Introduction
Bladder cancer (BCa) is the 10th most common form of
cancer worldwide and the 2nd most common urologic malignancy
(1). Of these, non-muscle invasive
BCa (NMIBC) accounts for 70-75% of patients with bladder urothelial
carcinoma (UC) at the time of initial diagnosis (2,3).
Histologically, BCa with variant histology (VH) represents 15-25%
of all patients who have undergone transurethral resection of
bladder tumor (TURBT) or radical cystectomy (RC) (4,5).
Prior to the 2016 World Health Organisation (WHO) histological
classification (6), there was a
notable scarcity of reports and analyses concerning VH. The 2016
classification has heightened the interest in the precise
morphological characterization of histological variants,
introducing the category of ‘invasive UC with divergent
differentiation’ for tumors exhibiting a combination of ‘usual
type’ UC and other morphologies. Although individual variants are
relatively rare, they collectively constitute a significant subset
of the disease. Consequently, studies have been conducted on
patients undergoing RC to evaluate the clinical significance of VH,
particularly in muscle invasive BCa (MIBC). Most studies have
reported that VH is associated with an increased risk of recurrence
and progression, as well as worse cancer-specific survival
(7-9).
Despite the increasing interest in the management of
VH in BCa, a limited number of studies have reported on the
management and prognosis of VH in NMIBC, and some of the findings
are controversial (10-16).
Most studies comparing the efficacy of RC with a bladder-sparing
approach in NMIBC with VH have mainly focused on micropapillary
variants (17-20).
Unfortunately, there is a lack of adequate information on other
types of VH. Furthermore, studies comparing the oncologic outcomes
of VH and conventional UC in NMIBC managed with a bladder-sparing
approach have concluded that VH is associated with worse survival
outcomes without considering other high-risk features, such as
carcinoma in situ, extensive stromal invasion and
significant tumor burden (11,21,22).
Even with this limited evidence, the American
Urological Association (AUA) risk stratification for NMIBC includes
any VH as high risk, and the National Comprehensive Cancer Network
(NCCN) guidelines recommend aggressive therapy, including RC, for
NMIBC with VH (23,24). However, there is a more recent view
that VH can be divided into aggressive variants (glandular,
squamous, microcystic, giant cell, nested) and highly aggressive
variants (micropapillary, plasmacytoid, sarcomatoid), based on
their pathologic aggressiveness, to determine whether a
bladder-sparing approach or aggressive treatment is appropriate
(25). In addition, the prompt
consideration of early RC in NMIBC with VH without considering the
tumor burden in each patient is controversial and may lead to
overtreatment. In this context, a propensity score matching (PSM)
analysis was performed to assess the prognostic value of VH in
NMIBC compared with conventional UC with a similar tumor
burden.
Materials and methods
Patients
The present study is based on a review of the
database of Busan Paik Hospital (Busan South Korea), which contains
information on 1,068 consecutive patients who underwent TURBT
between February 2010 and December 2020. The database consisted of
698 men and 370 women between the ages of 23 and 94. The inclusion
criteria for the study were as follows: i) Pathological diagnosis
of UC in the bladder; ii) pathological tumor stage a-1 with no
clinical evidence of lymph node or distant metastasis; and iii)
complete resection, meaning no visible tumor left behind and
bladder muscle clearly identifiable by the pathologist and free of
disease. The exclusion criteria were as follows: i) Patients with
previous or sequential second primary cancers, including NMIBC or
upper tract UC; ii) those with incomplete clinical data; and iii)
patients with nonurothelial variants, such as pure adenocarcinoma,
squamous cell carcinoma, and small-cell carcinoma. Ultimately, a
total 494 newly diagnosed patients with NMIBC were included in the
final analysis. The study protocol adhered to the ethical
guidelines of the 1975 Declaration of Helsinki and received prior
approval (approval no. BPIRB 2023-23-030) from the Institutional
Review Board of Inje University Busan Paik Hospital (Busan, South
Korea). The collected data included age, sex, tumor size and
multifocality, pathological tumor staging and grading, the presence
of VH, and disease recurrence status.
Patients with VH were matched with patients with
conventional UC on a 1:3 ratio. For this purpose, a PSM analysis
was performed based on the propensity of patients with VH. A
nearest neighbor PSM without replacement generated by logistic
regression was used to adjust for confounding factors between the
two groups (26,27). Pathologic stage and grade, tumor
size, tumor number and postoperative Bacillus Galmette-Guerin (BCG)
instillation were selected as covariates. After matching, all
standardized mean differences were found to be <0.1 for the
covariates and <0.15 for squares and two-way interactions
between covariates, indicating an adequate balance between the two
groups.
Pathologic evaluation
Tumor size was measured based on the largest
dimension determined by macroscopic examination at cystoscopy,
which was conducted no more than one month before the TURBT. The
diameter described by the operator before TURBT was also taken into
account. In the cases of multifocality, the largest tumor diameter
was used for analysis. All specimens were histologically confirmed
by a genitourinary pathologist with >20 years of experience at
the institution. Tumor staging was assessed according to the tumor,
node and metastasis classification systems of the 7th and 8th
American Joint Committee on Cancer, and grading was performed
according to the 2004 and 2016 WHO systems and the International
Society of Urological Pathology consensus classification (6,28-30).
VH was considered based on previous reports that are widely
accepted by the uropathological community and WHO classifications
(6,28,31).
The extent of VH was semiquantitatively assessed by visually
estimating the VH percentage in the initial TURBT specimens. VH
components of <25, 25-50%, and >50% of the total tumor
architecture were classified as focal, moderate, and extensive,
respectively. Carcinoma in situ and lymphovascular invasion
status were also evaluated. The histologic variants of NMIBC were
stratified into two groups based on to their pathologic
aggressiveness (25): Aggressive
variants (glandular differentiation, squamous differentiation,
microcystic variant, giant cell variant and nested variant) and
highly aggressive variants (micropapillary variant, plasmacytoid
variant and sarcomatoid variant).
Management and follow-up
Patients with high-risk features of AUA risk
stratification or VH were recommended to repeat TURBT and receive
intravesical BCG instillation (23). However, if the patient was
unwilling, these procedures were not performed according to
protocol after the first TURBT. BCG TICE (OncoTICE®;
MSD; Merck & Co., Inc.) instillation was typically initiated
2-4 weeks after the last TURBT. According to the European
Association of Urology (EAU) guidelines, patients would undergo a
6-week course of intravesical BCG induction followed by a standard
maintenance regimen (32).
Patients were generally followed up every 3 months for the first 2
years after TURBT, every 6 months for the 3rd to 5th year, and
annually thereafter. Follow-up examinations included cystoscopy,
serum laboratory tests and periodic thoracoabdominal computed
tomography scans or magnetic resonance imaging. Recurrence was
defined as the detection of new NMIBC at 3 months after complete
resection or 1.5 months after the induction course with BCG.
Pathologic progression was defined as the recurrence of a tumor
with features of MIBC after the first TURBT. Distant metastasis was
defined as the detection of a new extravesical lesion in the lymph
node or other organs on imaging or pathological examination at 3
months after the last TURBT.
Statistical analysis
Continuous variables were presented as either the
mean and standard deviation or the median and interquartile range
(IQR), while categorical variables were presented as frequencies
and percentages. The distribution of clinicopathological
characteristics according to the VH status before and after PSM
(Table I), as well as patient
characteristics and oncologic outcomes stratified by pathologic
aggressiveness of VH (Table II,
Fig. 1), were evaluated using
Pearson's chi-squared test, Fisher's exact test, and
linear-by-linear association for categorical variables, and
Student's t-test and one-way analysis of variance with Tukey's post
hoc tests for continuous variables. Recurrence-free survival (RFS)
and progression-free survival (PFS) were estimated using the
Kaplan-Meier method, and log-rank testing was used to assess
statistical differences. The restricted mean survival time (RMST)
analysis was used to compare the average survival from baseline to
a specific time point between study groups. A univariate logistic
regression model was employed to identify any clinicopathologic
factors that may have influenced RFS, with risk expressed as odds
ratios (ORs) and 95% confidence intervals (CIs) determined using
reference groups. Statistical analysis was performed using the SPSS
v.27.0 (IBM Corp.) and MedCalc v.22.0 (MedCalc Software Ltd.). PSM
was performed using R v.4.1.0 (R Foundation for Statistical
Computing) with the ‘MatchIt’ package (26). For all tests, a two-sided P-value
of <0.05 was considered to indicate a statistically significant
difference.
 | Table IClinicopathological characteristics
according to the variant histology status before and after
propensity score matching. |
Table I
Clinicopathological characteristics
according to the variant histology status before and after
propensity score matching.
| | Before propensity
score matching | After propensity
score matching |
|---|
| Clinicopathological
characteristics | Conventional UC
n=434 | Variant UC n=60 | P-value | Conventional UC
n=180 | Variant UC n=60 | P-value |
|---|
| Median age at
surgery, years (IQR) | 68.0 (40-93) | 71 (49-86) | 0.032 | 70 (43-92) | 71 (49-86) | 0.288 |
| Sex, n (%) | | | | | | |
|
Male | 345 (79.5) | 50 (83.3) | 0.486 | 144 (80.0) | 50 (83.3) | 0.570 |
|
Female | 89 (20.5) | 10 (16.7) | | 36 (20.0) | 10 (16.7) | |
| T stage | | | | | | |
|
Ta | 168 (38.7) | 3 (5.0) | <0.001 | 10 (5.6) | 3 (5.0) | 1.000 |
|
T1 | 266 (61.3) | 57 (95.0) | | 170 (94.4) | 57 (95.0) | |
| WHO grade
2004/2016 | | | | | | |
|
Low
grade | 179 (41.2) | 4 (6.7) | <0.001 | 11 (6.1) | 4 (6.7) | 1.000 |
|
High
grade | 255 (58.8) | 56 (93.3) | | 169 (93.9) | 56 (93.3) | |
| Concomitant
CIS | | | | | | |
|
No | 366 (84.3) | 45 (75.0) | 0.070 | 145 (80.6) | 45 (75.0) | 0.359 |
|
Yes | 68 (15.7) | 15 (25.) | | 35 (19.4) | 15 (25.) | |
| Median tumor size,
cm (IQR) | 1.5 (0.5-4.0) | 1.45 (0.5-4.8) | 0.223 | 1.75 (0.5-4.8) | 1.45 (0.5-4.8) | 0.695 |
| Tumor size | | | | | | |
|
<3
cm | 387 (89.2) | 48 (80.0) | 0.091 | 147 (81.7) | 48 (80.0) | 0.775 |
|
≥3 cm | 47 (10.8) | 12 (20.0) | | 33 (18.3) | 12 (20.0) | |
| Median tumor
number, n (IQR) | 1 (1-2) | 1.0 (1-3) | 0.489 | 1.0 (1-3) | 1.0 (1-3) | 0.604 |
| No. of tumors | | | | | | |
|
Single | 341 (78.6) | 39 (65.0) | 0.019 | 132 (73.3) | 39 (65.0) | 0.217 |
|
Multiple | 93 (21.4) | 21 (35.0) | | 48 (26.7) | 21 (35.0) | |
| Repeat TURBT | | | | | | |
|
No | 350 (80.6) | 38 (63.3) | 0.002 | 123 (68.3) | 38 (63.3) | 0.475 |
|
Yes | 84 (19.4) | 22 (36.7) | | 57 (31.7) | 22 (36.7) | |
| Postoperative BCG
instillation | | | | | | |
|
No | 296 (68.2) | 33 (55.0) | 0.128 | 102 (56.7) | 33 (55.0) | 0.822 |
|
Yes | 138 (31.8) | 27 (45.0) | | 78 (43.3) | 27 (45.0) | |
| Postoperative BCG
duration | | | | | | |
|
No | 296 (68.2) | 33 (55.0) | 0.105 | 102 (56.7) | 33 (55.0) | 0.751 |
|
<1
year | 64 (14.7) | 11 (18.3) | | 38 (21.1) | 11 (18.3) | |
|
≥1 year | 74 (17.1) | 10 (16.7) | | 40 (22.2) | 10 (16.7) | |
| AUA risk | | | | | | |
|
Low
risk | 106 (24.4) | 2 (3.3) | <0.001 | 5 (2.8) | 2 (3.3) | 0.922 |
|
Intermediate
risk | 89 (20.5) | 2 (3.3) | | 7 (3.9) | 2 (3.3) | |
|
High
risk | 239 (55.1) | 56 (93.3) | | 168 (93.3) | 56 (93.3) | |
|
aMarkedly
high-risk features | 0 (0) | 60(100) | | 0 (0) | 60(100) | |
 | Table IICharacteristics of patients with
variant histology stratified by pathologic aggressiveness of
variant histology in bladder cancer. |
Table II
Characteristics of patients with
variant histology stratified by pathologic aggressiveness of
variant histology in bladder cancer.
| Patient
characteristics | Aggressive
variantsa n=38
(63.3%) | Highly aggressive
variantsb n=22
(36.7%) | P-value |
|---|
| Median age at
surgery, years (IQR) | 70 (49-81) | 76 (54-83) | 0.133 |
| Males (%) | 31 (81.6) | 19 (86.4) | 0.732 |
| T stage a/1
(%) | 3/35 (8.6) | 0/22 (0) | 0.292 |
| High grade (%) | 34 (89.5) | 22(100) | 0.286 |
| Concomitant CIS
(%) | 12 (31.6) | 3 (13.6) | 0.122 |
| Median tumor size,
cm (IQR) | 1.4 (0.5-4.1) | 1.65 (0.5-4.8) | 0.281 |
| Tumor size, ≥3 cm
(%) | 6 (15.8) | 6 (27.3) | 0.327 |
| Median tumor no.
(IQR) | 1 (1-3) | 1 (1-3) | 0.965 |
| Multifocal tumor
(%) | 14 (36.8) | 7 (31.8) | 0.694 |
| Repeat TURBT
(%) | 24 (63.2) | 14 (63.6) | 0.970 |
| Variant extent | | | 0.738 |
|
Focal
(<25%) | 30 (81.1) | 20 (87.0) | |
|
Moderate
(25-50%) | 6 (16.2) | 2 (8.7) | |
|
Extensive
(>50%) | 1 (2.7) | 1 (4.3) | |
| Postoperative BCG
instillation (%) | 17 (44.7) | 10 (45.5) | 0.957 |
| Postoperative BCG
duration ≥1 year (%) | 11 (28.9) | 5 (22.7) | 0.600 |
| AUA high risk
(%) | 34 (89.5) | 22(100) | 0.140 |
Results
During the 10-year period, a total of 494 patients
were newly diagnosed with NMIBC, of whom 60 (12.1%) presented with
VH. The median age of the patients at the time of surgery was 68
years (IQR, 40-93 years). The study included 395 men (79.9%) and 99
women (20.1%). Patients with VH were found to have an older age at
the time of surgery (P=0.032), a higher tumor stage (P<0.001), a
higher tumor grade (P<0.001), and a higher prevalence of
multiple tumors (P=0.019) compared with patients diagnosed with
conventional UC. According to the AUA risk stratification for
NMIBC, a higher proportion of patients with VH were classified as
high-risk compared with patients with conventional UC [56 (93.3%)
vs. 239 (55.1%); P<0.001]. In the analyses with the 1:3
propensity-matched groups, there were no significant differences in
baseline clinicopathological characteristics between the VH and
conventional UC groups (Table I).
After matching, it was found that 195 (81.3%) patients had tumor
sizes <3 cm, and 171 (71.3%) patients had solitary tumors. These
findings indicated that most of the patients included in the
analysis had a lower tumor burden. Overall, 105 patients (43.8%)
received postoperative BCG instillation.
Among the 60 patients with VH, the different types
of VH identified were as follows: Glandular differentiation (n=28,
46.7%), micropapillary variant (n=17, 28.3%), squamous
differentiation (n=4, 6.7%), microcystic variant (n=4, 6.7%),
plasmacytoid variant (n=3, 5.0%), sarcomatoid variants (n=2, 3.3%),
giant cell variant (n=1, 1.7%) and nested variant (n=1, 1.7%). The
extent of VH was focal (<25%) in 50 patients (83.3%), moderate
(25-50%) in 8 patients (13.3%), and extensive (>50%) in 2
patients (3.3%). When the VH group was stratified based on its
pathological aggressive nature, there were no significant
differences in clinicopathological characteristics between the
aggressive and highly aggressive variants (Table II).
Among the 240 propensity score-matched patients, 85
(35.1%) experienced disease recurrence and 13 (5.4%) progressed to
MIBC during a median follow-up period of 42.5 months (IQR,
4.0-122.0 months). Distant metastasis occurred in 2 out of 180
patients with conventional UC (1.1%) and 1 out of 60 patients with
VH (1.7%). All these patients developed pathologic progression and
then distant metastasis. The median RFS and PFS were not reached in
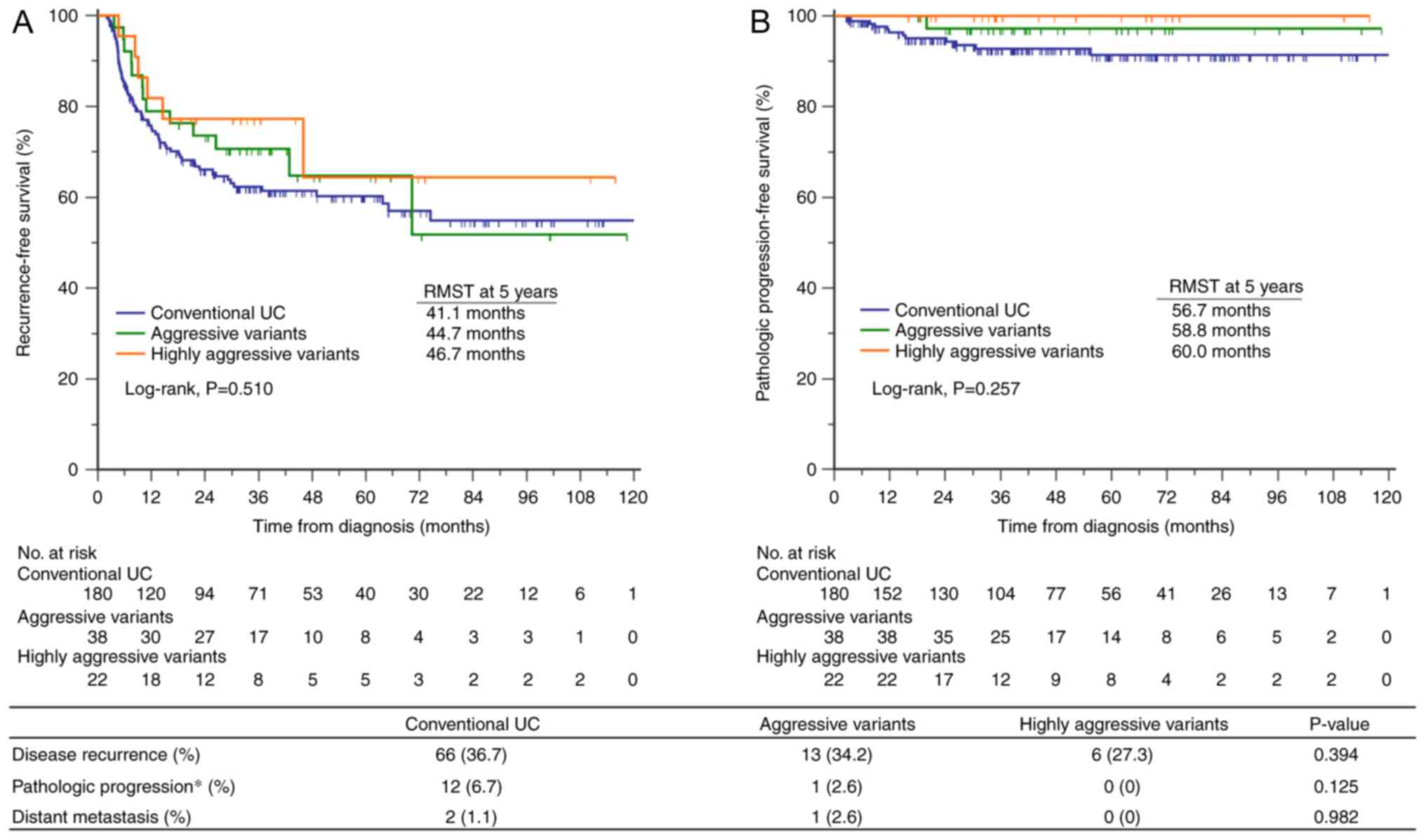
any of the groups (Fig. 1). There
was no significant difference in RFS (log-rank, P=0.510). The RMST
for RFS at 5 years was 41.1 months (95% CI, 37.4-44.8) for
conventional UC, 44.7 months (95% CI, 37.6-51.9) for aggressive
variants, and 46.7 months (95% CI, 37.7-55.7) for highly aggressive
variants. The difference in RMST for RFS was 3.6 months (95% CI,
-1.4-11.6; P=0.377) between conventional UC and aggressive variants
and 5.6 months (95% CI, 4.1-15.2; P=0.260) between conventional UC
and highly aggressive variants (Fig.
1A). Similarly, there was no significant difference in PFS
(log-rank, P=0.257). The RMST for PFS at 5 years was 56.7 months
(95% CI, 54.8-58.5), 58.8 months (95% CI, 56.7-61.0) and 60.0
months (95% CI, 60.0-60.0) for conventional UC, aggressive variants
and highly aggressive variants, respectively. The difference in
RMST for PFS was 2.1 months (95% CI, -0.6-4.9; P=0.129) between
conventional UC and aggressive variants and 3.3 months (95% CI,
1.4-5.1; P<0.001) between conventional UC and highly aggressive
variants (Fig. 1B). Univariate
analysis revealed that intravesical BCG treatment was the only
factor associated with a reduced risk of recurrence (OR, 8.20; 95%
CI, 1.94-34.75; P<0.001; Table
III). The results allowed a risk-adapted management of NMIBC
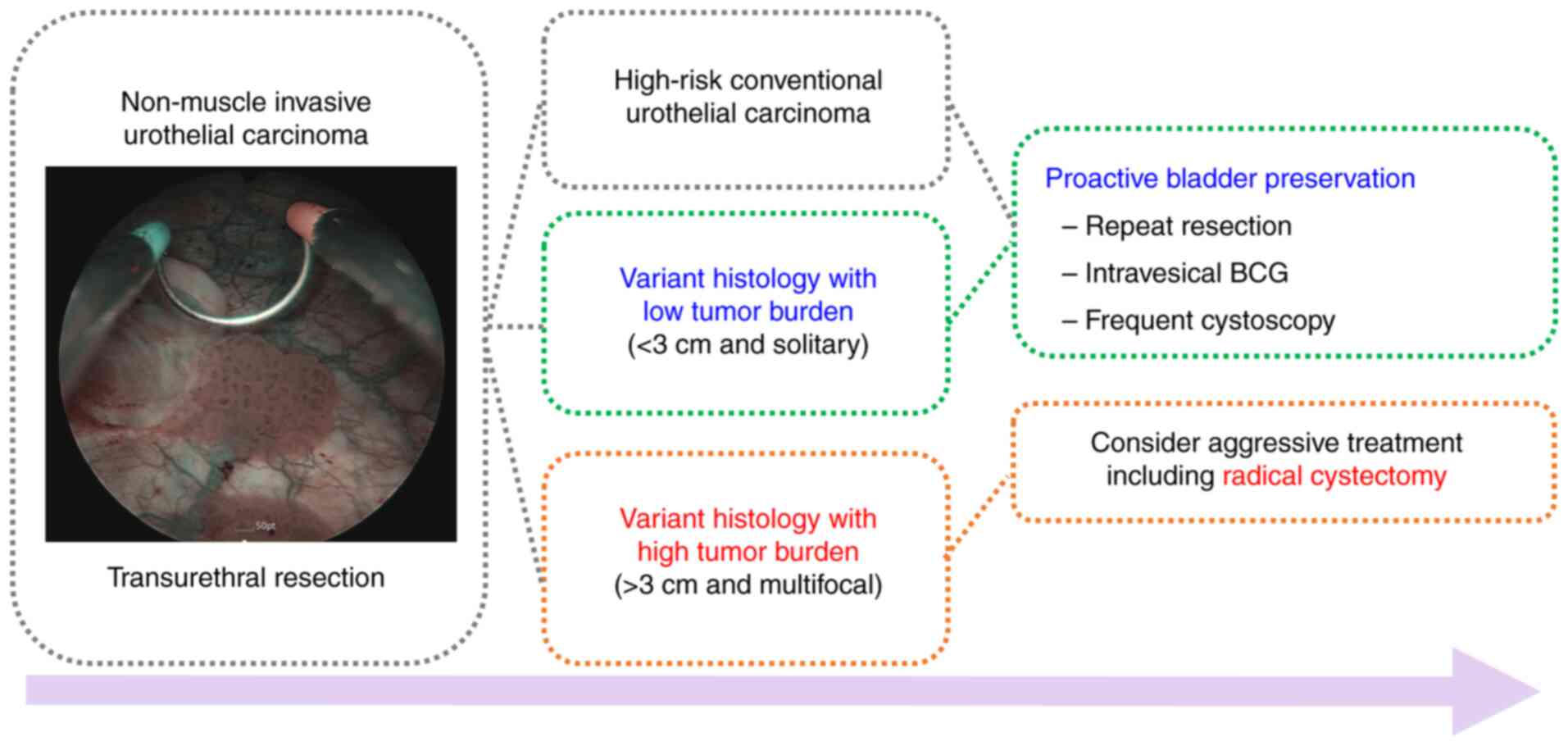
with VH based on tumor burden (Fig.
2).
 | Table IIIUnivariate logistic regression
analysis of factors influencing recurrence after diagnosis of
non-muscle invasive bladder cancer. |
Table III
Univariate logistic regression
analysis of factors influencing recurrence after diagnosis of
non-muscle invasive bladder cancer.
| | Univariate
analysis |
|---|
|
Characteristics | OR | 95% CI | P-value |
|---|
| Age (years) | 1.01 | 0.98-1.03 | 0.426 |
| Sex (male vs.
female) | 0.96 | 0.49-1.89 | 0.920 |
| T stage (Ta vs.
T1) | 1.24 | 0.37-4.18 | 0.719 |
| WHO grade 2004/2016
(low vs. high) | 1.54 | 0.47-5.01 | 0.467 |
| Concomitant CIS (no
vs. yes) | 0.82 | 0.42-1.60 | 0.570 |
| Tumor size | 0.85 | 0.67-1.08 | 0.209 |
| No. of tumors | 1.02 | 0.78-1.33 | 0.852 |
| Mutlifocal tumor
(no vs. yes) | 1.05 | 0.58-1.88 | 0.866 |
| Types of variant
histology | | | |
|
Conventional
UC | 1.00 | - | |
|
Aggressive
variants | 0.89 | 0.43-1.87 | 0.774 |
|
Highly
aggressive variants | 0.64 | 0.24-1.73 | 0.388 |
| Repeat TURBT (yes
vs. no) | 0.84 | 0.48-1.48 | 0.561 |
| AUA risk (low and
intermediate vs. high) | 1.30 | 0.72-2.33 | 0.371 |
| Intravesical BCG
(yes vs. no) | 8.20 | 1.94-34.75 | <0.001 |
Discussion
The aim of the present study was to evaluate the
prognostic value of VH in NMIBC compared with conventional UC with
a similar tumor burden. Over the last two decades, the importance
of VH in BCa has increased. Several studies have examined the
prognostic role of VH in MIBC, particularly in patients treated
with RC, and most have revealed that VH in NMIBC is associated with
worse survival outcomes (7-9).
However, the prognostic role of VH in NMIBC has been less studied.
Understanding the impact of VH in NMIBC is crucial, especially when
deciding between bladder preservation or RC based on the presence
and type of VH (33). Current
guidelines recommend an aggressive approach when VH is detected on
pathology (24,32). Therefore, initial RC is generally
favored for NMIBC with VH, as it is considered as a significantly
high-risk category for NMIBC according to both the EAU and AUA
guidelines (23,32). However, relying solely on the
presence of VH as an indication for RC without considering the
tumor burden and extent of VH in each patient may lead to
overtreatment and is not aligned with the current trend of
personalized medicine.
Similar to studies on MIBC series, several studies
have identified the presence of VH as a poor prognostic factor for
recurrence and disease progression in NMIBC. The reported
progression rates can be as high as 40% (10,17,34,35).
However, upon close examination of these studies, no balance was
observed between the VH with conventional UC (Table IV) (10-16,18-22,36-38).
The significant differences of this study compared with previous
studies are as follows. First, numerous previous studies did not
clearly present tumor size and multifocality (10,11,16,18,19,21,22).
In the present study, the prognosis and treatment outcomes of NMIBC
with conventional UC were compared based on a tumor size of 3 cm
and multifocality, which are representative criteria for tumor
burden. Additionally, the extent of VH, which is known to
significantly impact VH prognosis, was clearly presented.
Furthermore, most previous studies only compared a single variant
with conventional UC (14-16,18-21,36,37).
By contrast, the present study classified VH according to its
aggressiveness and performed a comprehensive analysis that included
most VH. In addition to tumor characteristics, treatment management
is also an important factor affecting prognosis. Numerous previous
studies did not evidently present whether and how often repeat
TURBT and intravesical treatment were performed (12-16,36).
However, the present study provided clear information on these
aspects. Therefore, without considering these various factors, it
is not appropriate to simply conclude that the presence of VH
indicates a poor prognosis in NMIBC. To the best of our knowledge,
the present study is the first to investigate the prognostic role
of VH in NMIBC using PSM to ensure baseline characteristics,
including tumor burden, are balanced between VH and conventional
UC.
 | Table IVClinical outcomes of VH in NMIBC
according to selected studies. |
Table IV
Clinical outcomes of VH in NMIBC
according to selected studies.
| First author,
year | Study design | Cases, n | Stage | VH, n (%) | Subtype, % | Treatment, % | Presentation of
tumor burden | Clinical
significance | (Refs.) |
|---|
| Present study | Single center,
retrospective comparison with conventional UC | 494 | Ta/T1 | 60 (12.1) | GD 46.7; MV 28.3;
SD 6.7; MC 6.8; PV 5.0; SV 3.3; GV 1.7; NV 1.7 | All patients:
Repeat TUR, 21.4; IVS BCG, 43.8; IVS CTx, N/A; RC, N/A | No.: Solitary,
86.2%; size: <3 cm, 88.0%; extent of VH: <25%, 83.3%; 25-50%,
13.3%; >50%, 3.3% | No difference in
RFS or pathologic PFS in VH with low-tumor burden compared with
conventional UC | - |
| Xu et al,
2017 | Single center,
retrospective comparison with conventional UC | 869 | Ta/T1 | 232 (26.7) | SD/GD, 100 | All patients:
Repeat TUR, N/A; IVS BCG, none; IVS CTx, 100; RC, N/A | No.: Solitary,
59.6%; size: <3 cm, 67.4%; extent of VH: N/A | Higher recurrence
rate and shorter RFS compared with conventional UC | (13) |
| Li et al,
2018 | Single center,
retrospective comparison with conventional UC | 426 | T1 | 213(50) | SD, 100 | All patients:
Repeat TUR, N/A; IVS BCG, none; IVS CTx, 100; RC, N/A | No.: Solitary,
70.4%; size: <3 cm, 67.4%; extent of VH: N/A | Prognostic factor
for poor recurrence and progression rate | (14) |
| Zhao et al,
2019 | Single center,
retrospective comparison with conventional UC | 248 | T1 | 82 (33.0) | GD, 100 | All patients:
Repeat TUR, N/A; IVS BCG, none; IVS CTx, 100; RC, N/A | No.: Solitary
64.5%; size: <3 cm, 71.3%; extent of VH: N/A; LVI+,
15.3% | Higher recurrence
and progression rates and poorer OS compared with conventional pT1
UC | (15) |
| Li et al,
2016 | Single center,
retrospective SD only | 206 | T1 | 206(100) | SD, 100 | All patients:
Repeat TUR, N/A; IVS BCG, N/A; IVS CTx, 100; Management after
recurrence (n=65): TUR, 42; RC, 58 | No.: Solitary,
50.8%; size: <3 cm, 70.4%; extent of VH, N/A; LVI+:
27.6% | -Shorter CSS rate
in LVI+ cases-Shorter median CSS after TURBT than after
RC in recurrent patients | (36) |
| Gofrit et
al, 2016 | Single center,
retrospective comparison with conventional UC | 181 | Ta/T1 | 41 (22.6) | MV, 34; SD, 32; GD,
22; NV, 17 | VH only: Repeat
TUR, 100; IVS BCG, 100; IVS CTx, N/A; RC, none | No.: N/A; size:
N/A; extent of VH: N/A | Shorter 5-year RFS,
PFS, DSS and OS compared with conventional high-grade UC | (11) |
| Shapur et
al, 2011 | Single center,
retrospective comparison with conventional UC | 166 | Ta/T1 | 22 (13.2) | SD, 32; NV, 27; GD,
18; MV, 18; SV, 5 | VH only: Repeat
TUR, 100; IVS BCG, 100; IVS CTx, 0; RC, 22.7 | No.: N/A; size:
Non-bulky (<4 cm), 100%; extent of VH: N/A | -Worse 2- and
5-year PFS and shorter median time to progression compared with
conventional UC -No differences in RFS and DSS | (10) |
| Fujii et al,
2017 | Single center,
retrospective comparison with conventional UC | 148 | T1 | 17 (11.5) | GD, 59; SD, 29; SD
+ GD, 12 | All patients:
Repeat TUR, N/A; IVS BCG, 33.1; IVS CTx, 46.6; RC, N/A | No.: Solitary,
42.6%; size: <3 cm, 75%; extent of VH: <25%, 58.8%; 25-50%,
29.4%; >50%, 11.7% | Shorter RFS and PFS
rates compared with conventional UC | (12) |
| Mally et al,
2017 | Single center,
retrospective comparison with conventional UC | 120 | T1 | 30(25) | NV, 100 | VH only: Repeat
TUR, 100; IVS therapy, 53.3; Early RC, 43.3 | No.: Solitary, 40%;
size: N/A; extent of VH: N/A | No difference in
metastasis-free survival and CSS compared with conventional UC | (21) |
| Lopez-Beltran et
al, 2022 | Single center,
retrospective comparison with conventional UC | 92 | T1 | 34 (36.9) | NV, 23.5; MV, 20.5;
GD, 5.8; SD, 4.7; MC, 2.9; inverted, 23.5; basaloid, 2.9; VL, 2.9;
LL, 2.9 | All patients:
Repeat TUR, N/A; IVS BCG, 92.4; IVS CTx, 7.6; RC, N/A | No.: N/A; size:
N/A; extent of VH: N/A | Worse cumulative
PFS and DSS in VH compared to conventional UC Conventional UC, MV,
NV, GD, Basaloid are high-risk of aggressive disease | (22) |
| Willis et
al, 2015 | Single center,
retrospective MV only | 72 | T1 | 72(100) | MV, 100 | VH only: Repeat
TUR, 100; IVS BCG, 55; IVS CTx, N/A; early RC, 36 | No.: N/A; size:
N/A; extent of VH: <25%, 65%; >25%, 26%; unknown, 8%;
LVI+, 17% | Higher 5-year DSS
in RC group compared with BCG or RC after recurrence | (20) |
| Suh et al,
2019 | Single center,
retrospective SD/GD only | 62 | Ta/T1 | 62(100) | SD/GD, 100 | All patients:
Repeat TUR, 56.4; IVS BCG, 48.3; IVS CTx, N/A; RC, 24.2 | No.: Solitary,
45.2%; size: <3 cm, 62.9%; Extent of VH: N/A | Similar 5-year OS
and CSS in both BCG and RC groups | (38) |
| Yorozuya et
al, 2018 | Multicenter,
retrospective SD/GD only | 47 | Ta/T1 | 47(100) | GD, 80.9; SD,
19.1 | VH only: Repeat
TUR, 49; IVS BCG, 42.5; IVS CTx, 12.7; early RC, 12.8 | No.: Solitary,
57.4%; size: <3 cm, 83.0%; extent of VH: <50%, 19.1%; ≥50%,
36.2%; unknown, 44.7% | Improved RFS, PFS,
and CSS after BCG compared with other treatments or no additional
treatment | (37) |
| Spaliviero et
al, 2014 | Single center,
retrospective MV only | 36 | T1 | 36(100) | MV, 100 | VH only: Repeat
TUR, 100; IVS BCG, 44.4; IVS CTx, N/A; early RC, 41.6 | No.: Solitary,
60.7%; size: N/A; extent of VH: <10%, 6.2%; 10-50%, 68.7%;
>50% 25% | No difference in
outcome between early RC and conservative management | (19) |
| Sangoi et
al, 2020 | Single center,
retrospective MV only | 20 | Ta | 20(100) | MV, 100 | VH only: Repeat
TUR, N/A; IVS BCG, 10; IVS CTx, 5; early RC, 5 | No.: N/A; size:
N/A; extent of VH: <25%, 50%; 25-50%, 25%; >50%, 25% | No difference in
PFS rates compared with conventional pTa high grade UC | (16) |
| Ghoneim et
al, 2011 | Single center,
retrospective MV only | 10 | Ta/T1 | 10(100) | MV, 100 | VH only: Repeat
TUR, N/A; IVS BCG, 70; IVS CTx, N/A; RC, 30 | No.: N/A; size:
N/A; extent of VH: N/A | All patients with
cTa/T1 who had undergone initial bladder-sparing therapy with BCG
had pathologically advanced disease at cystectomy | (18) |
The present study found no difference in the disease
recurrence rate between conventional UC and VH in NMIBC, and
intravesical treatment was the only factor that prevented
recurrence. Similarly, there was no difference in pathologic
progression or distant metastasis rates between conventional UC and
VH. Reviewing the treatment records of all patients with
progression revealed that all had multifocal tumors, and half did
not receive intravesical BCG or chemotherapy. These findings have
important implications for managing NMIBC with VH. In high-risk
NMIBC cases managed with intensive bladder preservation, the
presence of VH does not significantly determine progression or
distant metastasis. In other words, in low-volume NMIBC cases,
regardless of the presence of VH, proactive bladder preservation
treatment, including intravesical BCG, effectively prevents
recurrence and progression, avoiding more aggressive treatments
such as RC. These findings are consistent with a recent review
showing that VH does not significantly worsen survival compared
with conventional UC at the same disease stage (39).
In the present study, 80% of patients with VH had
tumors <3 cm, and 65% had solitary tumors. No significant
differences were observed in disease recurrence and progression
rates between VH and conventional UC. These findings support the
efficacy of a bladder preservation approach for low tumor burden
NMIBC with VH. Management of VH can be stratified by tumor burden,
defined by size and multifocality. For low tumor burden VH (≤3 cm
and solitary), a proactive bladder preservation approach, including
repeat resection, intravesical therapy, and frequent surveillance,
is viable. For high tumor burden VH (>3 cm and multifocal), an
aggressive treatment approach, including RC, is recommended to
prevent progression. The risk-adapted management protocol of the
authors aims to prevent overtreatment of low tumor burden VH in
real clinical practice.
In the present study, patients were categorized into
two groups: Aggressive VH and highly aggressive VH, based on the
clinical and pathologic aggressiveness of VH, as suggested by a
previous recommendation (25).
According to the current NCCN guidelines, immediate RC is
recommended for highly aggressive VH, such as micropapillary,
plasmacytoid and sarcomatoid (24). However, the findings of the present
study showed no significant difference in survival between the two
groups. This suggested that the current recommendation to divide
patients into these two groups for deciding between bladder
preservation and RC may not be useful in low-volume NMIBC with VH.
The discrepancy between the recommendation and the study findings
may be attributed to the nature of the landmark studies used as
references. For instance, a retrospective study at MD Anderson
Cancer Center focused on patients with T1 NMIBC with a
micropapillary variant and treated with BCG, and found that the
majority did not respond to BCG (89%) and experienced disease
progression (67%), including metastatic disease (22%) (17). However, this study cohort did not
provide information on tumor burden, such as tumor size and number,
despite indicating the extent of the micropapillary variant. On the
other hand, a retrospective study from Memorial Sloan Kettering
Cancer Center revealed no statistically significant difference in
disease-specific survival at 5 years between initial RC and bladder
sparing with repeat TURBT and intravesical BCG in T1 NMIBC with
micropapillary variant (19).
Therefore, considering the findings of these studies and the
current study, the treatment recommendations for highly aggressive
variants should take into account evidence-based considerations
regarding tumor burden.
The present study suggested that bladder
preservation may be a viable treatment option for low-burden NMIBC
with VH. However, it is important to recognize that VH in BCa is an
aggressive disease that requires intensive surveillance. While the
study design of the following studies was retrospective and lacked
correction for baseline characteristics such as tumor burden,
several studies have reported lower response rates to intravesical
BCG in patients with VH, leading to higher recurrence and
progression rates than conventional UC (10,11,38).
Therefore, close follow-up with frequent cystoscopy and pelvic
imaging is crucial in managing NMIBC with VH to reduce morbidity
and mortality from missed recurrence and progression.
Although the present study makes several important
contributions, it is important to acknowledge some limitations,
including its small sample size, single-institution setting and
retrospective design. There were also differences in baseline
characteristics between the VH and conventional UC groups, as VH is
often associated with unfavorable pathological features, including
T stage, high-grade tumors and large tumor burdens. In addition,
although postoperative intravesical therapy is the only method to
reduce the recurrence or progression of NMIBC regardless of VH, the
number of patients treated in the present study was relatively
limited. To minimize the impact of unbalanced baseline
characteristics on tumor burden and the effect of intravesical
treatment between the two groups, the PSM method was used. Finally,
although the present study showed no difference in oncological
outcomes in NMIBC regardless of VH status in cases of low tumor
burden, only a limited number of cases with VH were included for
analysis. In fact, including the current study there are limited
multicenter large cohort studies evaluating the oncologic outcome
of VH in NMIBC. Further studies based on multicenter larger cohorts
are required.
In conclusion, the prognostic significance of VH in
NMIBC with low-tumor burden has been evaluated. Despite the adverse
pathologic features often associated with NMIBC with VH at
diagnosis, the findings revealed no significant difference in RFS
or pathologic PFS compared with conventional UC with a similar
tumor burden. This suggests that careful bladder preservation
methods, such as intravesical BCG instillation, currently used for
high-risk conventional NMIBC, may also be effective for treating
low-tumor burden NMIBC with VH.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 2024 Inje
University Busan Paik Hospital Research Grant.
Availability of data and materials
The datasets generated and analyzed during the
current study are available from the corresponding author in
reasonable request.
Authors' contributions
HSL and CHL contributed to the concept and design of
the study. Clinical data on patients who underwent TURBT were
collected by KSM, WIS, SJS and CHL. SJJ provided the pathological
data. SJJ and JIC confirm the authenticity of all raw data. CHL,
KSM, WIS, SJS and CHL analyzed and interpreted the
clinicopathological data, and JIC assisted with all statistical
analyses. The first draft of the manuscript was written by HSL, and
all authors commented on earlier versions of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and was approved by the institutional
ethics review board of Inje University Busan Paik Hospital (BPIRB
2023-23-030). For this type of retrospective and/or observational
study formal consent is not required. Pursuant to the provisions of
the ethics committee and the ethic guideline in Korea, written
consent was not required in exchange for public disclosure of study
information in the case of retrospective and/or observational study
using a material such as the existing documentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nielsen ME, Smith AB, Meyer AM, Kuo TM,
Tyree S, Kim WY, Milowsky MI, Pruthi RS and Millikan RC: Trends in
stage-specific incidence rates for urothelial carcinoma of the
bladder in the United States: 1988 to 2006. Cancer. 120:86–95.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder Cancer: A Review. JAMA. 324:1980–1991. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lopez-Beltran A, Henriques V, Montironi R,
Cimadamore A, Raspollini MR and Cheng L: Variants and new entities
of bladder cancer. Histopathology. 74:77–96. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lonati C, Baumeister P, Ornaghi PI, Di
Trapani E, De Cobelli O, Rink M, Karnes RJ, Poyet C, Simone G,
Afferi L, et al: Accuracy of transurethral resection of the bladder
in detecting variant histology of bladder cancer compared with
radical cystectomy. Eur Urol Focus. 8:457–464. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO Classification of tumours of the
urinary system and male genital Organs-Part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Koguchi D, Matsumoto K, Ikeda M, Taoka Y,
Hirayama T, Murakami Y, Utsunomiya T, Matsuda D, Okuno N, Irie A
and Iwamura M: Histologic variants associated with biological
aggressiveness and poor prognosis in patients treated with radical
cystectomy. Jpn J Clin Oncol. 49:373–378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stroman L, Nair R, Russell B, Malik N,
Desai A, Chandra A, Thurairaja R, Dasgupta P, Khan MS and Malde S:
The impact of non-urothelial variant histology on oncological
outcomes following radical cystectomy. BJU Int. 124:418–423.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mori K, Abufaraj M, Mostafaei H, Quhal F,
Karakiewicz PI, Briganti A, Kimura S, Egawa S and Shariat SF: A
systematic review and meta-analysis of variant histology in
urothelial carcinoma of the bladder treated with radical
cystectomy. J Urol. 204:1129–1140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shapur NK, Katz R, Pode D, Shapiro A,
Yutkin V, Pizov G, Appelbaum L, Zorn KC, Duvdevani M, Landau EH and
Gofrit ON: Is radical cystectomy mandatory in every patient with
variant histology of bladder cancer. Rare Tumors.
3(e22)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gofrit ON, Yutkin V, Shapiro A, Pizov G,
Zorn KC, Hidas G, Gielchinsky I, Duvdevani M, Landau EH and Pode D:
The response of variant histology bladder cancer to intravesical
immunotherapy compared to conventional cancer. Front Oncol.
6(43)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujii N, Hoshii Y, Hirata H, Mori J,
Shimizu K, Kobayashi K, Kawai Y, Inoue R, Yamamoto Y, Matsumoto H,
et al: Impact of divergent differentiation in urothelial carcinoma
on oncological outcome in patients with T1 high-grade bladder
cancer. Jpn J Clin Oncol. 47:560–567. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu H, Xie L, Liu X, Zhang Y, Shen Z, Chen
T, Qiu X, Sha N, Xing C, Wu Z, et al: Impact of squamous and/or
glandular differentiation on recurrence and progression following
transurethral resection for non-muscle invasive urothelial
carcinoma of bladder. Oncol Lett. 14:3522–3528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li G, Hu J and Niu Y: Squamous
differentiation in pT1 bladder urothelial carcinoma predicts poor
response for intravesical chemotherapy. Oncotarget. 9:217–223.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao G, Wang C, Tang Y, Liu X, Liu Z, Li G
and Mei Y: Glandular differentiation in pT1 urothelial carcinoma of
bladder predicts poor prognosis. Sci Rep. 9(5323)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sangoi AR, Cox RM, Higgins JP, Quick CM
and McKenney JK: Non-invasive papillary urothelial carcinoma with
‘micropapillary’ architecture: Clinicopathological study of 18
patients emphasising clinical outcomes. Histopathology. 77:728–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kamat AM, Gee JR, Dinney CP, Grossman HB,
Swanson DA, Millikan RE, Detry MA, Robinson TL and Pisters LL: The
case for early cystectomy in the treatment of nonmuscle invasive
micropapillary bladder carcinoma. J Urol. 175:881–885.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ghoneim IA, Miocinovic R, Stephenson AJ,
Garcia JA, Gong MC, Campbell SC, Hansel DE and Fergany AF:
Neoadjuvant systemic therapy or early cystectomy? Single-center
analysis of outcomes after therapy for patients with clinically
localized micropapillary urothelial carcinoma of the bladder.
Urology. 77:867–870. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Spaliviero M, Dalbagni G, Bochner BH, Poon
BY, Huang H, Al-Ahmadie HA, Donahue TF, Taylor JM, Meeks JJ,
Sjoberg DD, et al: Clinical outcome of patients with T1
micropapillary urothelial carcinoma of the bladder. J Urol.
192:702–707. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Willis DL, Fernandez MI, Dickstein RJ,
Parikh S, Shah JB, Pisters LL, Guo CC, Henderson S, Czerniak BA,
Grossman HB, et al: Clinical outcomes of cT1 micropapillary bladder
cancer. J Urol. 193:1129–1134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mally AD, Tin AL, Lee JK, Satasivam P, Cha
EK, Donat SM, Herr HW, Bochner BH, Sjoberg DD and Dalbagni G:
Clinical outcomes of patients with T1 nested variant of urothelial
carcinoma compared to pure urothelial carcinoma of the bladder.
Clin Genitourin Cancer: Jul 14, 2017 doi:
10.1016/j.clgc.2017.07.002 (Epub ahead of print).
|
|
22
|
Lopez-Beltran A, Blanca A, Cimadamore A,
Montironi R, Luque RJ, Volavšek M and Cheng L: T1 bladder carcinoma
with variant histology: Pathological features and clinical
significance. Virchows Arch. 480:989–998. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chang SS, Boorjian SA, Chou R, Clark PE,
Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD,
et al: Diagnosis and treatment of Non-Muscle invasive bladder
cancer: AUA/SUO guideline. J Urol. 196:1021–1029. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Flaig TW, Spiess PE, Abern M, Agarwal N,
Bangs R, Boorjian SA, Buyyounouski MK, Chan K, Chang S, Friedlander
T, et al: NCCN Guidelines® Insights: Bladder Cancer, Version
2.2022. J Natl Compr Canc Netw. 20:866–878. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Seisen T, Compérat E, Léon P and Roupret
M: Impact of histological variants on the outcomes of nonmuscle
invasive bladder cancer after transurethral resection. Curr Opin
Urol. 24:524–531. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ho D, Imai K, King G and Stuart EA:
MatchIt: Nonparametric Preprocessing for Parametric Causal
Inference. J Statistical Software. 42:1–28. 2011.
|
|
27
|
Austin PC: A comparison of 12 algorithms
for matching on the propensity score. Stat Med. 33:1057–1069.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Montironi R and Lopez-Beltran A: The 2004
WHO classification of bladder tumors: A summary and commentary. Int
J Surg Pathol. 13:143–153. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lopez-Beltran A and Cheng L: Histologic
variants of urothelial carcinoma: Differential diagnosis and
clinical implications. Hum Pathol. 37:1371–1388. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Babjuk M, Burger M, Capoun O, Cohen D,
Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F,
Masson-Lecomte A, Mostafid AH, et al: European association of
urology guidelines on Non-muscle-invasive bladder cancer (Ta, T1,
and carcinoma in situ). Eur Urol. 81:75–94. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sanguedolce F, Calò B, Mancini V, Zanelli
M, Palicelli A, Zizzo M, Ascani S, Carrieri G and Cormio L:
Non-Muscle invasive bladder cancer with variant histology:
Biological features and clinical implications. Oncology.
99:345–358. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kamat AM, Dinney CP, Gee JR, Grossman HB,
Siefker-Radtke AO, Tamboli P, Detry MA, Robinson TL and Pisters LL:
Micropapillary bladder cancer: A review of the University of Texas
M. D. Anderson Cancer Center experience with 100 consecutive
patients. Cancer. 110:62–67. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wright JL, Black PC, Brown GA, Porter MP,
Kamat AM, Dinney CP and Lin DW: Differences in survival among
patients with sarcomatoid carcinoma, carcinosarcoma and urothelial
carcinoma of the bladder. J Urol. 178:2302–2307. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li G, Song H, Wang J, Bao Y and Niu Y:
Poor prognostic value of lymphovascular invasion for pT1 urothelial
carcinoma with squamous differentiation in bladder cancer. Sci Rep.
6(27586)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yorozuya W, Nishiyama N, Shindo T, Kyoda
Y, Itoh N, Sugita S, Hasegawa T and Masumori N: Bacillus
Calmette-Guérin may have clinical benefit for glandular or squamous
differentiation in non-muscle invasive bladder cancer patients:
Retrospective multicenter study. Jpn J Clin Oncol. 48:661–666.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Suh J, Moon KC, Jung JH, Lee J, Song WH,
Kang YJ, Jeong CW, Kwak C, Kim HH and Ku JH: BCG instillation
versus radical cystectomy for high-risk NMIBC with
squamous/glandular histologic variants. Sci Rep.
9(15268)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lobo N, Shariat SF, Guo CC, Fernandez MI,
Kassouf W, Choudhury A, Gao J, Williams SB, Galsky MD, Taylor JA
III, et al: What Is the significance of variant histology in
urothelial carcinoma? Eur Urol Focus. 6:653–663. 2020.PubMed/NCBI View Article : Google Scholar
|