Introduction
Bladder cancer (BLCA) is the most common malignancy
of the genitourinary system in China and ranking tenth worldwide
(1-3).
BLCA is classified into non-muscular-invasive BLCA (NMIBC) and
muscle-invasive BLCA (MIBC). NMIBC, which accounts for 75% of
BLCAs, progresses slowly and has a long survival, however it still
develops into MIBC in nearly 30% of NMIBC (4,5).
Treatment outcomes for MIBC are less favorable, with shorter
recurrence-free survival (RFS) and overall survival (OS) (6,7).
Early identification of BLCA is closely linked to prognosis.
However, patients with early BLCA often do not present with
specific symptoms. Therefore, exploring the molecules associated
with BLCA is of great importance to monitoring the incidence of
BLCA and improving the clinical treatment strategy.
Interleukin 1 receptor-like 2 (IL1RL2), also known
as IL36R, is part of the interleukin-1 receptor family. Alongside
four other family members interleukin-1 receptor type I,
interleukin-1 receptor type II, interleukin-1 receptor-like 1 and
interleukin-18 receptor 1-IL1RL2 forms a cluster of cell receptor
genes. Research on IL36R primarily focuses on its crucial role as a
mediator of inflammatory responses. The three receptor agonists,
IL-36α, IL-36β and IL-36γ, bind to the IL-36R complex and exert
pleiotropic effects, particularly in the context of inflammatory
bowel diseases (8-11).
The established interaction between inflammation and cancer
recognizes chronic inflammation as a hallmark of cancer (12). High expression of IL1RL2 has been
observed in colorectal cancer, demonstrating a pro-metastatic
effect and association with patient prognosis (13). IL1RL2 has also been revealed to
play a regulatory role in breast, gastric and lung cancer (14-16).
However, the relationship between IL1RL2 and BLCA is less
understood. Thus, the objective of the present study was to assess
the prognostic significance of IL1RL2 in BLCA and investigate its
correlation with clinical pathological features of the disease.
Materials and methods
Clinical specimens
A total of eight pairs of BLCA tissues and adjacent
non-cancerous tissues from Peking University First Hospital-Miyun
Hospital were collected between January 2018 and January 2023
(Beijing, China). Each pair of bladder tissue and adjacent
non-cancerous tissue came from the same patient. Additionally, 17
pairs of BLCA and adjacent non-cancerous tissues, along with 112
paraffin-embedded BLCA tissue blocks at various stages, were
gathered. Data from 112 patients with bladder cancer were
incorporated into the Miyun cohort. All patients were
pathologically diagnosed with urothelial carcinoma, and the
histological characteristics of the samples were confirmed by
experienced urological pathologists using hematoxylin-eosin
staining. The present study was approved (approval no.
2023-029-001) by the Medical Ethics Committee of Peking University
First Hospital-Miyun Hospital (Beijing, China). Clinicopathological
characteristics of patients (including sex and age distribution)
are listed in Table I.
 | Table IClinicopathologic analysis of IL1RL2
expression in bladder cancer. |
Table I
Clinicopathologic analysis of IL1RL2
expression in bladder cancer.
| | Expression level of
IL1RL2 (%) | |
|---|
| Variables | Low | High | P-value |
|---|
| Age, n (%) | | | 0.287 |
|
<65 | 25 (34.7%) | 10 (25.0%) | |
|
≥66 | 47 (65.3%) | 30 (75.0%) | |
| Sex, n (%) | | | 0.091 |
|
Male | 63 (87.5%) | 30 (75.0%) | 0.392 |
|
Female | 9 (12.5%) | 10 (25.0%) | |
| Body mass
index | 24.83±2.72 | 24.31±3.60 | |
| Smoking, n (%) | | | 0.700 |
|
Yes | 22 (31.0%) | 11 (27.5%) | |
|
No | 49 (69.0%) | 29 (72.5%) | |
| Hypertension, n
(%) | | | 0.527 |
|
Yes | 31 (43.7%) | 15 (37.5%) | |
|
No | 40 (56.3%) | 25 (62.5%) | |
| Diabetes, n
(%) | | | 0.493 |
|
Yes | 6 (8.4%) | 5 (12.5%) | <0.001 |
|
No | 65 (91.6%) | 35 (87.5%) | |
| Tumor diameter,
cm | 2.95±2.26 | 4.79±3.35 | |
| Tumor number, n
(%) | | | 0.858 |
|
≥3 | 48 (66.7%) | 26 (65.0%) | |
|
<3 | 24 (33.3%) | 14 (35.0%) | |
| Pathological T, n
(%) | | | <0.001 |
|
≤ pT2 | 69 (95.8%) | 20 (50.0%) | |
|
≥ pT3 | 3 (4.2%) | 20 (50.0%) | |
| Histological grade,
n (%) | | | 0.006 |
|
G1-2 | 31 (43.1%) | 7 (17.5%) | |
|
G3-4 | 41 (56.9%) | 33 (82.5%) | |
| Pathological N, n
(%) | | | <0.001 |
|
Yes | 0 (0.0%) | 8 (20.0%) | |
|
No | 72 (100.0%) | 32 (80.0%) | |
| State of survival,
n (%) | | | <0.001 |
|
Death | 7 (9.72%) | 15 (37.5%) | |
|
Survival | 65 (90.3%) | 25 (62.5%) | |
| Survival time,
days | 674.11± 315.92 | 617.91± 371.54 | 0.408 |
| State of recurrent,
n (%) | | | 0.739 |
|
Recurrent | 16 (22.2%) | 10 (25.0%) | |
|
Recurrence-free | 56 (77.8%) | 30 (75.0%) | |
| Recurrence-free
survival time, days | 601.44± 347.13 | 701.24± 560.84 | 0.212 |
In silico analysis of IL1RL2 using
online datasets
Transcriptome and clinical data from the The Cancer
Genome Atlas (TCGA)-BLCA datasets, encompassing 410 patients with
bladder carcinoma, were downloaded from UCSC XENA (https://xena.ucsc.edu/) (17). The Xiantao tool (https://www.xiantao.love/) was used for visualization
and analysis of expression differences, prognosis and enrichment
(18).
Cell culture
Human ureteral epithelial cells (SV-HUC-1) and human
BLCA cell lines (T24, J82, UMUC3 and SW780) were obtained from the
American Type Culture Collection and cultured according to the
manufacturer's protocols. SV-HUC-1 cells were maintained in F-12K
medium (Gibco; Thermo Fisher Scientific, Inc.), while T24, J82,
UMUC3 and SW780 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). All media were supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
1% penicillin G-streptomycin (Sigma-Aldrich; Merck KGaA). Cultures
were kept at 37˚C with 5% CO2.
Reverse transcription-quantitative
(RT-q) PCR
RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA synthesis was performed using a reverse transcription
system (Tiangen Biotech Co., Ltd.) following the manufacturer's
protocol (42˚C for 15 min, 95˚C for 3 min, 4˚C maintenance).
RT-qPCR was conducted on the 7500 Reverse transcription PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.), with GAPDH as
the internal reference. The thermocycling conditions were as
follows: pre-denaturation at 95˚C for 3 min, denaturation at 95˚C
for 30 sec, annealing extension at 60˚C for 30 sec, deformation and
annealing extension for 40 cycles. The dissolution curve was
increased by 0.5˚C every 2 cycles to 95˚C. The primer sequences
were as follows: IL1RL2 forward, 5'-TCCCGAAGAGTTGTGTTTTGG-3, and
reverse, 5'-TGAGTGTGTCAGTATGGCTTGA-3'; and GAPDH forward,
5'-GGAGCGAGATCCCTCCAAAAT-3, and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. The 2-ΔΔCq method was
used for quantification (19).
Western blotting
The primary antibody was incubated at 4˚C overnight,
and the secondary antibody was incubated at room temperature for 1
h. Total protein was extracted using NP-40 lysis buffer (Beyotime
Institute of Biotechnology) and quantified by the BCA method
(Pierce; Thermo Fisher Scientific, Inc.). Proteins were separated
by SDS-PAGE (10% gel concentration) and transferred to PVDF
membranes. After blocking with 5% skimmed milk for 1 h at room
temperature, membranes were incubated with IL1RL2/IL36R antibody
(1:1,000; cat. no. 10090; ABclonal Biotech Co., Ltd.) followed by a
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(1:2,000; cat. no. sc-2004/sc-2005; Santa Cruz Biotechnology,
Inc.). GAPDH (1:1,000; cat. no. sc-47724; Santa Cruz Biotechnology,
Inc.) served as the internal reference. Immunoreactive bands were
visualized using the ECL Plus kit (Applygen Technologies Inc.).
Immunohistochemistry (IHC)
Paraffin samples were sliced to a thickness of 5 µm.
All paraffin samples were cut by the same pathologist and were of
similar thickness. IHC staining was performed using the PV-6000
universal kit (cat. no. IB000088; ZSGB-BIO; OriGene Technologies,
Inc.). Sections were heated at 70˚C for 1 h, deparaffinized in
xylene and rehydrated (95% anhydrous ethanol), followed by antigen
retrieval with citrate repair solution at 110˚C for 10 min. Slides
were incubated at room temperature for 20 min away from light) with
endogenous peroxidase inhibitor blocking agent and sheep serum
(cat. no. ZU-9022). IL1RL2/IL36R antibody (1:500; cat. no. 10090;
ABclonal Biotech Co., Ltd.) was applied (incubated overnight at 4˚C
in the dark), followed by incubation with a biotinylated secondary
antibody (cat. no. 32020; Thermo Fisher Scientific, Inc.; room
temperature for 20 min away from light) and staining with the DAB
substrate kit (cat. no. ZU-9019; ZSGB-BIO; OriGene Technologies,
Inc.). When the background of the slide was brown, reaction was
terminated in tap water. Blue was reversed in running tap water for
30 min. Then gradient dehydration with ethanol and xylene was
carried out. Finally, it was sealed with neutral resin. IL1RL2
expression was graded based on staining intensity (1, no staining;
2, weak; 3, moderate; 4, strong) and the percentage of reactive
cells (1, 0-25%; 2, 26-50%; 3, 51-75%; 4, >75%). The final score
(range 1-16) was the product of these variables. The degree of
dyeing has several intermediate stages, and the pink brown is also
one of them, representing the degree of dyeing. Images were
observed using a light microscope.
Statistical analysis
Data were analyzed using GraphPad Prism 9.0
(Dotmatics) or SPSS 20.0 (IBM). Results are expressed as the mean ±
SD. Continuous variables were compared using paired and unpaired
Student's t-test or one-way ANOVA followed by Bonferroni's post hoc
test (for multiple-group comparison) single factor analysis.
Pearson's chi-square test or Fisher's exact test were used for
correlation analysis. Survival rates were analyzed using the
Kaplan-Meier method and log-rank P-tests. Prognostic correlations
between clinicopathological and IHC data were assessed by
univariate and multivariate Cox regression analyses.
*P<0.05 was considered to indicate a statistically
significant difference.
Results
Il1RL2 is upregulated in BLCA and
associated with tumor stage
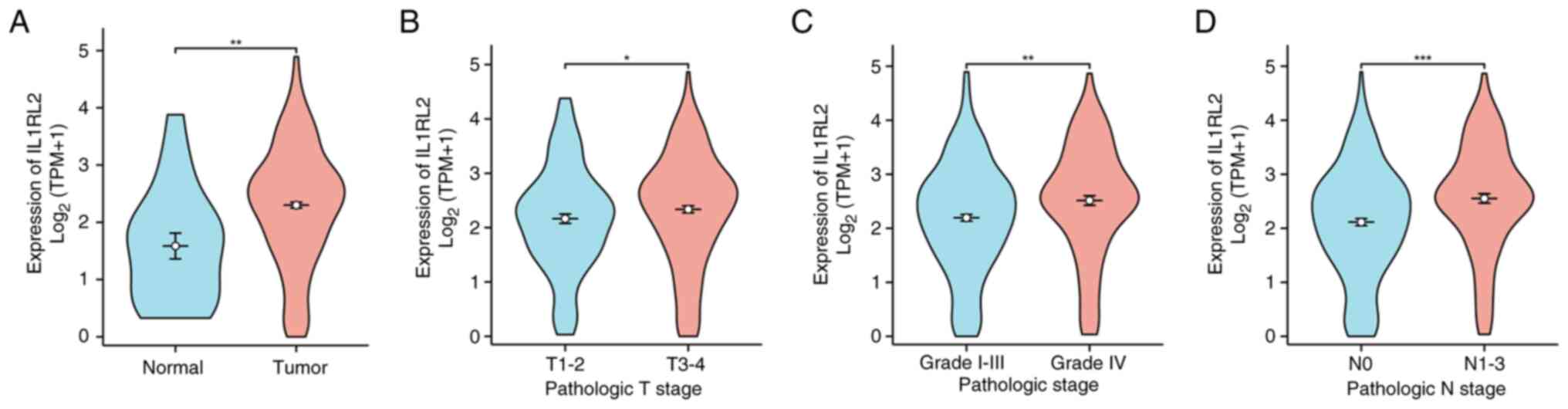
To investigate the expression of IL1RL2 in BLCA,
IL1RL2 mRNA expression was examined using data from the TCGA
database. As illustrated in Fig.
1A, IL1RL2 expression in BLCA tissues from TCGA was
significantly elevated compared with normal tissues. Data from TCGA
indicated upregulated expression of IL1RL2 mRNA in high-stage and
high-grade BLCA tissues (Fig. 1B
and C). Additionally, IL1RL2 mRNA
was also highly expressed in patients with BLCA and lymph node
metastasis (Fig. 1D).
IL1RL2 demonstrates elevated
expression in both BLCA cell lines and tissues
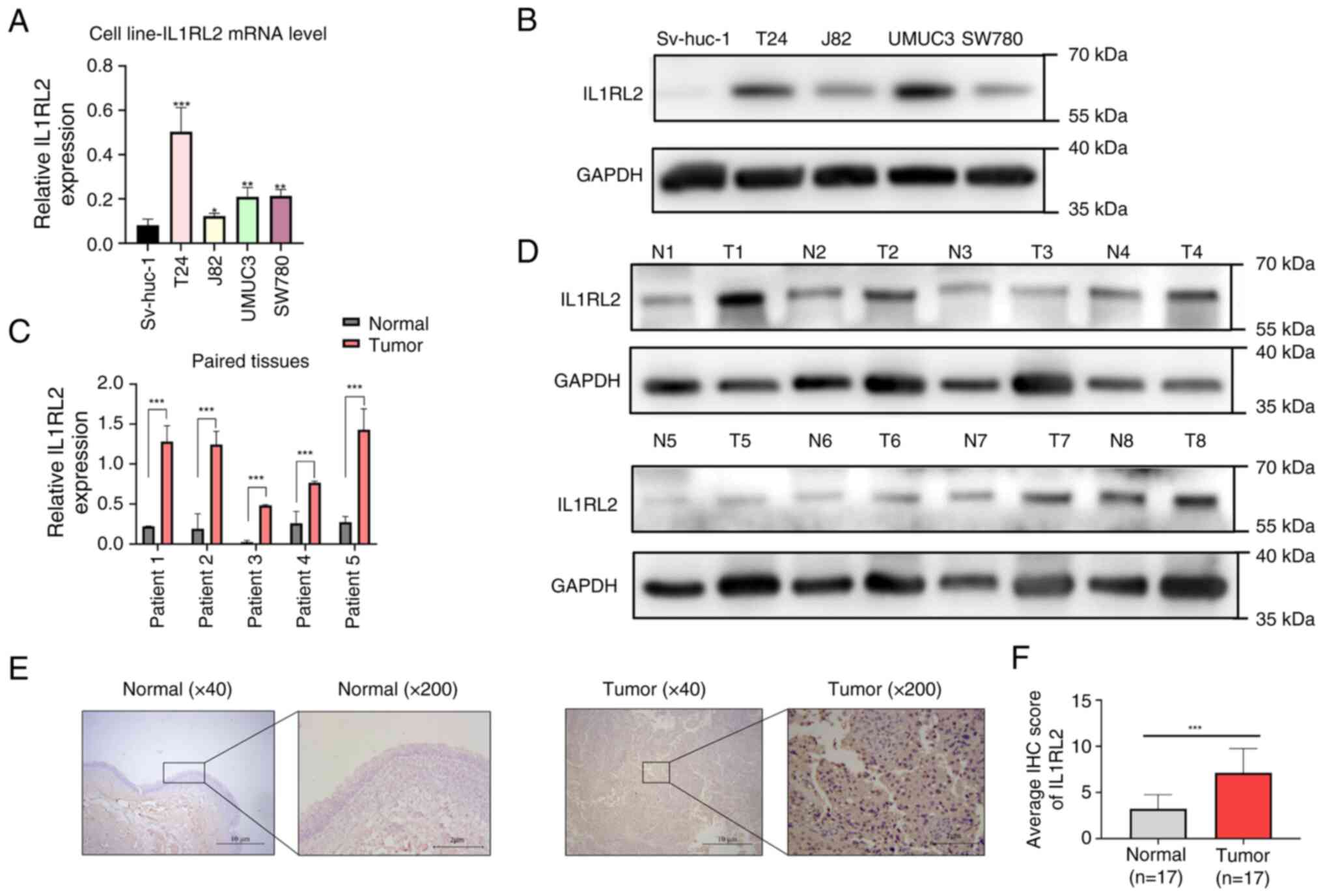
RT-qPCR was employed to assess IL1RL2 mRNA levels
specifically in BLCA cell lines. The results revealed higher IL1RL2
mRNA expression in the tumor cell lines compared with SV-HUC-1
cells, as depicted in Fig. 2A.
Meanwhile, the mRNA expression level of IL1RL2 in BLCA tissues was
higher than that in adjacent normal tissues (Fig. 2C). Furthermore, western blot
analysis demonstrated an upregulation of IL1RL2 protein expression
in both BLCA cell lines (Fig. 2B)
and tissues (Fig. 2D). To further
understand the IL1RL2 presence in BLCA tissues, IHC was performed
on 17 pairs of BLCA tissues and adjacent normal tissues. The
results revealed that IL1RL2 was highly expressed in BLCA tissues
compared with adjacent normal tissues (Fig. 2E and F).
IL1RL2 is highly expressed in
high-stage and high-grade BLCA
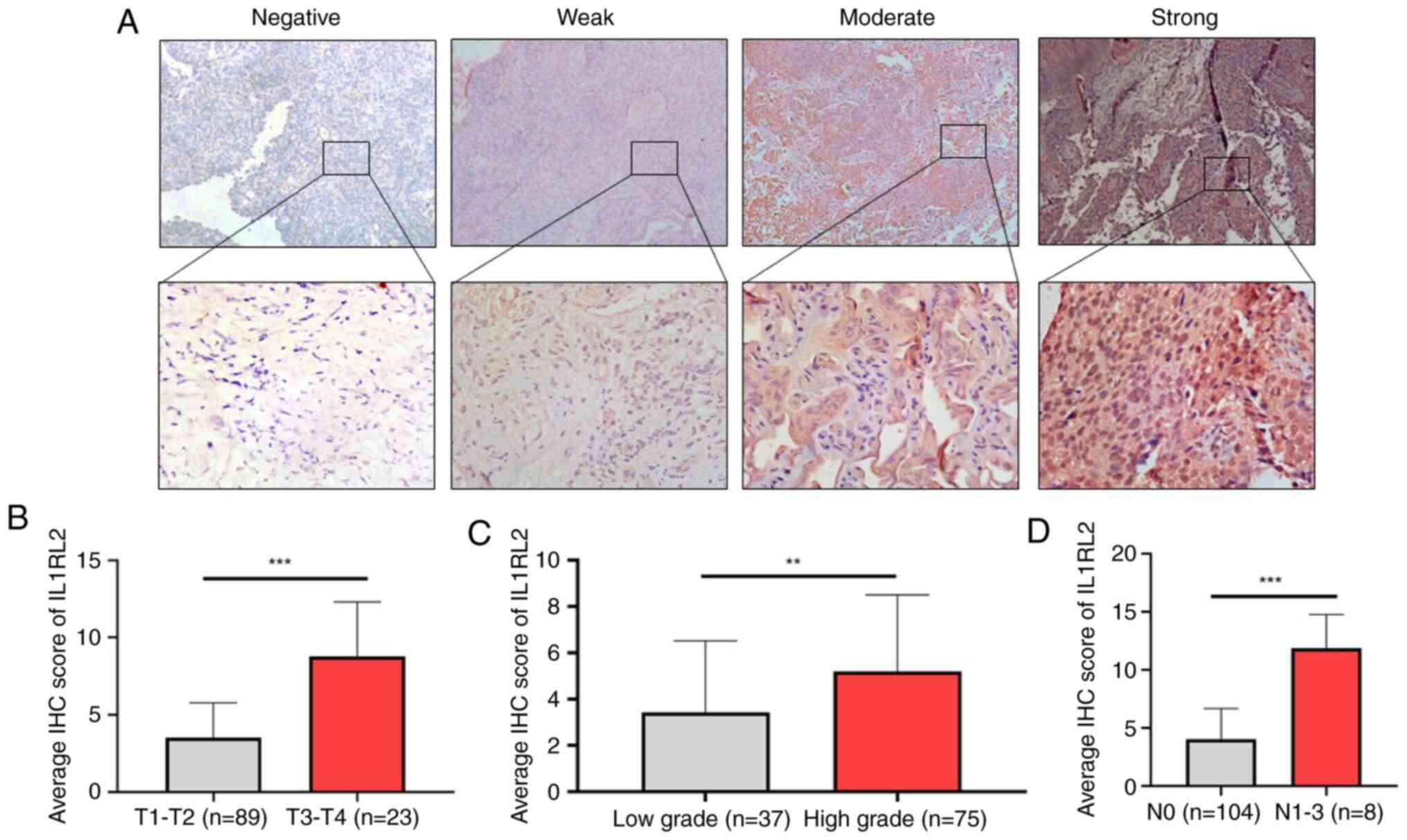
IHC scoring based on different staining intensities
(Fig. 3A) and areas was performed.
The average IHC score of IL1RL2 expression in patients with
high-stage and high-grade BLCA was significantly higher than in
low-stage (P<0.001; Fig. 3B)
and low-grade BLCA (P<0.01; Fig.
3C; Table I). Patients with
BLCA with lymph node metastasis demonstrated a significantly higher
average IHC score of IL1RL2 expression compared with non-metastatic
patients (P<0.001; Fig.
3D).
High IL1RL2 expression is associated
with improved OS in patients with BLCA
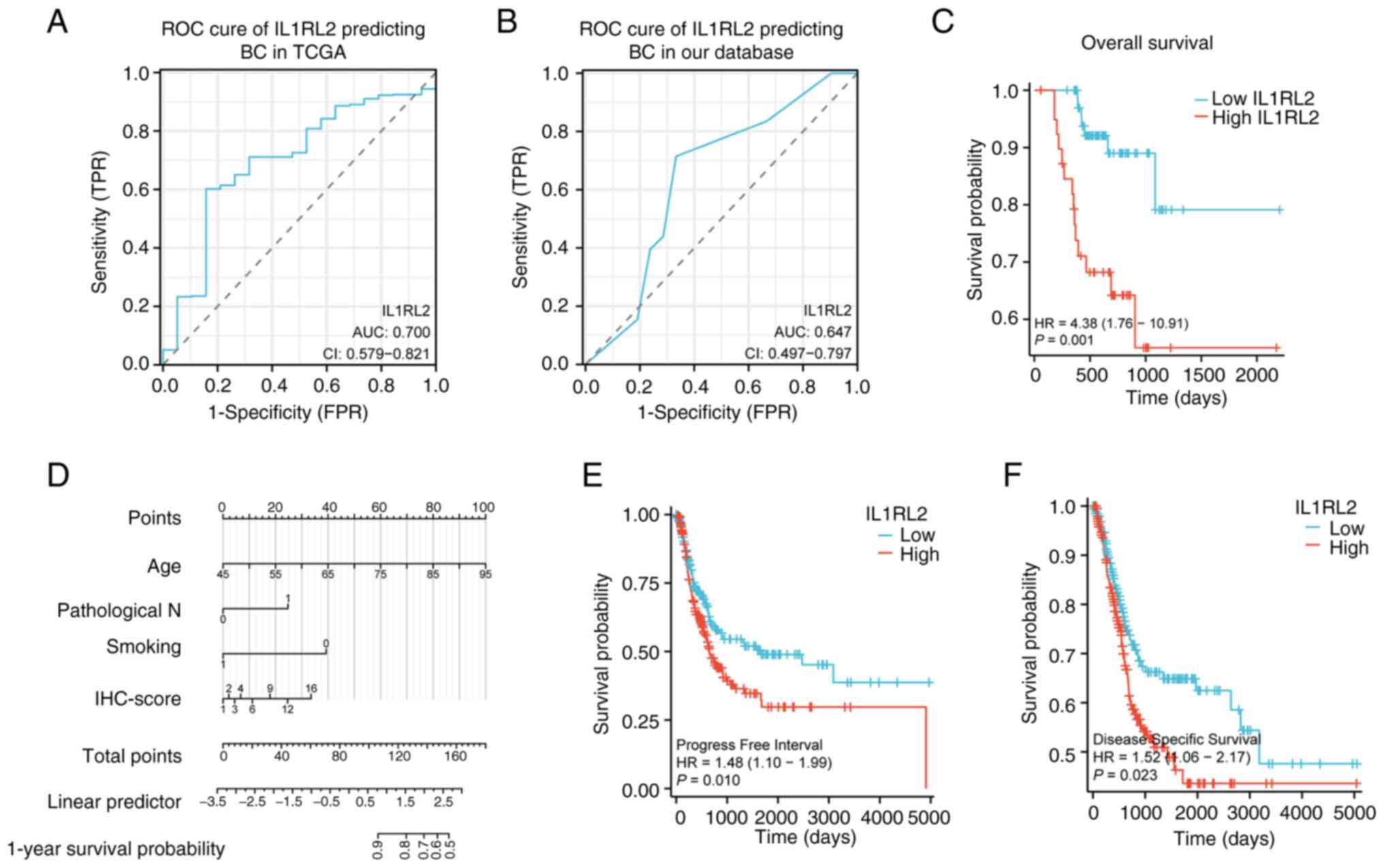
To evaluate the diagnostic potential of IL1RL2, the
differential expression in BLCA vs. adjacent normal tissues was
analyzed, generating receiver operator characteristic (ROC) curves
using data from the TCGA database and a cohort of 112 patients with
BLCA. The ROC curves revealed that IL1RL2 expression distinguished
tumors from adjacent normal tissues with an AUC of 0.700 (95% CI:
0.579-0.821) in TCGA and 0.647 (95% CI: 0.497-0.797) in Miyun chart
database (Fig. 4A and B). IHC analysis of 112 patients with BLCA
at different stages and grades revealed a correlation between OS
time and IL1RL2 expression (Fig.
4C). For OS analysis, in univariate analysis, IL1RL2 expression
was negatively correlated with OS (95% CI: 0.072-0.552; P=0.002);
and smoking (95% CI: 1.091-22.834; P=0.038), age (95% CI:
1.035-1.175; P=0.003) and lymph node metastasis (95% CI:
0.024-0.502; P=0.004) were also factors influencing OS (Table II). In multivariate analysis,
smoking (95% CI: 1.024-28.661; P=0.047), age (95% CI: 1.028-1.190;
P=0.007) and IL1RL2 expression (95% CI: 0.089-0.988; P=0.048) were
associated with poorer OS. A line chart predicting survival was
constructed based on smoking, age and IL1RL2 (Fig. 4D). For RFS analysis, in univariate
analysis, sex (95% CI: 0.116-0.939; P=0.038) and pathological stage
(95% CI: 0.136-0.989; P=0.047) were factors influencing RFS, while
IL1RL2 was not a factor for RFS (Table SI). In multivariate analysis, sex
(95% CI: 0.332 (0.116-0.949; P=0.0039) was associated with poorer
RFS, suggesting that sex may be an independent prognostic factor
for patients with BLCA. In TCGA database, High levels of IL1RL2
expression were associated with lower disease-specific survival and
progression-free survival (Fig. 4E
and F).
 | Table IIUnivariate analysis and multivariate
analysis of overall survival of bladder cancer in Miyun chart
database. |
Table II
Univariate analysis and multivariate
analysis of overall survival of bladder cancer in Miyun chart
database.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| IL1RL2 level, high
vs. low | 0.200
(0.072-0.552) | 0.002 | 0.296
(0.089-0.988) | 0.048 |
| Smoking, yes vs.
no | 4.994
(1.091-22.834) | 0.038 | 5.417
(1.024-28.661) | 0.047 |
| Hypertension, yes
vs. no | 2.000
(0.711-5.625) | 0.189 | | |
| Diabetes, yes vs.
no | 0.585
(0.141-2.426) | 0.46 | | |
| Tumor diameter | 0.981
(0.826-1.166) | 0.83 | | |
| Tumor number, ≥3
vs. <3 | 1.603
(0.608-4.231) | 0.34 | | |
| Body mass
index | 0.920
(0.787-1.076) | 0.298 | | |
| Age | 1.103
(1.035-1.175) | 0.003 | 1.106
(1.028-1.190) | 0.007 |
| Sex, male vs.
female | 0.839
(0.247-2.847) | 0.778 | | |
| Pathological T, T3
+ T4 vs. T1 + T2 | 0.789
(0.225-2.440) | 0.681 | | |
| Pathological N,
N1-3 vs. N0 | 0.109
(0.024-0.502) | 0.004 | 0.210
(0.035-1.258) | 0.088 |
| Histological grade,
high vs. low | 1.033
(0.378-2.824) | 0.949 | | |
To explore the functional implications of IL1RL2 in
BLCA, enrichment analysis of Gene Ontology (GO) terms and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways were performed
using genes co-expressed with IL1RL2 obtained from the TCGA BLCA
database. This analysis aimed to uncover the biological roles
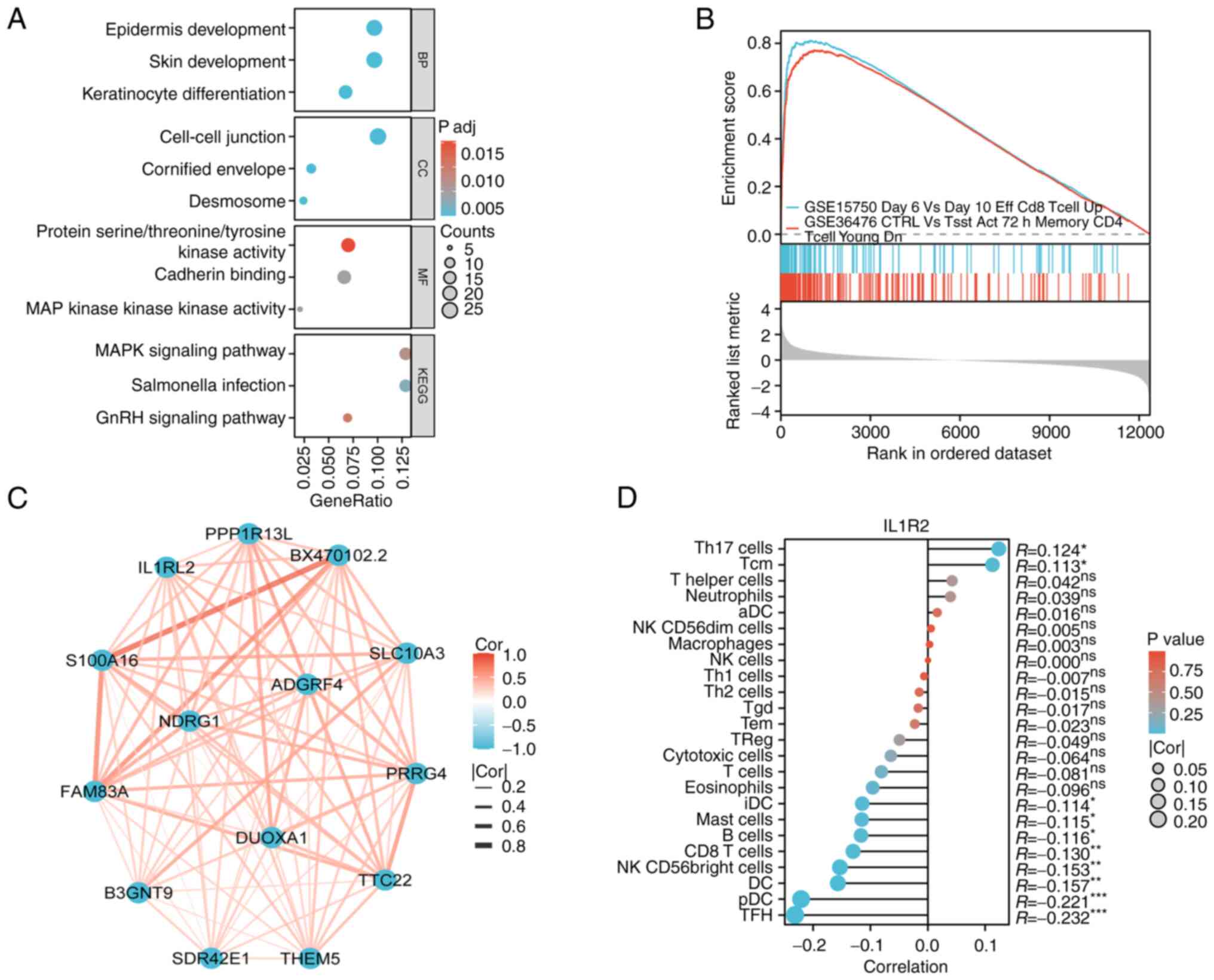
associated with IL1RL2 in BLCA. In GO enrichment analysis,
cell-cell junction, epidermis development and MAP kinase activity
were enriched (Fig. 5A). KEGG
enrichment highlighted MAPK signaling pathway and Salmonella
infection (Fig. 5A). Additionally,
Gene Set Enrichment Analysis (GSEA) in BLCA indicated that elevated
IL1RL2 expression was linked to several critical processes that
promote tumorigenesis, including tumor immune infiltration-related
processes such as CD8 T cells and memory CD4 (Fig. 5B), suggesting that IL1RL2 may
facilitate bladder tumor development. Protein-protein interaction
analysis revealed that potential targets of IL1RL2 were primarily
genes associated with cell proliferation and migration (Fig. 5C). Immuno-infiltration analysis of
IL1RL2 in BLCA revealed potential associations with plasmacytoid
dendritic cells (pDC) and T follicular helper cells (TFH) (Fig. 5D). Overall, these results suggested
that IL1RL2 may regulate tumor development through modulation of
the tumor immune microenvironment and MAPK signaling pathway.
Discussion
BLCA is a common malignancy with a high recurrence
rate and potential for metastasis (20). Despite continuous advancements in
medical care over the past decades, there has been limited
improvement in the treatment outcomes and diagnostic methods for
BLCA (21). Identifying specific
genes expressed at the molecular level in BLCA could contribute to
its diagnosis and treatment. Currently, there is no reported
research on the role of IL1RL2 in BLCA. The present study revealed
that IL1RL2 is upregulated in BLCA and correlated with the stage,
grade, lymph node album and prognosis of BLCA.
Early diagnosis and monitoring of BLCA are crucial
for improving patient prognosis. Numerous diagnostic biomarkers
have been identified in previous studies. Lokeshwar et al
(22) found that BTA testing has
high sensitivity and specificity in patients with BLCA.
Additionally, Grossman et al (23) demonstrated that NMP22 testing can
effectively distinguish patients with BLCA from healthy
individuals. However, current diagnostic methods are still limited,
and there is a need to improve both sensitivity and
specificity.
IL1RL2 has been identified as a novel diagnostic and
prognostic marker in various cancer types, such as breast, gastric,
colorectal and lung cancer, suggesting its potential as both a
diagnostic marker and therapeutic target (19,24-27).
Baker et al (27) found
that IL1RL2 agonists promote the progression of human and murine
lung cancer, leading to tumor cell proliferation and migration. The
team also discovered high expression of IL1RL2 in colorectal
cancer, with a concurrent role in promoting colorectal cancer
metastasis (13). These results
suggested that IL1RL2 plays a crucial role in tumorigenesis and
development.
In the present study, through analysis of TCGA and
112 samples of patients with BLCA database, the abundance of IL1RL2
in BLCA and its clinical significance were explored and confirmed.
The results indicated that IL1RL2 is upregulated in both BLCA
tissues and cells. The IL1RL2 level is significantly correlated
with tumor stage, grade and metastasis. Furthermore, IHC analysis
revealed that IL1RL2 expression is an independent risk factor for
OS. Additionally, Kaplan-Meier survival analysis using TCGA and 112
patients with BLCA from Miyun chart database revealed that
upregulated IL1RL2 status is associated with lower disease-specific
survival and progression-free interval rates. These data suggested
that IL1RL2 is associated with BLCA survival and tumor
progression.
To further analyze the oncogenic role of IL1RL2 in
BLCA, a bioinformatics analysis was performed for functional
prediction. Enrichment analysis suggested that IL1RL2 may
participate in the MAPK signaling pathway, a pathway crucial for
tumor proliferation, migration and invasion (28-30).
GSEA enrichment revealed the enrichment of immune cells, such as
CD4 and CD8, indicating that IL1RL2 may regulate tumor progression
through modulation of the tumor immune microenvironment.
Immuno-infiltration analysis of IL1RL2 in BLCA suggested potential
associations with pDC and TFH.
While the present study has identified the
expression profile of IL1RL2 in BLCA, the oncogenic functions of
IL1RL2 in BLCA need further clarification both in vitro and
in vivo. Additionally, the specific molecular mechanisms
through which IL1RL2 operates require further exploration. IL1RL2
has the potential to serve as a molecular marker for the assessment
of BLCA prognosis and help clinicians develop more individualized
treatment strategies. The present study provided new ideas and
potential molecular targets for the early diagnosis, prognosis
assessment and individualized treatment of BLCA.
Supplementary Material
Univariate analysis and multivariate
analysis of recurrence-free survival of bladder cancer in our
database.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH, YL, LS and WG conceptualized and designed the
present study. LS and WG provided administrative support. XH, YL,
ZG, JC, YN, LS, WG provided the study materials or recruited
patients. WG collected and assembled the data. XH and YL analyzed
and interpreted the data. XH and YL confirm the authenticity of all
the raw data. All authors contributed to manuscript writing, and
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki (as revised in 2013) and was approved
(approval no. 2023-029-001) by the Ethics Committee of Peking
University First Hospital-Miyun Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
Globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li K and Lin T: Chinese Bladder Cancer
Consortium. Xue W, Mu X, Xu E, Yang X, Chen F, Li G, Ma L, et al:
Current status of diagnosis and treatment of bladder cancer in
China-analyses of Chinese bladder cancer consortium database. Asian
J Urol. 2:63–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lobo N, Afferi L, Moschini M, Mostafid H,
Porten S, Psutka SP, Gupta S, Smith AB, Williams SB and Lotan Y:
Epidemiology, screening, and prevention of bladder cancer. Eur Urol
Oncol. 5:628–639. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen H, Yang W, Xue X, Li Y, Jin Z and Ji
Z: Integrated analysis revealed an inflammatory cancer-associated
fibroblast-based subtypes with promising implications in predicting
the prognosis and immunotherapeutic response of bladder cancer
patients. Int J Mol Sci. 23(15970)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Babjuk M, Burger M, Capoun O, Cohen D,
Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F,
Masson-Lecomte A, Mostafid AH, et al: European association of
urology guidelines on non-muscle-invasive bladder cancer (Ta, T1,
and carcinoma in situ). Eur Urol. 81:75–94. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stein JP and Skinner DG: Radical
cystectomy for invasive bladder cancer: Long-term results of a
standard procedure. World J Urol. 24:296–304. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Witjes JA, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A,
Neuzillet Y, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2020
guidelines. Eur Urol. 79:82–104. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dale M and Nicklin MJ: Interleukin-1
receptor cluster: Gene organization of IL1R2, IL1R1, IL1RL2
(IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human
chromosome 2q. Genomics. 57:177–179. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ngo VL, Kuczma M, Maxim E and Denning TL:
Il-36 cytokines and gut immunity. Immunology. 163:145–154.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tomuschat C, O'Donnell AM, Coyle D and
Puri P: Altered expression of IL36γ and IL36 receptor (IL1RL2) in
the colon of patients with Hirschsprung's disease. Pediatr Surg
Int. 33:181–186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scheibe K, Kersten C, Schmied A, Vieth M,
Primbs T, Carlé B, Knieling F, Claussen J, Klimowicz AC, Zheng J,
et al: Inhibiting interleukin 36 receptor signaling reduces
fibrosis in mice with chronic intestinal inflammation.
Gastroenterology. 156:1082–1097.e11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baker KJ, Brint E and Houston A:
Transcriptomic and functional analyses reveal a tumour-promoting
role for the IL-36 receptor in colon cancer and crosstalk between
IL-36 signalling and the IL-17/IL-23 axis. Br J Cancer.
128:735–747. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Poudel M, Bhattarai PY, Shrestha P and
Choi HS: Regulation of interleukin-36γ/IL-36R signaling axis by
PIN1 in epithelial cell transformation and breast tumorigenesis.
Cancers (Basel). 14(3654)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang P, Yang W, Guo H, Dong HP, Guo YY,
Gan H, Wang Z, Cheng Y, Deng Y, Xie S, et al: IL-36γ and IL-36Ra
reciprocally regulate NSCLC progression by modulating GSH
homeostasis and oxidative stress-induced cell death. Adv Sci
(Weinh). 8(e2101501)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kovach MA, Che K, Brundin B, Andersson A,
Asgeirsdottir H, Padra M, Lindén SK, Qvarfordt I, Newstead MW,
Standiford TJ and Lindén A: IL-36 cytokines promote inflammation in
the lungs of long-term smokers. Am J Respir Cell Mol Biol.
64:173–182. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Goldman MJ, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al:
Visualizing and interpreting cancer genomics data via the Xena
platform. Nat Biotechnol. 38:675–678. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo Q, Zhao L, Yan N, Li Y, Guo C, Dang S,
Shen X, Han J and Luo Y: Integrated pan-cancer analysis and
experimental verification of the roles of tropomyosin 4 in gastric
cancer. Front Immunol. 14(1148056)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dyrskjøt L, Hansel DE, Efstathiou JA,
Knowles MA, Galsky MD, Teoh J and Theodorescu D: Bladder cancer.
Nat Rev Dis Primers. 9(58)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lokeshwar VB and Soloway MS: Current
bladder tumor tests: Does their projected utility fulfill clinical
necessity? J Urol. 165:1067–1077. 2001.PubMed/NCBI
|
|
23
|
Grossman HB, Messing E, Soloway M, Tomera
K, Katz G, Berger Y and Shen Y: Detection of bladder cancer using a
point-of-care proteomic assay. JAMA. 293:810–816. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wen S, He L, Zhong Z, Mi H and Liu F:
Prognostic model of colorectal cancer constructed by eight
immune-related genes. Front Mol Biosci. 7(604252)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen YC, Gonzalez ME, Burman B, Zhao X,
Anwar T, Tran M, Medhora N, Hiziroglu AB, Lee W, Cheng YH, et al:
Mesenchymal stem/stromal cell engulfment reveals metastatic
advantage in breast cancer. Cell Rep. 27:3916–3926.e5.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hao M, Li H, Yi M, Zhu Y, Wang K, Liu Y,
Liang X and Ding L: Development of an immune-related gene
prognostic risk model and identification of an immune infiltration
signature in the tumor microenvironment of colon cancer. BMC
Gastroenterol. 23(58)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Baker KJ, Buskiewicz E, Finucane M,
Chelliah A, Burke L, Houston A and Brint E: IL-36 expression is
increased in NSCLC with IL-36 stimulation of lung cancer cells
promoting a pro-tumorigenic phenotype. Cytokine.
165(156170)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Drosten M and Barbacid M: Targeting the
MAPK pathway in KRAS-driven tumors. Cancer Cell. 37:543–550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21(1102)2020.PubMed/NCBI View Article : Google Scholar
|