Introduction
Adenomyosis is a benign uterine disease whereby the
endometrial glands and stroma are pathologically demonstrated in
the uterine myometrium. Before menopause, patients with adenomyosis
typically exhibit various symptoms, such as excessive menstruation,
dysmenorrhea, pain during sexual intercourse and infertility
(1). However, cases of uterine
corpus cancer developing from the malignant transformation of
adenomyosis have been reported over the past decade (2). In total, 16 cases of uterine corpus
cancer arising from cystic adenomyosis, which is a rare variation
of adenomyosis, have been reported, of which 8 were clear cell
carcinoma. By contrast, the most common histological subtypes of
cancer arising from uterine diffuse adenomyosis were endometrioid
carcinoma, followed by serous carcinoma and clear cell carcinoma
(2). Based on this difference, we
hypothesized that different mechanisms are at work when diffuse
adenomyosis and cystic adenomyosis become malignant. However, to
the best of our knowledge, there have been no reports describing
the mechanism of malignant transformation of cystic adenomyosis or
why clear cell carcinoma occurs more frequently than diffuse
adenomyosis.
In the present report, a case of clear cell
carcinoma arising from cystic adenomyosis over a long period of
time was documented. Cystic adenomyosis and endometriotic cysts are
characterized by the retention of bleeding contents within the cyst
for a long period of time. Furthermore, of the 16 cases of
malignant cystic adenomyosis that have been reported, 8 cases were
clear cell carcinomas, which is consistent with the most common
histological type of ovarian cancer derived from endometriotic
cysts (3). The present report
focused on these similarities and further proposed some of the
mechanisms underlying the malignant transformation of cystic
adenomyosis in this case.
Case report
A 73-year-old woman, gravida 2, para 2, had been
diagnosed with leiomyoma 17 years ago at another hospital. Because
she was already menopausal and had no symptoms, she stopped
receiving regular checkups. She was then referred to our hospital,
Kanazawa university hospital because of a suspected malignancy on
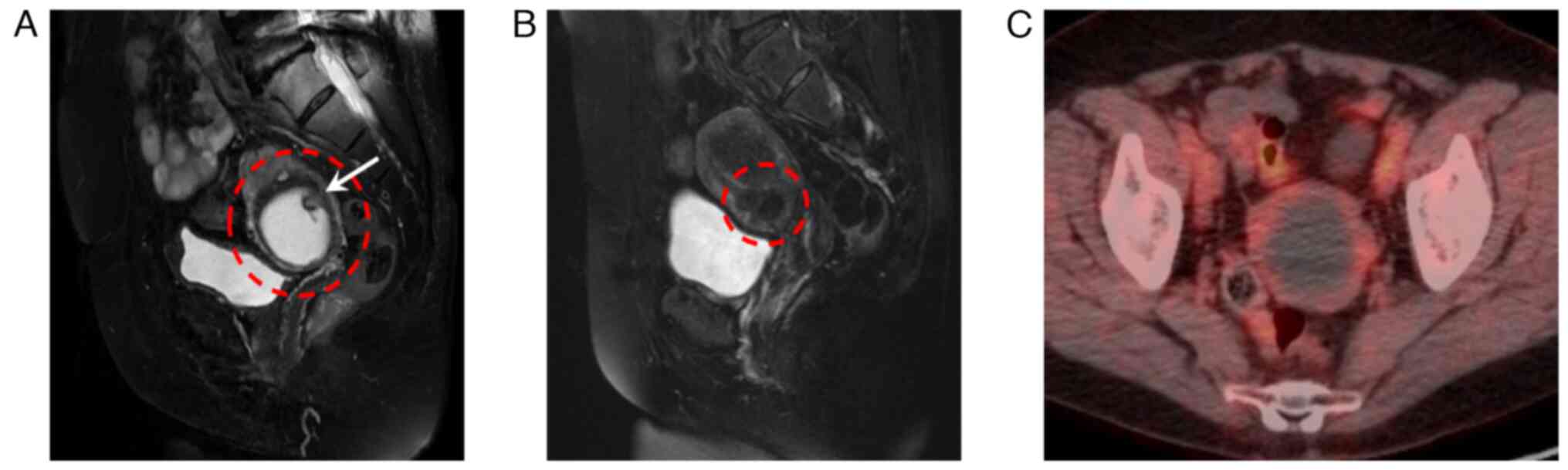
ultrasound and magnetic resonance imaging (MRI) examination. A 5-cm
cystic lesion above the uterine cervix was found, with a 1.5-cm
solid part inside the cyst on ultrasound. Endometrial cytology was
negative. MRI revealed a 5.2-cm cystic mass within the myometrium,
where bloody internal fluid was suggested in T2-weighted images,
leading to a diagnosis of cystic adenomyosis (Fig. 1A). MRI conducted 17 years ago
suggested a 1.5-cm myoma with bleeding between the myometrium and
submucosa (Fig. 1B). Positron
emission tomography-computed tomography (PET-CT) showed no abnormal
accumulation of fluorodeoxyglucose (FDG) in the cyst wall or the
solid part (Fig. 1C). Based on the
aforementioned findings, a final diagnosis of cystic adenomyosis
was made.
Since the size of the cystic adenomyosis increased
with the development of a solid part, malignant transformation was
suspected. Therefore, simple abdominal hysterectomy and bilateral
salpingo-oophorectomy was performed. Written consent to participate
in the study or to use her tissue and for publication was obtained
from the patient according to the principles of the Declaration of
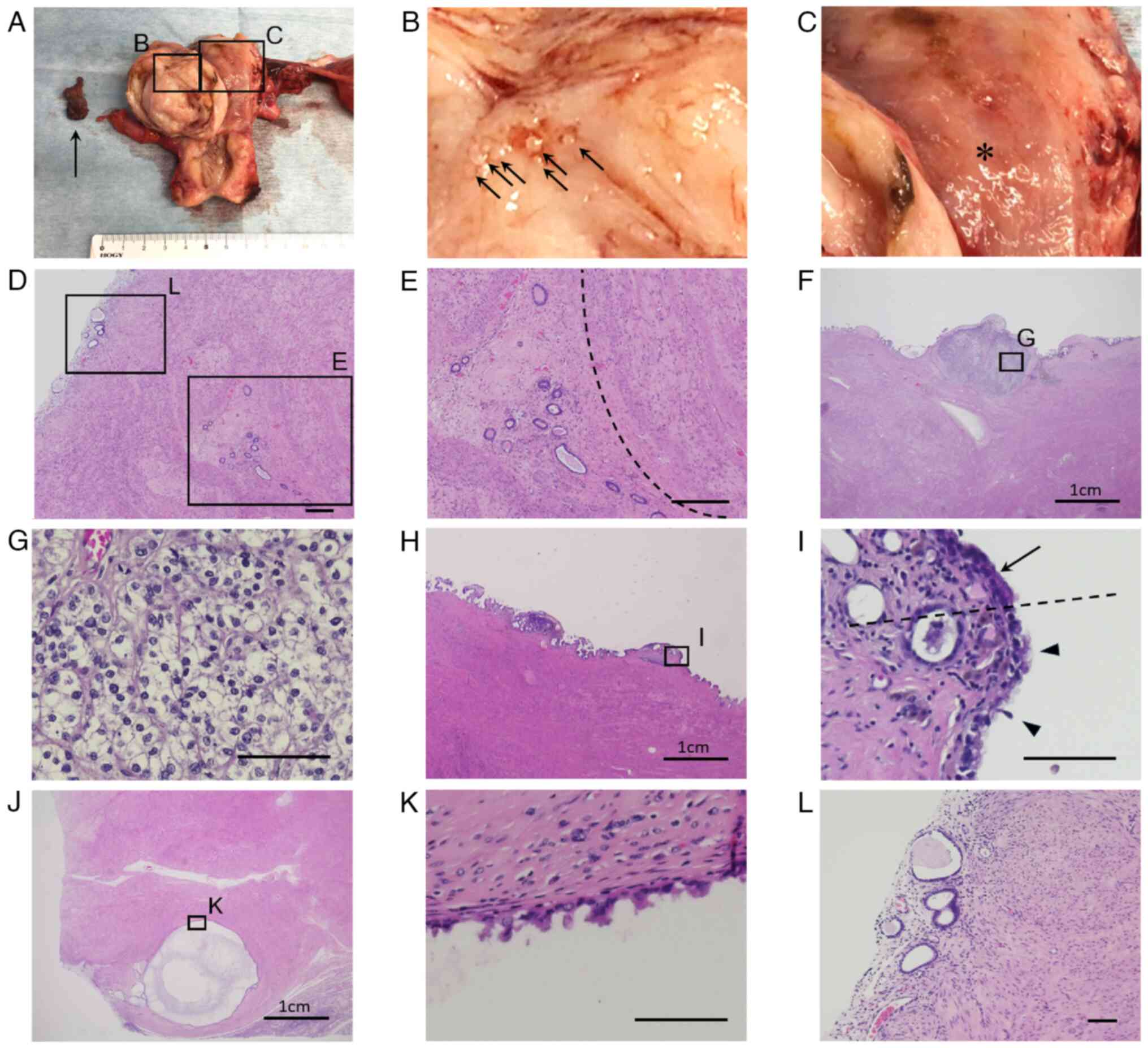
Helsinki. Macroscopically, a thick-walled cystic lesion was
observed on the posterior myometrial wall of the uterine corpus.
The cyst contained brown blood and an organized hematoma, which
corresponded to the solid part observed on the preoperative MRI
(Fig. 2A). Numerous small, raised
lesions were observed in the cyst wall (Fig. 2B). In the uterine cavity, the
endometrium was smooth, but tumorous lesions were not observed
(Fig. 2C).
On microscopic examination, the cyst wall consisted
of smooth muscle cells and hyaline stroma, accompanied by
adenomyosis, which was compatible with cystic adenomyosis (Fig. 2D and E). On the surface of the cyst wall, mural
nodules of ~3 mm that containing nests of clear cell carcinoma
cells were detected (Fig. 2F and
G). In another region of the main
cyst wall, a transformation site from columnar epithelium to
hobnail-shaped epithelial cells was observed (Fig. 2H and I). In addition, the other intramural
small cysts adjacent to the main cystic adenomyosis lesion had
hobnail-shaped atypical cells (Fig.
2J and K), suggesting that the
atypical transformation from the epithelial cells of cystic
adenomyosis had occurred. The depth of tumor invasion from the
surface of the cyst wall was within ≤3 mm. Invasion outside the
adenomyosis or vascular invasion was not observed. In addition,
there were no atypical cells in the endometrium (Fig. 2L).
Subsequently, the degree of oxidative stress in
cystic adenomyosis and cancer cell lesions was evaluated by the
immunohistochemical staining of 8-hydroxy-20-deoxyguanosine
(8-OHdG; a marker of DNA oxidative stress) and 4-hydroxy-2-nonenal
(4-HNE; a marker of late-stage of lipid peroxidation).
Immunohistochemical staining for 8-OHdG was performed using the
standard avidin-biotin complex peroxidase method, as described
previously with a minor modification (3). Briefly, endogenous peroxidase
blocking with 3% hydrogen peroxide was not performed because the
hydroxy radicals generated from hydrogen peroxide can react
directly with the normal deoxyguanosine in tissues to produce
8-OHdG. The primary antibody used in the present case was a mouse
anti-8-OHdG monoclonal antibody (clone N45.1, catalog#MOG-020P;
Japan Institute for the Control of Aging, NIKKEN SEIL Co, Ltd.) at
a dilution of 1:200. Immunohistochemical staining for 4-HNE was
performed using the same method as 8-OHdG. The primary antibody
used in the present case was a mouse anti-4-HNE monoclonal antibody
(clone HNEJ2, catalog#MHN-020P; Japan Institute for the Control of
Aging, NIKKEN SEIL Co, Ltd.) at a dilution of 1:50. Positive
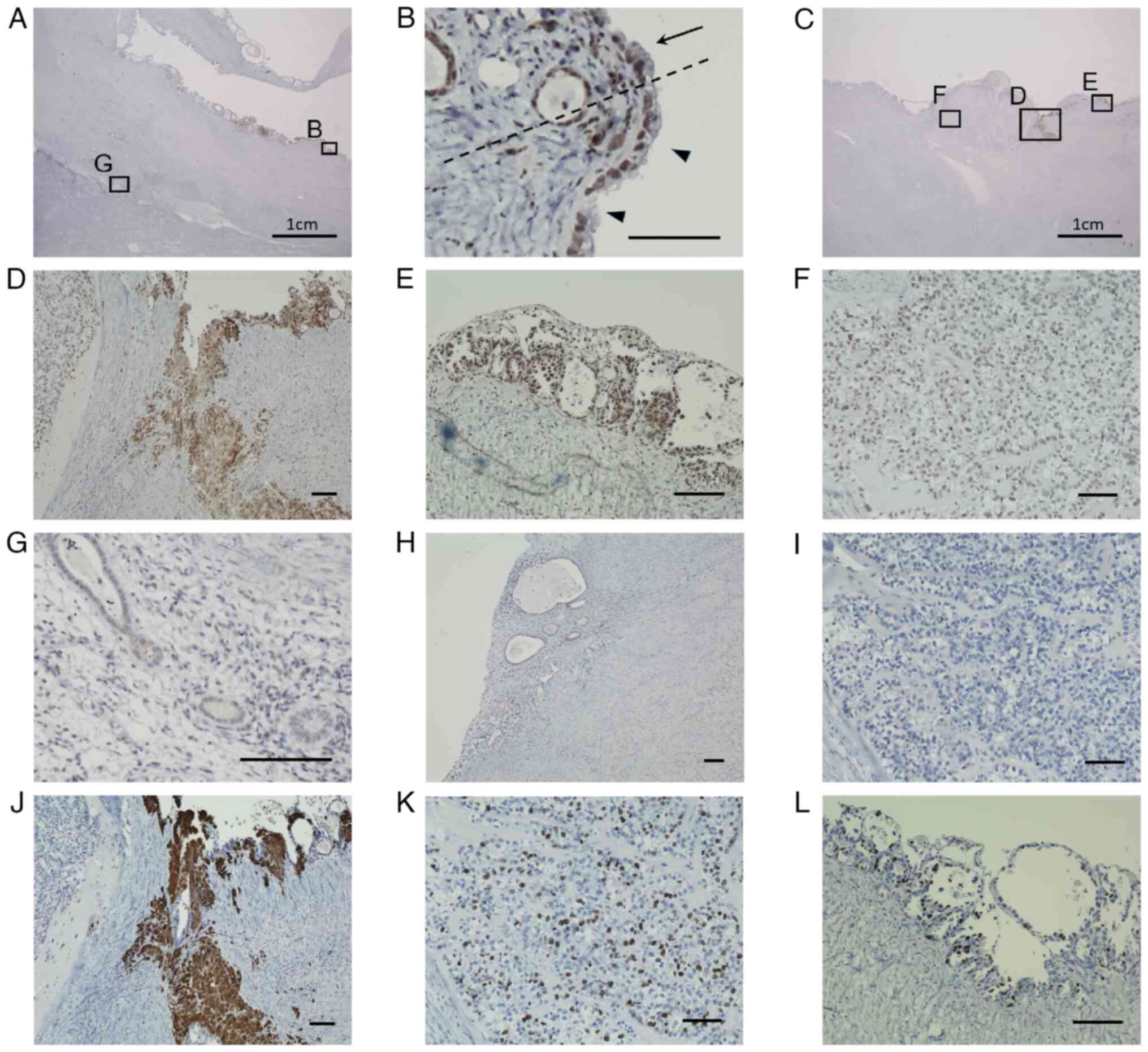
expression of 8-OHdG was observed in the epithelium within cystic
adenomyosis (Fig. 3A). At the
transition site, both columnar epithelial and hobnail-shaped cells
were stained positive for 8-OHdG (Fig.
3B). Almost all of the hobnail-shaped epithelial cells of the
cyst wall stained positive for 8-OHdG (Fig. 3C). Inside the cyst wall, 8-OHdG
staining was observed throughout the area where hemosiderin was
deposited (Fig. 3D). 8-OHdG was
also staining strongly positive in the small cysts that were
layered with atypical cells. They appeared to be in the early
stages of cancerous transformation (Fig. 3E). Although 8-OHdG staining was
negative in the majority of the solid cancer nests, clear cell
carcinoma cells located on the cyst wall side did stain positive
(Fig. 3F). A number of the
adenomyotic lesions around cystic adenomyosis also stained positive
for 8-OHdG, albeit weakly (Fig.
3G). By contrast, there were no 8-OHdG-positive cells in the
normal endometrial epithelium (Fig.
3H). The majority of the clear cell carcinoma cells in the
solid cancer nest stained negative for 4-HNE (Fig. 3I). However, 4-HNE staining was
positive in areas immediately adjacent to the cancer nest where
hemosiderin was deposited (Fig.
3J). The extent of cell proliferation in the cancer cell
lesions were next evaluated by the immunohistochemical staining of
Ki67. Immunohistochemical staining for Ki67 was performed using the
same method as 8-OHdG. The primary antibody used was a rabbit
anti-Ki67 monoclonal antibody (clone sp6, catalog#MA5-14520; Thermo
Fisher Scientific, Inc.) at a dilution of 1:200. Positive
expression of Ki67 was observed in the clear cell carcinoma cells
(Fig. 3K), and the atypical cells
located on the cyst wall (Fig.
3L).
The final diagnosis was uterine corpus cancer, clear
cell carcinoma, pT1aNXM0, stage IA. Since this case was type 2
uterine corpus cancer, which was revealed after total hysterectomy,
the necessity of additional retroperitoneal lymph node dissection
and adjuvant chemotherapy was considered. However, since there were
no tumor lesions in the endometrium, invasion outside the cyst or
vascular invasion, the wishes of the patient and her family were
considered and no additional treatments were conducted. The patient
is currently undergoing out-patient follow-up observation. No
recurrence could be observed 6 months after surgery.
Discussion
The diagnostic criteria for the malignant
transformation of adenomyosis were first proposed by Sampson
(4), before Colman and Rosenthal
(5) modified Sampson's criteria.
They were as follows: (i) Absence of carcinoma in the endometrium
or elsewhere in the pelvis; (ii) demonstration of carcinoma arising
from the epithelium of adenomyosis and not invading from other
sites; and (iii) presence of endometrial stromal cells surrounding
the epithelial glands to support the diagnosis of adenomyosis. The
pathological findings of the present case fulfilled (i) and (iii)
of the aforementioned criteria. However, atypical cells migrated
from the normal epithelium of cystic adenomyosis and no invasion of
cancer from other sites could be observed. Therefore, the cancer
was concluded to be derived from cystic adenomyosis.
Mori et al (6) previously described the cystic
adenomyosis as a rare variation of adenomyosis, which develops when
there is bleeding into the ectopic islands of endometrial glandular
tissues surrounded by the myometrium. Repeated hemorrhage during
menstruation was proposed to be a cause of extensive cyst formation
(6). To the best of our knowledge,
no reports demonstrating such a mechanism by which cystic
adenomyosis forms exist. However, in the present case, multiple
glandular ducts were observed in the area of adenomyosis, where
blood accumulated and formed small cysts (Fig. 2J), meaning that there was no
contradiction with the findings of Mori et al (6). In total, 16 cases of uterine corpus
cancer arising from cystic adenomyosis have been previously
reported. However, to the best of our knowledge, there have been no
reports describing the mechanism by which clear cell carcinoma
develops from cystic adenomyosis. Table SI shows the characteristics of the
present case and the 16 previous cases where detailed information
is available. The long axis of the cysts was 5 cm or more in most
cases. Imaging findings showed that many cases had a solid part in
the cyst. Epidemiologically, the two main histological subtypes
were clear cell carcinoma (9/17, 52.9%) and endometrioid carcinoma
(5/17, 29.4%). Coincidentally, they are also the two main
histological subtypes of ovarian cancer derived from the
endometriotic cyst, occurring at 69.7% for clear cell carcinoma and
24.2% for endometrioid carcinoma (7). By contrast, the most common
histological subtypes of cancer arising from uterine diffuse
adenomyosis were endometrioid carcinoma (76.1%), followed by serous
carcinoma (15.2%) and clear cell carcinoma (6.5%) (2). Therefore, it was hypothesized that
the cystic adenomyosis became malignant through the same mechanism
as that of endometriotic cysts. Although endometriosis and
adenomyosis are diseases that both originate from the ectopic
endometrium, they are reported to utilize different pathways for
their formation. According to Sampson (8), retrograde menstruation corresponds to
a ‘seed’ growing in the ‘soil’ of the peritoneal wall. The cause of
endometriosis remains poorly understood. However, the report by
Sampson, the presence of immune cells and stem cells in the
regurgitated menstrual blood has been found, suggesting that these
cells may be a one of the causes of endometriosis. However, how the
ectopic endometrium invades the myometrium is important for the
formation of adenomyosis. Periodic bleeding from the ectopic
endometrium inevitably initiates tissue damage and repair
processes, inducing fibrosis. In adenomyosis, high levels of
estrogen can stimulate peristalsis of the muscle fibers of the
myometrium, causing stress in the endometrial-myometrial junctional
zone (9,10). Therefore, it is speculated that the
combination of hyperestrogenism and hyperperistalsis causes tissue
self-trauma in the endometrial-myometrial junctional zone, favoring
the translocation of basal endometrium into the myometrium.
Although there are some differences in the etiology
of adenomyosis and endometriosis, they have similarities that they
develop from ectopic endometrium, where cysts are formed by the
cyclic menstrual blood from ectopic endometrium.
Malignant endometriotic cyst transformation has been
reported to be caused by reactive oxygen species accumulation due
to oxidative stress as a result of free iron within the hemorrhagic
cyst (11,12), which in turn causes genetic
mutations (13,14). Oxidative stress is induced by the
decomposition products of blood cells, such as iron, heme, and
thrombin, which can produce free radicals. Iron is known to
generate a hydroxy radicals through the following Fenton reaction:
Fe2+ + H2O2 → Fe3+ + HO• (hydroxy radical) +
OH-.
To validate this oxidative stress hypothesis,
immunohistochemistry for 8-OHdG, which is a marker of DNA oxidative
stress and 4-HNE, a marker of late-stages of lipid peroxidation,
was performed. Both markers are reported to be stain positively in
endometriosis-associated ovarian clear cell carcinoma (15,16).
Positive staining for 8-OHdG in the atypical cells and in the
normal epithelium of cystic adenomyosis was detected. In various
adenomyotic lesions around the cystic adenomyosis, the degree of
oxidative stress varied depending on the time elapsed since blood
had accumulated in the glandular ducts, as some of the ducts were
stained weakly for 8-OHdG. 4-HNE positively was also found in the
atypical cells of cystic adenomyosis, especially in the area where
hemosiderin was deposited. However, 4-HNE stained negative in clear
cell carcinoma cells in the cancer nests. Immunohistochemical
staining for Ki67 was performed, which found its expression in both
atypical cells and clear cell carcinoma cells in the cancer nest.
These findings suggest that chronic oxidative stress occurred in
the epithelial cells of the cyst wall that were directly exposed to
hemorrhage for a long period of time. Marí-Alexandre et al
(17) previously reported that
endometriotic cysts exhibited stronger staining for 8-OHdG compared
with adjacent clear cell carcinoma, suggesting that oxidative
stress is involved in the process of initiating
endometriosis-associated ovarian cancer (17). In the present case, although the
majority of the clear cell carcinoma cells within the nodule were
negative for 8-OHdG and 4-HNE, the malignant cells located on the
cyst wall side were positive for 8-OHdG, supporting the hypothesis
that the hemorrhagic contents within the cyst were likely
responsible for oxidative stress. Furthermore, Ki67 was found to be
expressed in the clear cell carcinoma cells in the cancer nodules
and the atypical epithelial cells within the cyst wall. These
findings also suggested that after malignant transformation, clear
cell carcinoma proliferates regardless of oxidative stress. If we
experience a similar case in the future, we would like to confirm
the expression of proliferating cell nuclear antigen (PCNA; a
marker of cell proliferation). If PCNA is expressed in clear cell
carcinoma after malignant transformation, our hypothesis would be
further supported.
Oxidative stress has been implicated in the
development and progression of cancer through various mechanisms.
In endometriosis-associated ovarian cancer, reactive oxygen species
can rapidly activate Polo-like kinases (PLK), a mitotic regulator,
by regulating DNA replication under stressful conditions, thereby
promoting genome stability. PLK phosphorylates early mitotic
inhibitor-1 (Emi1) to promotes S-phase and mitosis entry, which
suppresses anaphase-promoting complex/cyclosome (APC/C).
Overexpression of Emi1 causes mitotic catastrophe and genome
instability, which promotes tumorigenesis (18). The Emi1/APC/C pathway has been
suggested to be upregulated during occult clear cell carcinoma
tumorigenesis during atypical endometriosis (19). These aforementioned previous
reports of endometriosis-related ovarian cancer suggest that DNA
damage caused by oxidative stress can lead to cell cycle
dysregulation and activation of oncogenic signaling pathways
(18). Therefore, DNA damage
caused by oxidative stress may be a trigger for malignant
transformation, and is thought to be an important mechanism in the
malignant transformation of cystic adenomyosis. This same mechanism
of oxidative stress may also mediate the malignant transformation
of endometriotic cysts. Therefore, the use of oral contraceptives,
which can prevent the malignant transformation of endometriotic
cysts, may also prevent the malignant transformation of cystic
adenomyosis (20). Hysterectomy is
also recommended if the cystic adenomyosis increase in size after
menopause.
One limitation of the present report was the lack of
genetic analysis using laser microdissection. Further examination
of sequential changes in gene mutations during the transforming
process will contribute to clarify the precise roles of oxidative
stress in the carcinogenesis of cystic adenomyosis. If we
experience a similar case in the future, we would like to perform
genetic analysis via laser microdissection.
In conclusion, the present case report proposes the
possible involvement of chronic oxidative stress in the malignant
transformation from cystic adenomyosis to clear cell carcinoma.
Consistent with the present case, previous reports also
demonstrated the late onset of tumor progression and a solid-part
appearance after menopause, which suggests the risk of chronic
exposure to oxidative stress. If the size of the cystic adenomyosis
increases after menopause, the possibility of malignant
transformation should be considered in the same manner as ovarian
endometriotic cysts.
Supplementary Material
Characteristics of 17 cases of uterine
corpus cancer arising from cystic adenomyosis.
Acknowledgements
The authors would like to thank Dr Ayumi Matsuoka
(Department of Obstetrics and Gynecology, Graduate School of
Medical Sciences, Kanazawa University, Kanazawa, Japan) for her
valuable contribution to clinical management and discussion
regarding the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NH, KK, TI and HF contributed to the study
conception and design. NH and KK drafted the first manuscript, and
all authors commented on previous versions of the manuscript. SH,
SN, KK, MN and TI performed clinical management of the patient. KK
conducted immunohistochemistry. NH, KK, TI and HF discussed the
results. KK and NH confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All study methods were conducted in accordance with
the Declaration of Helsinki. Preoperative written informed consent
was obtained from the patient prior to the collection of clinical
data from their medical record.
Patient consent for publication
Written consent for publication of this case report
including all images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peric H and Fraser IS: The symptomatology
of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20:547–555.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Machida H, Maeda M, Cahoon SS, Scannell
CA, Garcia-Sayre J, Roman LD and Matsuo K: Endometrial cancer
arising in adenomyosis versus endometrial cancer coexisting with
adenomyosis: Are these two different entities? Arch Gynecol Obstet.
295:1459–1468. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iizuka T, Ono M, Masumoto S, Mitani Y,
Yamazaki R and Fujiwara H: Amniotic epithelial cells damage by
oxidative stress in cases of diffuse chorioamniotic hemosiderosis.
J Obstet Gynaecol Res. 45:2095–2099. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sampson JA: Endometrial carcinoma of the
ovary, arising in endometrial tissue in that organ. Arch Surg.
10:1–72. 1925.
|
|
5
|
Colman HI and Rosenthal AH: Carcinoma
developing in areas of adenomyosis. Obstet Gynecol. 14:342–348.
1959.PubMed/NCBI
|
|
6
|
Mori M, Furusawa A, Kino N, Uno M, Ozaki Y
and Yasugi T: A rare case of endometrioid adenocarcinoma arising
from cystic adenomyosis. J Obstet Gynaecol Res. 41:324–328.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taniguchi F: New knowledge and insights
about the malignant transformation of endometriosis. J Obstet
Gynaecol Res. 43:1093–1100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into venous circulation. Am J Pathol. 3:93–110. 43.
1927.PubMed/NCBI
|
|
9
|
Leyendecker G, Herbertz M, Kunz G and Mall
G: Endometriosis results from the dislocation of basal endometrium.
Hum Reprod. 17:2725–2736. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leyendecker G, Wildt L and Mall G: The
pathophysiology of endometriosis and adenomyosis: Tissue injury and
repair. Arch Gynecol Obstet. 280:529–538. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamaguchi K, Mandai M, Toyokuni S,
Hamanishi J, Higuchi T, Takakura K and Fujii S: Contents of
endometriotic cysts, especially the high concentration of free
iron, are a possible cause of carcinogenesis in the cysts through
the iron-induced persistent oxidative stress. Clin Cancer Res.
14:32–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mandai M, Matsumura N, Baba T, Yamaguchi
K, Hamanishi J and Konishi I: Ovarian clear cell carcinoma as a
stress-responsive cancer: Influence of the microenvironment on the
carcinogenesis and cancer phenotype. Cancer Lett. 310:129–133.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu TX, Zhao SZ, Dong M and Yu XR: Hypoxia
responsive miR-210 promotes cell survival and autophagy of
endometriotic cells in hypoxia. Eur Rev Med Pharmacol Sci.
20:399–406. 2016.PubMed/NCBI
|
|
15
|
Niiro E, Kawahara N, Yamada Y, Yoshimoto
C, Shimada K, Sudo T and Kobayashi H: Immunohistochemical
expression of CD44v9 and 8-OHdG in ovarian endometrioma and the
benign endometriotic lesions adjacent to clear cell carcinoma. J
Obstet Gynaecol Res. 45:2260–2266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsuchimochi S, Wada-Hiraike O, Urano Y,
Kukita A, Yamaguchi K, Honjo H, Taguchi A, Tanikawa M, Sone K,
Mori-Uchino M, et al: Characterization of a fluorescence imaging
probe that exploits metabolic dependency of ovarian clear cell
carcinoma. Sci Rep. 13(20292)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Marí-Alexandre J, Carcelén AP, Agababyan
C, Moreno-Manuel A, García-Oms J, Calabuig-Fariñas S and
Gilabert-Estellés J: Interplay between MicroRNAs and oxidative
stress in ovarian conditions with a focus on ovarian cancer and
endometriosis. Int J Mol Sci. 20(5322)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hsu JY, Reimann JD, Sørensen CS, Lukas J
and Jackson PK: E2F-dependent accumulation of hEmi1 regulates S
phase entry by inhibiting APC(Cdh1). Nat Cell Biol. 4:358–366.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Gütgemann I, Lehman NL, Jackson PK and
Longacre TA: Emi1 protein accumulation implicates misregulation of
the anaphase promoting complex/cyclosome pathway in ovarian clear
cell carcinoma. Mod Pathol. 21:445–454. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang C, Liang Z, Liu X, Zhang Q and Li S:
The association between endometriosis, tubal ligation, hysterectomy
and epithelial ovarian cancer: Meta-Analyses. Int J Environ Res
Public Health. 13(1138)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mori Y, Inoue Y, Sakai K, Koizumi M,
Okamura N, Sato K and Mizuuchi H: Primary clear cell carcinoma
arising in cystic adenomyosis of the uterus-a case report. Nihon
Sanka Fujinka Gakkai Zasshi. 46:915–917. 1994.PubMed/NCBI(In Japanese).
|
|
22
|
Shinozaki H, MR Ishizuka Y, Sugiura K,
Nishii H, Watanabe A, Ochiai K and Tanaka T: A case of malignant
transformation of cystic adenomyosis in which fine-needle
aspiration cytology led to the diagnosis. Nihon Rinsho Saibo Gakkai
Zasshi. 39(370)2000.(In Japanese).
|
|
23
|
Tsunota C, MK Motoyama T, Yasuda J and
Tatebe A: A case of uterine corpus cancer thought to have arisen
from cystic adenomyosis. Shojinkai Igaku Zasshi. 43:151–155.
2004.(In Japanese).
|
|
24
|
Ohta Y, Hamatani S, Suzuki T, Ikeda K,
Kiyokawa K, Shiokawa A, Kushima M and Ota H: Clear cell
adenocarcinoma arising from a giant cystic adenomyosis: A case
report with immunohistochemical analysis of laminin-5 gamma2 chain
and p53 overexpression. Pathol Res Pract. 204:677–682.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heo SH, Lee KH, Kim JW and Jeong YY:
Unusual manifestation of endometrioid adenocarcinoma arising from
subserosal cystic adenomyosis of the uterus: Emphasis on MRI and
positron emission tomography CT findings. Br J Radiol.
84:e210–e212. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Okuyama D, OM Kotani M and Suzuki A: A
case of clear cell adenocarcinoma of the uterine corpus suspected
to have developed from cystic adenomyosis. Tokyo J Ob-Gyn.
62:131–137. 2013.(In Japanese).
|
|
27
|
Ito M, SK Owada M, Suzuki H and Saito A: A
case of clear cell adenocarcinoma suspected to have developed from
cystic adenomyosis. Fukushima Journal of Medical Science. 64:29–33.
2014.(In Japanese).
|
|
28
|
Ohta Y, MK Yonezawa M and Mizuno K: A case
of uterine corpus cancer arising from cystic adenomyosis. Kanto J
Obst Gynec. 53:553–559. 2016.(In Japanese).
|
|
29
|
Baba A, Yamazoe S, Dogru M, Ogawa M,
Takamatsu K and Miyauchi J: Clear cell adenocarcinoma arising from
adenomyotic cyst: A case report and literature review. J Obstet
Gynaecol Res. 42:217–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ueda M, KJ Okabe M, Morikawa K, Miyahara
Y, Katayama Y, Matsuoka T, Sekino K, Masumoto K and Komatsu R: Two
cases of uterine corpus cancer thought to have arisen from
adenomyosis. Gendai Sanfujinka. 64:303–308. 2016.(In Japanese).
|
|
31
|
Amimoto S, UT Tohyama A, Atsui C, Kurita
T, Kagami S, Kawagoe T, Matsuura Y and Hachisuka T: A case of
uterine corpus cancer thought to have arisen from adenomyosis. Acta
Obstetrica et Gynaecologica Japonica. 70(760)2020.(In
Japanese).
|
|
32
|
Sakata K, YT Jingi A, Kawamura T, Hirata T
and Kihira T: A case of clear cell adenocarcinoma suspected to have
developed from cystic adenomyosis. The Tokai Journal of Obstetrics
and Gynecology. 53:205–211. 2017.(In Japanese).
|
|
33
|
Okamoto S, HN Mizusaki M, Hamada K, Kita
K, Ichikawa H, Takahashi T, Kato I, Katayama H and Sengoku K: A
case of clear cell adenocarcinoma suspected to have developed from
cystic adenomyosis. Journal of the Hokkaido Obstetrical and
Gynecological Society. 64(106)2020.(In Japanese).
|
|
34
|
Gomez NF, Still MA, Akki AS,
Carbajal-Mamani SL and Cardenas-Goicoechea J: Uterine clear cell
carcinoma arising from cystic adenomyosis: A case report. J Obstet
Gynaecol Res. 41:1175–1177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dateki M, OA Yamaguchi T, Ueno N, Fujisaki
A, Kawagoe Y and Samejima H: A case of endometrial cancer arising
from cystic adenomyosis. Acta Obstetrica et Gynaecologica Japonica.
73(396)2021.(In Japanese).
|