Introduction
Fournier's gangrene (FG) is defined as necrotizing
fasciitis of the perineal, genital, and/or perianal regions
(1,2). FG is an extremely rare emergency, and
the incidence is only 1.6/100,000(3). The average age of patients is 50-60
years (4). Sepsis and shock may
occur if FG is not promptly diagnosed and surgically debrided, with
a mortality rate between 5-65% and the incidence rate exhibits a
male-to-female ratio of 10:1 (4,5). The
cause of the disease can be identified in 90% of cases. The common
risk factors include diabetes mellitus, morbid obesity, alcoholism
and immunosuppression. The presence of comorbidities, such as heart
disease, renal failure, was described as related to an increased
risk of mortality (4). Despite the
advancements made in etiology and pathophysiology, the high
mortality associated with FG has remained unchanged over the past
decades (6). The common
identifiable sources of infection include the skin, genitourinary
tract and lower gastrointestinal tract (7). Among these sources, FG originating
from anorectal disease carries the worst prognosis (1). The majority of anorectal sources are
benign, such as perianal abscesses and fistula. FG due to rectal
cancer is very rare, with only a few cases having been reported. In
the setting of rectal cancer, the therapeutic options are more
complex than for other forms of FG. The causative rectal tumor
should be removed, but the timing of this is a complex clinical
decision (8,9).
In the present study, the case of a rectal cancer
patient with severe FG was reported and the features of this rare
disease were summarized with an overview of the literature. This
case highlights the rare presentations including severe FG
associated with rectal cancer. Comprehensive examination should be
carried out to reduce the occurrence of misdiagnosis. The present
report also provided a clinical reference to facilitate
perioperative management of rectal cancer-induced FGs. The case is
presented in accordance with the CARE reporting checklist (2016)
(10).
Case report
Main complaints
A 57-year-old man had intermittent hematochezia for
two years and sudden perianal pain for 12 days.
History of present illness
Two years prior, the patient started having
intermittent hematochezia without obvious causes. At that time, he
had no abdominal pain, altered bowel habits or weight loss. The
patient considered it was hemorrhoids, and symptomatic treatment
was administered. In the following years, hematochezia appeared at
irregular intervals. A total of 12 days prior to diagnosis, the
patient felt sharp perianal pain with a high fever that resulted in
an emergency department visit. His body temperature was 38.8˚C, and
his heart rate was 96 beats per minute (bpm). On physical exam, his
perianal region, perineum and scrotum were reddening, and swelling
with skin temperature arose. The patient was misdiagnosed with
extensive perianal abscess without further imaging examinations. A
combination of prolonged antibiotics and emergent surgery were
applied immediately. An abscess was observed reaching from the left
lower back and inguinal area into Scarpa's fascia and descending
into the scrotum. During surgical exploration, an unexpected rectal
mass was found. The diagnosis of well-differentiated rectal
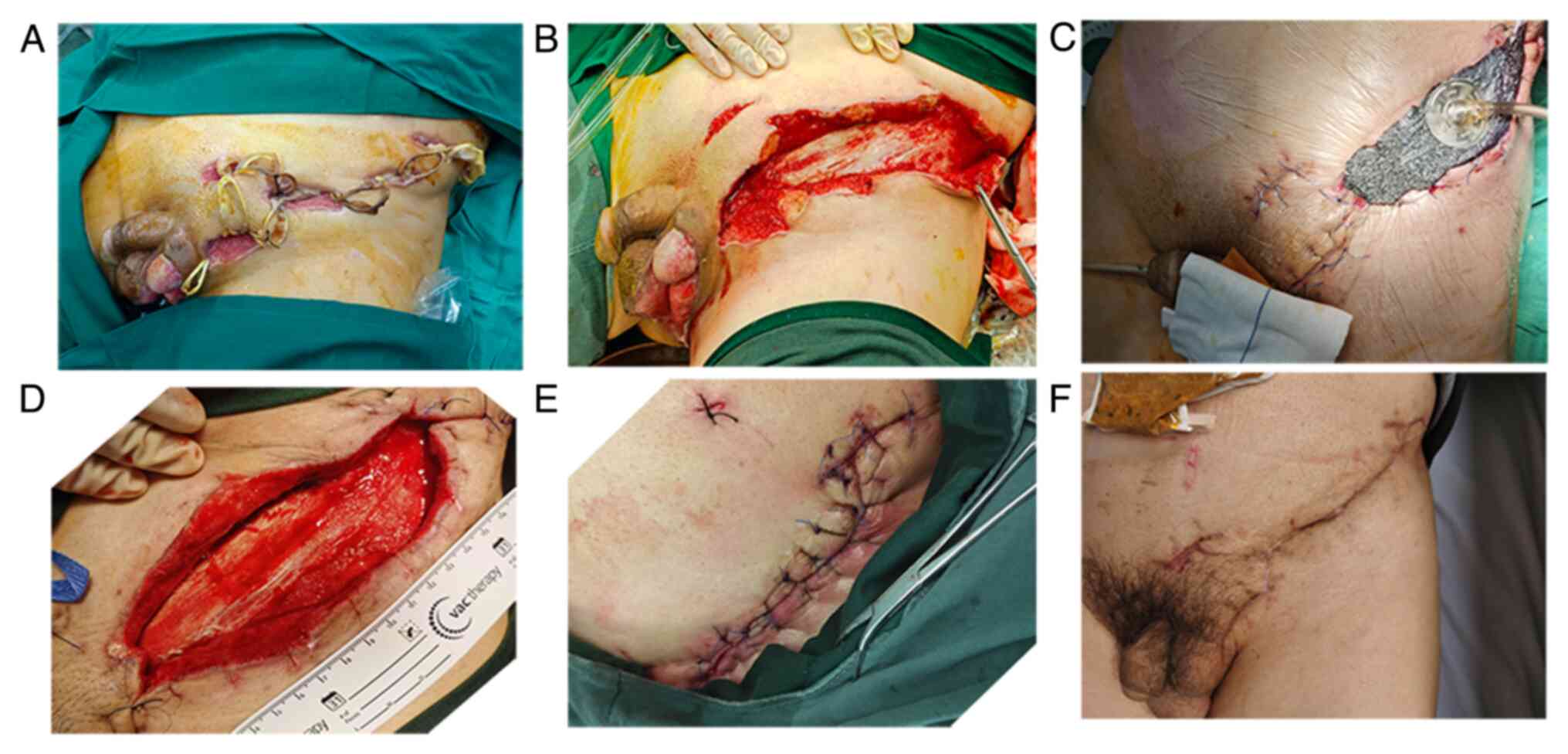
adenocarcinoma was established after frozen biopsy (Fig. S1). Incision, debridement and
drainage were carried out systematically. A total of seven
incisions, 2-3 cm each, were made on the abscess. The abscess was
probed, and pus was drained as much as possible. After repeated
irrigations, several loop drains were retained. An absorbent
dressing was applied over the loop and changed regularly. After
three days, the patient's vital signs returned to normal limits,
and he was transferred to Peking University People's Hospital for
further treatment. Since the onset, his food intake decreased by
50% in the past week and lost 2 kg of weight.
History of past illness
The patient reported no remarkable history of past
illness.
Personal and family history
The patient smoked 10 cigarettes per day for 20
years and quit smoking 7 years prior. He drank a glass of wine per
day (~15 g of ethanol per day). No significant personal or family
history was noted.
Physical examination
The patient's vital signs were within normal limits.
Physical examination revealed a body temperature of 36.5˚C, heart
rate of 78 bpm, blood pressure of 138/89 mm Hg, and respiration of
18 breaths per minute. He looked pale, and the nutrition risk score
was 3 points. Detailed scoring criteria were as follows: Malignant
tumor (1 point); Food intake decreased by 1/2-3/4 in the past week
(2 points); Age ≤70 (0 point). The incision and loop drainage of
the first surgery were observed. Skin defects in the left scrotum
and perineum were observed, and the external anal sphincter was
exposed. A suspected cancer of the lower position was palpated by
digital rectal examination under anesthesia.
Laboratory examinations
The patient's infectious indicators were elevated.
The white blood cell count was 11.30x109/l (reference
values: 4.0-10.0x109/l), C-reactive protein was 15.2
mg/l (reference values: 0-10 mg/l), and erythrocyte sedimentation
rate was 36 mm/H (reference values: 0-15 mm/l). The carcinoma
embryonic antigen level was 37.4 ng/ml (reference values: 0~5.0
ng/ml). Other laboratory findings were unremarkable.
Imaging examinations
Magnetic resonance imaging (MRI) revealed that the
full layers of the rectum were infiltrated. Mesorectal fascia and
extramural vascular invasion were possibly positive. The right
levator ani was invaded. Enhanced computed tomography showed
circumferential thickening of the bowel from the lower rectum to
the upper anal canal. The left inguinal and scrotal defects were
also observed (Fig. 1).
Final diagnosis
Rectal cancer-induced FG was diagnosed.
Treatment
Considering the patient's malnutrition and high risk
of infection, immediate radical resection was denied after a
multidisciplinary meeting. Broad-spectrum empirical antibiotic
treatment was initiated with imipenem (500 mg q12h), linezolid (600
mg q12h) and metronidazole (the initial dose was 1,000 mg and
maintenance doses were 500 mg q6h) via an intravenous drip. Blood
and tissue were collected for bacterial culture and drug
sensitivity tests. Escherichia coli was positive in the
bacterial cultivation and no anaerobic bacteria were detected.
According to the drug susceptibility test, the patient was
sensitive to imipenem and linezolid, thus these two antibiotics
were maintained. With the infection indicators improving,
antibiotics were gradually degraded. A total of four days after
admission, a transverse loop colostomy was performed after a
thorough examination. Benefiting from fecal diversion, the source
of infection was under control. Enteral nutrition and parenteral
nutrition were combined to improve his nutritional status. At the
same time, a vacuum-assisted closure (VAC) device was applied to
accelerate wound healing. The negative pressure was 100 mm Hg, with
5 min of suction followed by 2 min of rest. The prescribed dressing
change period for VAC was three days. Each time, wounds were
serially debrided under local anesthesia until healthy and viable
tissue was visible (Fig. 2). A
total of 12 days later, laparoscopic extra levator abdominoperineal
excision (ELAPE) was performed. The distal sigmoid was closed
without transverse colostomy reversal. The pelvic floor was
reconstructed with biological mesh. The skin flap was transplanted
by the plastic surgeon to repair scrotal defects. Pathological
results indicated a moderately differentiated adenocarcinoma with
perforation. No positive lymph nodes were identified. The proximal
and distal margins were negative, but the circumferential resection
margin was positive. The pathological TNM stage was T4N0M0. The
paraffin-embedded sections from the primary tumor were cut at 4 µm
thickness and attached onto slides. The sections were incubated
with the specific primary antibody (1:100 dilution) at 37˚C for 2
h. Then the anti-rabbit IgG (1:500) was applied onto the sections
and then observed by light microscopy. IHC indicated MLH1(+),
PMS2(+), MSH2(+), MSH6(+), P53(-) and CerB-2(-) (Fig. 3). Next-generation sequencing
indicated mutations in APC, ASXL1, FAT4, FBXW7, KRAS, SMAD3 and
SOX9. The patient was discharged from the hospital on day 15
without any complications. Post-operative chemoradiotherapy was
administered. Radiotherapy of 5,000 cGy was delivered in 25
fractions of 200 cGy five times per week for a total of 5 weeks. A
total of six cycles of the XELOX regimen (130 mg/m2
oxaliplatin on day 1 and 1,000 mg/m2 capecitabine bid
from day 1 to day 14) were applied.
Outcome and follow-up
The wound healed well, and no sign of recurrence was
observed during follow-up for 22 months. The patient was satisfied
with his recovery.
Discussion
There is a lack of successful clinical references
for rectal cancer-induced FG. Rectal cancer-induced FG has several
specific challenges. First, radical resection may not be possible
because of severe infection. Second, the large area of open wounds
increases the risk of tumor dissemination. Third, radical surgery
is complicated because anaplasty is commonly needed for the
reconstruction of perineal and scrotal defects. Finally,
post-operative adjuvant chemoradiotherapy is usually needed, which
may increase the incidence of post-operative complications
(1).
FG is a very insidious disease, with 40% of patients
being asymptomatic. Due to its rarity without typical signs, early
diagnosis of rectal cancer-induced FG is difficult, and
misdiagnosis is common. The initial symptoms include but are not
limited to perianal pain along with tenderness, usually with edema
of the overlying skin, pruritus, crepitus and fever (11). Manifestations of rectal
cancer-induced FG do not differ from the other FGs. Once the
symptoms appear, accompanied by hematochezia or changes in bowel
habits, rectal cancer-induced FG should be considered. Digital
rectal examination is recommended for all FGs originating from
anorectal disease. Diagnosis is usually made clinically, but
radiological diagnostics, such as ultrasound, CT, or MRI, can
determine the extent of the disease.
Sufficient multidisciplinary specialists are
associated with the downward mortality trend of FG (12). In the current case, doctors from
colorectal surgery, infectious disease departments, urinary
surgery, plastic surgery, radiology departments, anesthesiology
departments, intensive care units and radiotherapy departments
participated in the multidisciplinary meeting. These experts'
collaboration contributed to the optimal treatment plan.
Expedited treatment with liquid resuscitation,
broad-spectrum antibiotic therapy and prompt debridement are the
cornerstones of the initial management. Empiric antimicrobial
therapy should start when the diagnosis is suspected. Antibiotic
de-escalation should be based on the results of cultured pathogens
and drug sensitivity tests (13).
Wound care after debridement is essential and lays a foundation for
wound closure. Different strategies have been proposed for wound
care of FG, but their efficacy has not been fully elucidated. VAC
promotes blood supply, inflammatory cell migration and granulation
tissue formation. Current evidence supports that VAC therapy is
effective in the management of large wounds with less pain, lower
discomfort and greater mobility (14). For patients with disseminated FG,
VAC offers an advantage in wound healing and survival (15). The authors' experience confirmed
the safety and efficacy of VAC in the management of rectal
cancer-induced FG.
Diversional stomas in FG did not reduce the risk of
mortality; by contrast, this is a risk factor for poor outcomes
(16). However, in the current
case, rectal cancer invaded the external sphincter, anus-preserving
surgery was unsuitable, and colostomy was inevitable. Loop
colostomy can avoid persistent stool infection and provide
conditions for oral feeding. Perineal defects following
transitional abdominoperineal resection (APR) are a challenge for
anaplasty (17). ELAPE was based
on precise anatomy and conformed to the principle of radical
resection of low rectal cancer. ELAPE could reduce the occurrence
of post-operative complications and chronic perianal pain when
compared with traditional APR. In addition, it may further decrease
the local recurrence rate and improve survival (18). Biological mesh was applied to
reduce perineal hernia, perineal wound complications and
post-operative radiation pelvic disease (19).
FG Severity Index is a numeric score developed in
1995 to stratify risk for FG patients (20). Since then, several modified scales
have been proposed to optimize the accuracy of prognostic
prediction. Recent studies have reported that age, diabetes,
alcoholic liver disease, bedridden status, delayed hospital
presentation, delta neutrophil index and hyperbaric oxygen therapy
are prognostic factors of FG (21-23).
However, their effects on rectal cancer-induced FGs require further
investigation.
Several limitations exist in the present case
report. First, the pictures before incision and drainage were
unavailable. Second, the follow-up period was only 22 months, and
the long-term outcome still needs further evaluation. Third,
large-sample cohort studies are needed to summarize the regularity
of rectal cancer-induced FGs.
In conclusion, the present case highlights the
occurrence of FG as an extremely rare but life-threatening
complication as a result of rectal cancer. In the setting of rectal
cancer, the therapeutic options are more complex than for other
forms of FG. The causative rectal tumor should be removed, but the
timing of this is a complex clinical decision. The results of this
case indicated that multidisciplinary evaluation, early
intervention, staged management and close follow-up lead to
successful treatment for rectal cancer-induced FG.
Supplementary Material
Result of biopsy: Well-differentiated
rectal adenocarcinoma [High-power field of H&E staining
(magnification, x100)].
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Beijing Science
and Technology Planning Project (grant no. 2144000118).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have contributed significantly to the
content of the study. SH and YY confirm the authenticity of all the
raw data. SH and BC collected and organized the patient's clinical
data. SH wrote the first draft. KS designed the study. ZG and FL
contributed to conceptualization and supervision. YY reviewed the
manuscript. All authors were responsible for ensuring that the
descriptions are accurate, and read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Peking University People's Hospital. Approval number:
2022PHB053-001.
Patient consent for publication
Informed written consent was obtained from the
patient for publication of this case report and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eke N: Fournier's gangrene: A review of
1726 cases. Br J Surg. 87:718–728. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hughes T, Bowen D, Saeed K, Juliebø-Jones
P and Somani B: Management of Fournier's gangrene: A practical
guide for clinicians. Br J Hosp Med (Lond). 84:1–9. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hagedorn JC and Wessells H: A contemporary
update on Fournier's gangrene. Nat Rev Urol. 14:205–214.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sumisławski P, Kołecki J, Piotrowska M,
Kotowski M, Szemitko M and Sieńko J: Utility of diagnostic imaging
in the early detection and management of the fournier gangrene.
Diagnostics (Basel). 12(2320)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tufano A, Dipinto P, Passaro F, Anceschi
U, Franco G, Flammia RS, Proietti F, Antonelli L, Di Pierro GB,
Prata F, et al: The value of Fournier's gangrene scoring systems on
admission to predict mortality: A systematic review and
meta-analysis. J Pers Med. 13(1283)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Radcliffe RS and Khan MA: Mortality
associated with Fournier's gangrene remains unchanged over 25
years. BJU Int. 125:610–616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ballard DH, Mazaheri P, Raptis CA, Lubner
MG, Menias CO, Pickhardt PJ and Mellnick VM: Fournier gangrene in
men and women: Appearance on CT, ultrasound, and MRI and what the
surgeon wants to know. Can Assoc Radiol J. 71:30–39.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bruketa T, Majerovic M and Augustin G:
Rectal cancer and Fournier's gangrene-current knowledge and
therapeutic options. World J Gastroenterol. 21:9002–9020.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoshino Y, Funahashi K, Okada R, Miura Y,
Suzuki T, Koda T, Yoshida K, Koike J, Shiokawa H, Ushigome M, et
al: Severe Fournier's gangrene in a patient with rectal cancer:
Case report and literature review. World J Surg Oncol.
14(234)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riley DS, Barber MS, Kienle GS, Aronson
JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG,
Sox H, et al: CARE guidelines for case reports: Explanation and
elaboration document. J Clin Epidemiol. 89:218–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lewis GD, Majeed M, Olang CA, Patel A,
Gorantla VR, Davis N and Gluschitz S: Fournier's gangrene diagnosis
and treatment: A systematic review. Cureus.
13(e18948)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lin TY, Su CC, Chang YC, Chen IH, Ou CH
and Cheng YS: The sufficient multidisciplinary specialists under a
government-led health care system associated with the downward
mortality trend of Fournier's gangrene in Taiwan. Int J Urol.
30:182–189. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tarasconi A, Perrone G, Davies J, Coimbra
R, Moore E, Azzaroli F, Abongwa H, De Simone B, Gallo G, Rossi G,
et al: Anorectal emergencies: WSES-AAST guidelines. World J Emerg
Surg. 16(48)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yanaral F, Balci C, Ozgor F, Simsek A,
Onuk O, Aydin M and Nuhoglu B: Comparison of conventional dressings
and vacuum-assisted closure in the wound therapy of Fournier's
gangrene. Arch Ital Urol Androl. 89:208–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Iacovelli V, Cipriani C, Sandri M,
Filippone R, Ferracci A, Micali S, Rocco B, Puliatti S, Ferrarese
P, Benedetto G, et al: The role of vacuum-assisted closure (VAC)
therapy in the management of FOURNIER'S gangrene: A retrospective
multi-institutional cohort study. World J Urol. 39:121–128.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sarofim M, Di Re A, Descallar J and Toh
JWT: Relationship between diversional stoma and mortality rate in
Fournier's gangrene: A systematic review and meta-analysis.
Langenbecks Arch Surg. 406:2581–2590. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meuli JN, Hubner M, Martineau J, Oranges
CM, Guillier D, Raffoul W and di Summa PG: Impact of etiology
leading to abdominoperineal resection with anterolateral thigh flap
reconstruction: A retrospective cohort study. J Surg Oncol.
127:40–47. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qi XY, Cui M, Liu MX, Xu K, Tan F, Yao ZD,
Zhang N, Yang H, Zhang CH, Xing JD and Su XQ: Extralevator
abdominoperineal excision versus abdominoperineal excision for low
rectal cancer: A meta-analysis. Chin Med J (Engl). 132:2446–2456.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zaheer Ahmad N, Abbas MH, Al-Naimi NMAB
and Parvaiz A: Meta-analysis of biological mesh reconstruction
versus primary perineal closure after abdominoperineal excision of
rectal cancer. Int J Colorectal Dis. 36:477–492. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Laor E, Palmer LS, Tolia BM, Reid RE and
Winter HI: Outcome prediction in patients with Fournier's gangrene.
J Urol. 154:89–92. 1995.PubMed/NCBI
|
|
21
|
Arora A, Rege S, Surpam S, Gothwal K and
Narwade A: Predicting mortality in fournier gangrene and validating
the fournier gangrene severity index: Our experience with 50
patients in a tertiary care center in India. Urol Int. 102:311–318.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shin IS, Gong SC, An S and Kim K: Delta
neutrophil index as a prognostic factor for mortality in patients
with Fournier's gangrene. Int J Urol. 29:1287–1293. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mladenov A, Diehl K, Müller O, von Heymann
C, Kopp S and Peitsch WK: Outcome of necrotizing fasciitis and
Fournier's gangrene with and without hyperbaric oxygen therapy: A
retrospective analysis over 10 years. World J Emerg Surg.
17(43)2022.PubMed/NCBI View Article : Google Scholar
|