and adiponectin in pathological processes. Cancer
Metastasis Rev. 29:641–653. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Orecchioni S, Reggiani F, Talarico G and
Bertolini F: Mechanisms of obesity in the development of breast
cancer. Discov Med. 20:121–128. 2015.PubMed/NCBI
|
|
30
|
Riolfi M, Ferla R, Del Valle L,
Piña-Oviedo S, Scolaro L, Micciolo R, Guidi M, Terrasi M, Cetto GL
and Surmacz E: Leptin and its receptor are overexpressed in brain
tumors and correlate with the degree of malignancy. Brain Pathol.
20:481–489. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gonzalez-Perez RR, Lanier V and Newman G:
Leptin's pro-angiogenic signature in breast cancer. Cancers
(Basel). 5:1140–1162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Andò S and Catalano S: The multifactorial
role of leptin in driving the breast cancer microenvironment. Nat
Rev Endocrinol. 8:263–275. 2011.PubMed/NCBI View Article : Google Scholar
|