Introduction
Lung cancer was the leading cause of globally cancer
death (1). The incidence rate of
non-small cell lung cancer (NSCLC) was about 85% of lung cancers
(2). NSCLC was a heterogeneous
disease invading the lung and adjacent tissues, eventually
progressing and spreading to distant sites (3). A computed tomographic (CT) scan and a
positron emission tomographic scan using
18F-fluorodeoxyglucose was used for the staging of NSCLC
(3,4). Whilst chances for a complete
remission or a cure were highest in early-stage cancers, survival
rates decreased when the cancer was locally advanced (3,5).
Proper treatment of locally advanced NSCLC can result in a cure or
long-term survival (3). A locally
advanced lung tumor was resectable if surgery was sufficient to
achieve complete tumor removal and if the patient was able to
tolerate the surgery (3). For
patients with unresectable locally advanced NSCLC who were
medically or surgically inoperable, chemoradiotherapy (CRT) was
recommended (6). Typically, the
unresectable patients were managed with concurrent CRT using a
platinum-based doublet with a standard radiation dose of 60 Gy
(7-9).
After the initial treatment, patients without disease progression
were treated by consolidation durvalumab (7,10).
Patients who were likely to undergo radiation therapy (RT) should
be assessed for the risk of lung toxicity secondary to radiation
(3,11). The mean lung dose and the volume of
healthy lungs receiving ≥20 Gy radiation doses were good indicators
of the risk of radiation-induced lung injury (3,11).
For the treatment planning of RT, selective nodal irradiation was
recommended (12). From the
European Society for Radiotherapy and Oncology (ESTRO) guideline,
two options were recommended for the definition of nodal clinical
target volume (CTV): (a) geometric expansion, with CTV including
the nodal gross tumor volume (GTV) plus 5 mm margin, and (b) lymph
node (LN) stations, with CTV including the affected LN stations
(13). Conventionally, the option
for geometric expansion was known as involved-field irradiation
(IFI). It was expected that CTVs using the option of LN stations
were larger than those with the other option because LN stations
were frequently wider than the 5 mm margin from GTVs (14). However, it was unclear which option
was more advantageous for reducing the normal tissue dose to avoid
radiation toxicities.
Therefore, we retrospectively evaluated whether
dosimetric differences existed in the nodal CTV using the two
options for locally advanced NSCLC.
Patients and methods
Type of study
This single-institutional study was retrospectively
conducted at our hospital. This study was carried out in accordance
with the Declaration of Helsinki. Our institutional review board
(The Research Ethics Committee of Kagawa University Faculty of
Medicine, Kagawa, Japan) approved this study (approval number:
2022-165). After the approval, we investigated the patients who
were treated at our hospital between 2017 and 2022.
Patients
The inclusion criteria of this study were as
follows: patients over 20 years old; patients who had locally
advanced NSCLC with cT4N2M0(15);
patients who underwent RT; and patients who were treated between
2017 and 2022 at our department. The exclusion criteria were as
follows: patients who refused to participate in this study. Written
informed consent was obtained from each patient before treatment
planning. The patient consent for publication in written form was
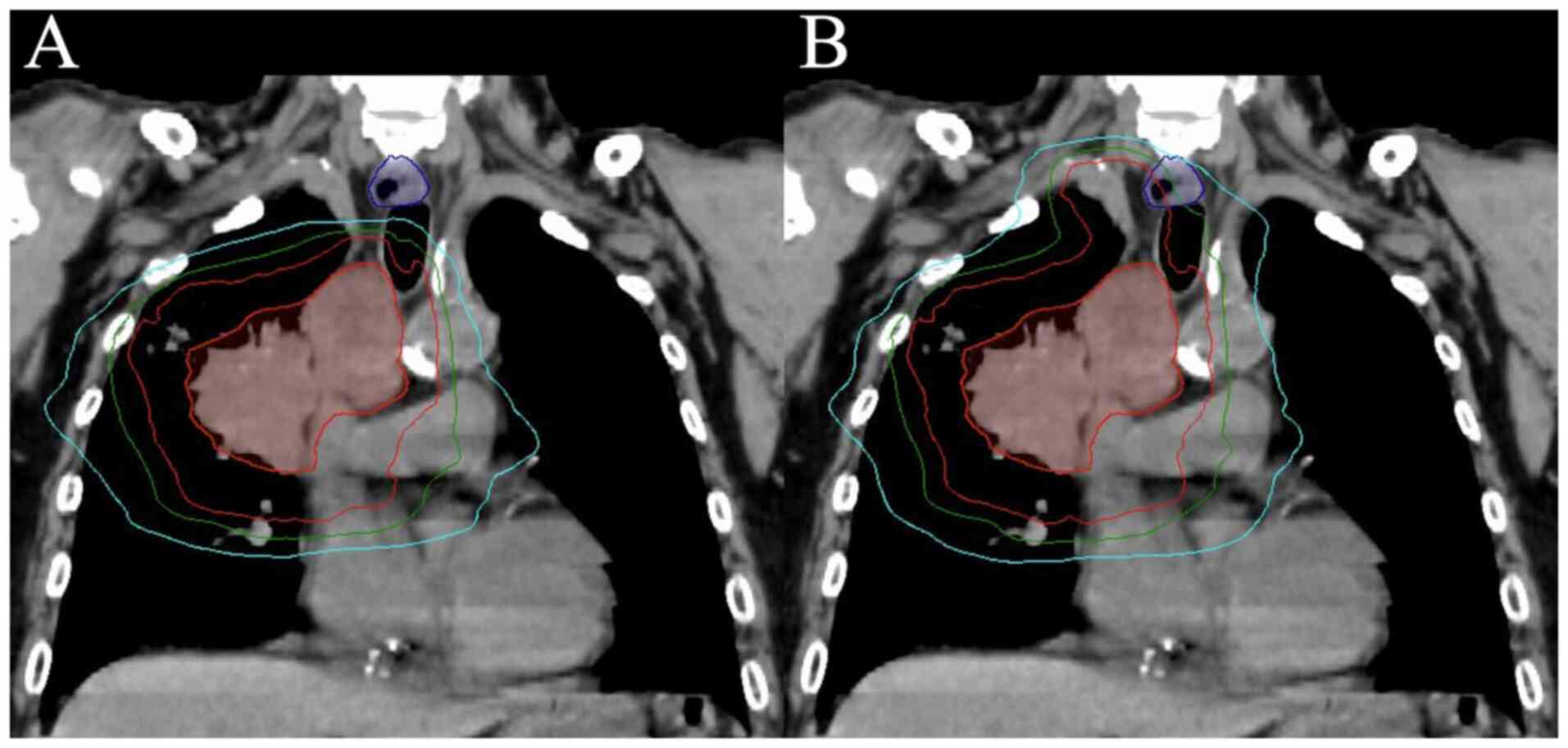
obtained regarding the anonymized CT images in Fig. 1. In total, 17 patients met the
selection criteria without refusal, and we used their treatment
planning CT images for this study.
Treatment planning
A radiation treatment planning system (Eclipse™ v16;
Varian Medical Systems) was used. We contoured nodal CTVs based on
the ESTRO guideline's options of: (a) geometric expansion, with CTV
including the nodal GTV plus 5 mm margin, and (b) LN stations, with
CTV including the affected LN stations (13). The 5 mm margins for planning target
volume (PTV) were added to the nodal and primary tumors' CTVs.
Treatment planning of 60 Gy in 30 fractions to the PTV
D50% was performed using volumetric modulated arc
therapy; Dn% was the irradiated dose to n% of
volumes of the structure; Dncc was the irradiated dose
received by the highest irradiated n cc volumes of the
structure; Dmean was the mean irradiated dose to the
structure; VnGy was the percentage of volumes of the
structure at least irradiated n Gy. Our goals of normal
tissue dose constraints were as follows: D0.1cc of the
spinal cord, ≤45 Gy; V20Gy, V5Gy, and
Dmean of the lungs, ≤40%, ≤65%, and ≤20 Gy,
respectively; V60Gy and Dmean of the
esophagus, ≤17% and ≤34 Gy, respectively; V50Gy and
Dmean of the heart, ≤25% and ≤20 Gy, respectively.
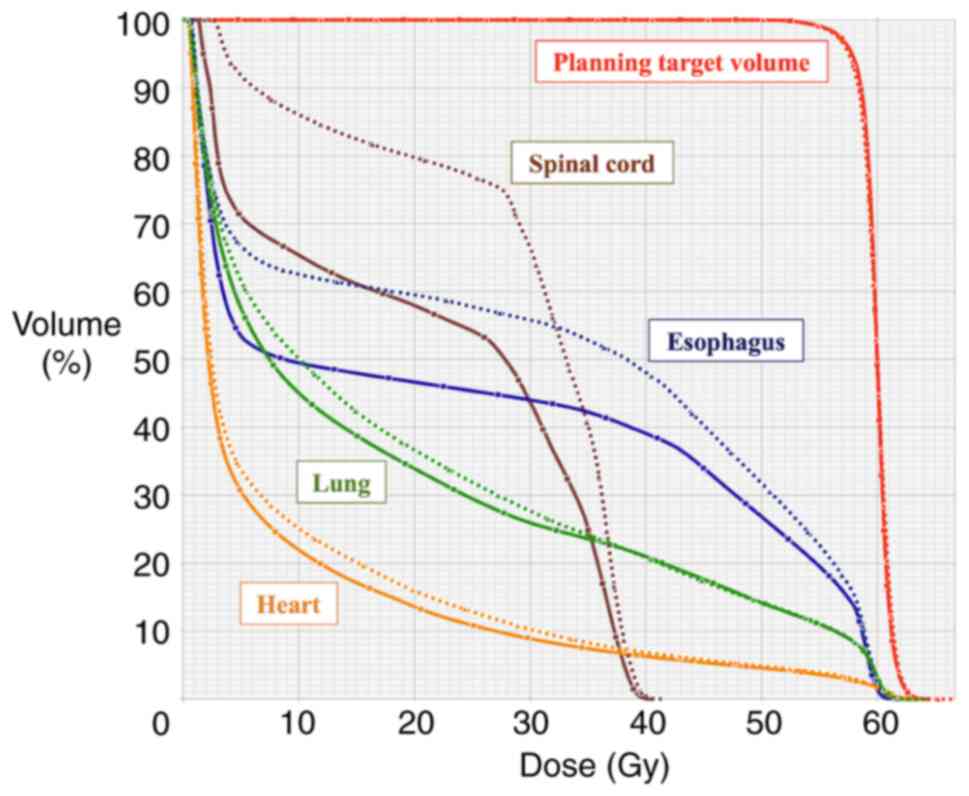
Examples of the dose distribution and the dose-volume histogram
(DVH) are shown in Figs. 1 and
2, respectively.
Statistical analysis
We compared DVH parameters between the two options
using the Wilcoxon signed-rank test. V20Gy of the lungs
was the most common DVH parameter for NSCLC (11). We evaluated the difference of
V20Gy of the lungs between the two options in patients
with or without LN metastases in stations 2 or 3 because stations 2
or 3 were wider for cranial-caudal direction than other stations
(14). For the difference of
V20Gy of the lungs between the two options, patients
with and without LN metastases in these stations were analyzed as
separate groups using the Wilcoxon rank sum test. Statistical
significance was defined as P<0.05. The software program JMP Pro
15 (SAS Institute) was used for the statistical analyses.
Results
Patient characteristics are listed in Table I. Stations 2, 3, 4, 5, 6, 7, 10,
and 11 were involved in 6, 4, 11, 4, 4, 7, 12, and 12 patients,
respectively (Table II). The DVH
parameters between the two options are listed in Table III, and the target coverage of
PTV D95% was comparable between the two options. The
normal tissue dose constraints of the spinal cord, lungs,
esophagus, and heart were fulfilled in both options. The option of
geometric expansion was associated with a significantly lower
V60Gy and Dmean of the esophagus,
V20Gy, V5Gy and Dmean of the
lungs, and Dmean of the heart than the option of LN
stations (P=0.017, P<0.001, P<0.001, P<0.001, P<0.001
and P=0.029, respectively).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Value |
|---|
| Age, years | |
|
Median | 68 |
|
Range | 45-77 |
| Sex, n (%) | |
|
Male | 14(82) |
|
Female | 3(18) |
| Histology, n (%) | |
|
Squamous
cell carcinoma | 9(53) |
|
Adenocarcinoma | 6(35) |
|
Non-small
cell carcinoma | 2(12) |
| Tumor laterality, n
(%) | |
|
Right | 12(71) |
|
Left | 5(29) |
| Tumor location, n
(%) | |
|
Upper
lobe | 13(76) |
|
Lower
lobe | 4(24) |
| c-stagea IIIB with cT4N2M0 | 17(100) |
| Gross tumor volume,
cc | |
|
Median | 213 |
|
Range | 39-812 |
 | Table IIInvolved lymph node stations in each
patient. |
Table II
Involved lymph node stations in each
patient.
| Patient no. | Involved station
nos. |
|---|
| 1 | 2, 6 and 10 |
| 2 | 4 and 7 |
| 3 | 4 and 11 |
| 4 | 2, 3, 4, 7, 10 and
11 |
| 5 | 2 and 4 |
| 6 | 4 and 10 |
| 7 | 7 and 11 |
| 8 | 4, 5, 10 and 11 |
| 9 | 5, 6, 10 and 11 |
| 10 | 4, 7, 10 and 11 |
| 11 | 2, 4, 10 and 11 |
| 12 | 7 and 11 |
| 13 | 3, 5, 6, 10 and
11 |
| 14 | 2, 4, 7, 10 and
11 |
| 15 | 4, 10 and 11 |
| 16 | 3, 5, 6 and 10 |
| 17 | 2, 3, 4, 7, 10 and
11 |
 | Table IIIComparison of dose-volume histogram
parameters between the two options in all patients. |
Table III
Comparison of dose-volume histogram
parameters between the two options in all patients.
| Parameter | Geometric expansion
(n=17) | Lymph node stations
(n=17) | P-value |
|---|
| PTV, volume
(cc) | 569 (149-2005) | 635 (184-2109) | <0.001 |
| PTV,
D95% (Gy) | 58.2
(57.5-58.7) | 58.1
(57.5-58.7) | 0.260 |
| Spinal cord,
D0.1cc (Gy) | 39.1
(30.2-40.3) | 39.1
(32.8-40.5) | 0.998 |
| Lung,
V20Gy (%) | 20.5
(14.8-33.9) | 24.0
(15.1-36.7) | <0.001 |
| Lung,
V5Gy (%) | 40.4
(25.8-57.6) | 45.4
(32.6-61.7) | <0.001 |
| Lung,
Dmean (Gy) | 12.2
(8.7-18.4) | 13.5
(9.5-19.4) | <0.001 |
| Esophagus,
V60Gy (%) | 0.0 (0.0-1.4) | 0.2 (0.0-2.6) | 0.017 |
| Esophagus,
Dmean (Gy) | 12.2
(6.6-24.9) | 16.1
(13.6-30.5) | <0.001 |
| Heart,
V50Gy (%) | 0.7 (0.0-11.4) | 0.8 (0.0-11.5) | 0.284 |
| Heart,
Dmean (Gy) | 3.3 (0.6-19.1) | 4.6 (0.7-18.9) | 0.029 |
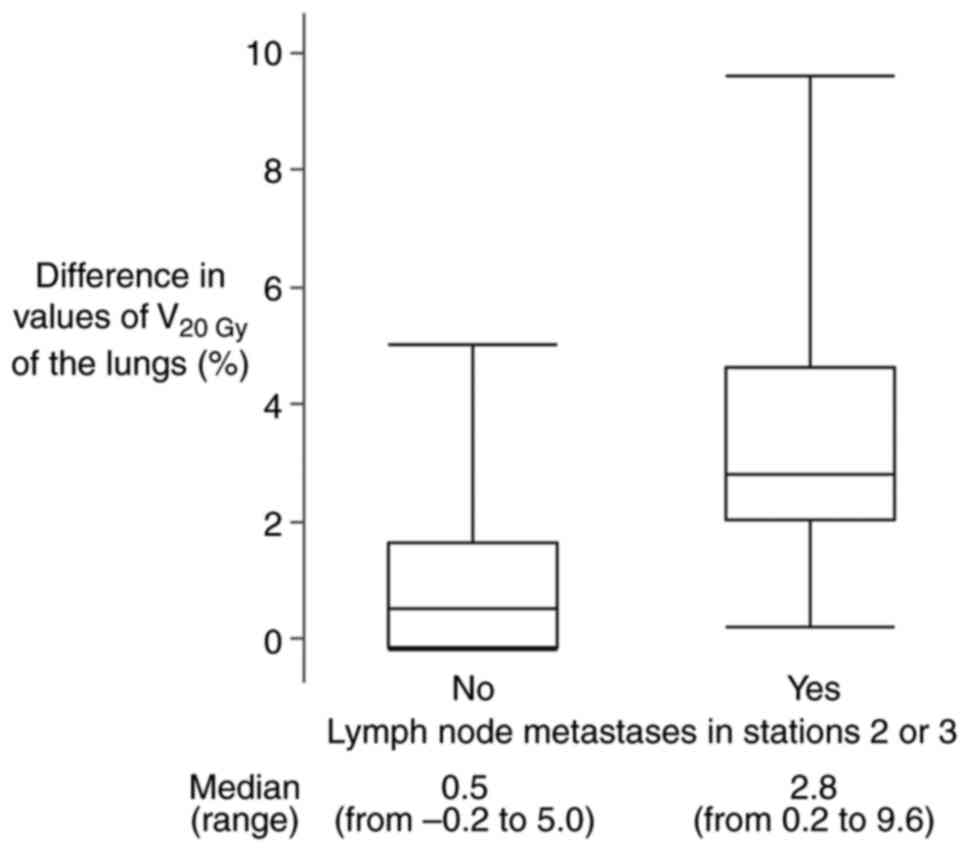
For the V20Gy of the lungs, the eight
patients (47%) with LN metastases in stations 2 or 3 had
significantly larger difference values between the two options than
the nine patients (53%) without those metastases (Fig. 3); median values of the difference
were 2.8% (range, from 0.2 to 9.6%) and 0.5% (range, from -0.2 to
5.0%) with and without LN metastases in stations 2 or 3,
respectively (P=0.027).
Discussion
In this study, we identified the merits of the
option for geometric expansion to reduce the irradiated dose to the
esophagus, lungs and heart in all patients with locally advanced
NSCLC as compared with the option for LN stations. In daily
clinical practice, if the dose constraints of the esophagus, lungs
and heart were not fulfilled with the options for LN stations, we
should try using the option for geometric expansion.
Esophagitis was one of the major toxicities after RT
for locally advanced NSCLC. In a large phase 3 trial using CRT for
locally advanced NSCLC, grades 2 and 3 esophagitis after a
radiation dose of 60 Gy in 30 fractions occurred in 24% and 7% of
patients, respectively (8). In
long-term results of the trial, the maximum grade of esophagitis
was one of the factors that affected overall survival (OS) on a
multivariable analysis (9).
Therefore, our finding might be important for prolonging OS through
reducing the irradiated dose to the esophagus in all patients.
Pneumonitis was also one of the major adverse events
after thoracic RT. As the V20Gy increased, the incidence
rate of fatal pneumonitis rose in proportion in an international
individual patient data meta-analysis: V20Gy <20%,
0.0% fatal rate; 20-29.99%, 1.0%; 30-39.99%, 2.9%, respectively
(11). In the meta-analysis, the
V20Gy was one of the predictors of fatal pneumonitis
(11). Therefore, our finding
might be important for avoiding fatal pneumonitis through reducing
the irradiated dose to the lungs in all patients and especially in
patients with LN metastases in stations 2 or 3. Stations 2 or 3
were wider for cranial-caudal direction than other stations
(14). In stations 2 or 3, a 5 mm
margin might be more advantageous to reduce the irradiated dose to
the lungs than including the affected LN stations.
Recently, cardiac toxicities after RT for locally
advanced NSCLC has been a topic. Pooled analysis of six prospective
trials showed that the heart dose was associated with symptomatic
cardiac events in multivariate analysis (16). The article concluded that heart
doses should be minimized (16).
Therefore, the present finding might be important for avoiding
symptomatic cardiac events through reducing the irradiated dose to
the heart in all patients.
To the best of our knowledge, no other study has
compared the options for geometric expansion and LN stations, to
date. For a somewhat modified comparison, a randomized phase 2
trial was conducted for no geometric expansion without CTV margins
vs. LN stations (17). The trial
showed that the option without CTV margins had significantly lower
DVH parameters for the esophagus, lung, and heart than the option
of LN stations (17). However,
this CTV definition without margins was not mentioned in the ESTRO
guideline (13). Therefore, it was
difficult to compare the previous knowledge with our findings.
Conventionally, the option for geometric expansion was known as
IFI. Compared to elective nodal irradiation that irradiated
mediastinal LN stations without LN metastases, IFI reduced the dose
to the lungs and the incidence rate of pneumonitis (18). It was our new finding that IFI in
all patients reduced the lung dose compared to the option of LN
stations.
Due to its retrospective nature, our study has
certain limitations, such as the small number of samples analyzed
and its single-institutional design. A multi-center study was
preferable to a single-center study to enhance the reliability and
generalizability of our findings.
In conclusion, using the option for geometric
expansion might help reduce the V60Gy and
Dmean of the esophagus, V20Gy,
V5Gy and Dmean of the lungs, and
Dmean of the heart in all patients, and the
V20Gy of the lungs in patients with LN metastases in
stations 2 or 3. A further large-scale study is needed to support
our findings. Moreover, further research is necessary whether the
option for geometric expansion reduces the incidence of
esophagitis, pneumonitis and symptomatic cardiac events compared
with the option for LN stations.
Acknowledgements
This abstract was presented at the 65th Annual
Meeting of the American Society for Radiation Oncology (San Diego,
CA, USA; October 1-4, 2023), and was published as Abstract no.
2136.
Funding
Funding: The present study was supported by JSPS KAKENHI (grant
no. JP20K16790).
Availability of data and materials
The data generated in the present study are not
publicly available due to the restricted permission for the current
study by the institutional review board (Research Ethics Committee
of Kagawa University Faculty of Medicine, Kagawa, Japan) but may be
requested from the corresponding author.
Authors' contributions
ST conceived and designed the study. ST, MA, TK and
TN acquired data. ST and MA confirmed the authenticity of all the
raw data. ST analyzed the data. ST, MA, TK, TN and TS interpreted
the data. ST drafted the manuscript. Article revision was
critically done by all authors. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of Kagawa University
Faculty of Medicine (Kagawa, Japan) approved the present
retrospective study (approval no. 2022-165). Written informed
consent for participation was obtained before treatment
planning.
Patient consent for publication
Written patient consent for publication was obtained
for the anonymized CT images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kinoshita FL, Ito Y and Nakayama T: Trends
in lung cancer incidence rates by histological type in 1975-2008: A
population-based study in Osaka, Japan. J Epidemiol. 26:579–586.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aoun-Bacha Z, Bitar N, Saleh WA, Assi H,
Bahous J, Boukhalil P, Chami H, Dabar G, El Karak F, Farhat F, et
al: Diagnosis and management of patients with stage III non-small
cell lung cancer: A joint statement by the Lebanese Society of
Medical Oncology and the Lebanese Pulmonary Society (Review). Oncol
Lett. 25(113)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Covington MF, Koppula BR, Fine GC, Salem
AE, Wiggins RH, Hoffman JM and Morton KA: PET-CT in Clinical Adult
Oncology: II. Primary Thoracic and Breast Malignancies. Cancers
(Basel). 14(2689)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Balata H, Fong KM, Hendriks LE, Lam S,
Ostroff JS, Peled N, Wu N and Aggarwal C: Prevention and early
detection for NSCLC: Advances in thoracic oncology 2018. J Thorac
Oncol. 14:1513–1527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Daly ME, Singh N, Ismaila N, Antonoff MB,
Arenberg DA, Bradley J, David E, Detterbeck F, Früh M, Gubens MA,
et al: Management of Stage III Non-Small-Cell Lung Cancer: ASCO
Guideline. J Clin Oncol. 40:1356–1384. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Simone CB II, Bradley J, Chen AB, Daly ME,
Louie AV, Robinson CG, Videtic GMM and Rodrigues G: ASTRO radiation
therapy summary of the ASCO guideline on management of stage III
non-small cell lung cancer. Pract Radiat Oncol. 13:195–202.
2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with or
without cetuximab for patients with stage IIIA or IIIB
non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bradley JD, Hu C, Komaki RR, Masters GA,
Blumenschein GR, Schild SE, Bogart JA, Forster KM, Magliocco AM,
Kavadi VS, et al: Long-Term Results of NRG Oncology RTOG 0617:
Standard-versus high-dose chemoradiotherapy with or without
cetuximab for unresectable stage III non-small-cell lung cancer. J
Clin Oncol. 38:706–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-Year survival outcomes from the PACIFIC
Trial: Durvalumab after chemoradiotherapy in stage III
Non-Small-Cell Lung Cancer. J Clin Oncol. 40:1301–1311.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Palma DA, Senan S, Tsujino K, Barriger RB,
Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, et al:
Predicting radiation pneumonitis after chemoradiation therapy for
lung cancer: An international individual patient data
meta-analysis. Int J Radiat Oncol Biol Phys. 85:444–450.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
De Ruysscher D, Faivre-Finn C, Moeller D,
Nestle U, Hurkmans CW, Le Péchoux C, Belderbos J, Guckenberger M
and Senan S: Lung Group and the Radiation Oncology Group of the
European Organization for Research and Treatment of Cancer (EORTC).
European Organization for Research and Treatment of Cancer (EORTC)
recommendations for planning and delivery of high-dose, high
precision radiotherapy for lung cancer. Radiother Oncol. 124:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nestle U, De Ruysscher D, Ricardi U, Geets
X, Belderbos J, Pöttgen C, Dziadiuszko R, Peeters S, Lievens Y,
Hurkmans C, et al: ESTRO ACROP guidelines for target volume
definition in the treatment of locally advanced non-small cell lung
cancer. Radiother Oncol. 127:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itazawa T, Tamaki Y, Komiyama T, Nishimura
Y, Nakayama Y, Ito H, Ohde Y, Kusumoto M, Sakai S, Suzuki K, et al:
The Japan Lung Cancer Society-Japanese Society for Radiation
Oncology consensus-based computed tomographic atlas for defining
regional lymph node stations in radiotherapy for lung cancer. J
Radiat Res. 58:86–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gospodarowicz MK, Wittekind C and Brierley
JD (eds): Lung. In: TNM Classification of Malignant Tumours. 8th
edition. Wiley-Blackwell, New Jersey, pp106-112, 2016.
|
|
16
|
Wang K, Eblan MJ, Deal AM, Lipner M, Zagar
TM, Wang Y, Mavroidis P, Lee CB, Jensen BC, Rosenman JG, et al:
Cardiac toxicity after radiotherapy for stage III non-small-cell
lung cancer: Pooled analysis of dose-escalation trials delivering
70 to 90 gy. J Clin Oncol. 35:1387–1394. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cui T, Zhang A, Cui J, Chen L, Chen G, Dai
H, Qin X, Li G and Sun J: Feasibility of omitting the clinical
target volume under PET-CT guidance in unresectable stage III
non-small-cell lung cancer: A phase II clinical trial. Radiother
Oncol. 181(109505)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li
J, Liu X, Wang R, Li B, et al: A randomized study of involved-field
irradiation versus elective nodal irradiation in combination with
concurrent chemotherapy for inoperable stage III nonsmall cell lung
cancer. Am J Clin Oncol. 30:239–244. 2007.PubMed/NCBI View Article : Google Scholar
|