Introduction
Intravascular large B-cell lymphoma (IVLBCL) is a
rare subtype of extranodal large B-cell lymphoma, characterized by
the almost exclusive growth of large lymphoma cells within the
lumina of blood vessels; particularly the capillaries, although not
in large arteries or veins (1-3).
One peculiar feature of IVLBCL is the lack of lymphadenopathy
and/or mass formation. Non-specific symptoms such as fever, dyspnea
and fatigue, have also been reported. Delayed diagnosis is common
in patients with IVLBCL, and postmortem diagnosis is not unusual,
even today. The early diagnosis of this subtype of malignant
lymphoma is important to improve patient prognosis.
The current gold standard for the diagnosis of
IVLBCL is the detection of lymphoma cells within the lumina of
blood vessels (2,3). However, a precise diagnosis may not
be straightforward as, typically, only a few lymphoma cells are
detected in the specimens and the biopsy sites may be difficult to
determine. Random skin biopsies performed on normal-appearing skin,
including subcutaneous fatty tissues, have been found to be useful
in the diagnosis of IVLBCL (4-7);
however, the detection rates of IVLBCL in random skin biopsies have
been reported to be no >22%, which are not necessarily high
(6,8). Therefore, more effective diagnostic
methods are needed for patients with suspected IVLBCL. A few cases
of IVLBCL involving cherry angiomas have been previously described
(9-26),
and the diagnostic utility of skin biopsy for cherry angiomas in
patients suspected of having IVLBCL has also been reported
(17).
Cherry angioma, also known as senile hemangioma or
Campbell de Morgan spot, is a common vascular lesion that presents
as multiple tiny red papules on the skin in middle-aged and older
individuals (27). Patients
suspected of having IVLBCL are usually middle-aged or older, and
may have cherry angiomas, making it easier to perform skin biopsy
for the diagnosis of IVLBCL. The present study describes the 21st
reported case of IVLBCL involving a cherry angioma, with a focus on
the diagnostic strategies for this rare subtype of malignant
lymphoma.
Case report
An 82-year-old Japanese man presented to a local
clinic with mild lightheadedness and anorexia in June 2023. A few
days later, the patient developed fever and penile enlargement. His
medical history included epilepsy for 45 years, which was being
treated with medication, and surgical resection for renal cell
carcinoma with lung metastasis 3.5 years prior to his presentation,
which was under observation without recurrence. The patient was
suspected of having an infectious disease and was prescribed
antibiotics; however, his symptoms did not improve. Approximately 1
week after his initial presentation, the patient was admitted to
Daiichi Towakai Hospital (Takatsuki, Japan). As laboratory tests
revealed elevated levels of soluble interleukin-2 receptor
(sIL-2R), lactate dehydrogenase (LD) and C-reactive protein (CRP),
malignant lymphoma was suspected (Table I). Contrast-enhanced computed
tomography (CT) revealed no lymphadenopathy or mass lesions
suggestive of malignancy throughout the body. In June 2023, ~3
weeks after the initial presentation, the patient was referred to
Osaka Medical and Pharmaceutical University (Takatsuki, Japan) for
further evaluation of fever of unknown origin. The aforementioned
medical information and laboratory data of the previous hospital
were obtained from the referral letter in the patient's medical
record.
 | Table ILaboratory data. |
Table I
Laboratory data.
| Parameter | 11 days before
admissiona | Reference
rangea | On
admissionb | Day 13b | Day 17b | Reference
rangeb |
|---|
| Hematocrit, % | 28.5 | 39.8-51.8 | 30.5 | 25.6 | 20.9 | 40.7-50.1 |
| Hemoglobin, g/dl | 9.4 | 13.5-17.6 | 10.1 | 7.8 | 7 | 13.7-16.8 |
| White-cell count,
/µl | 4,100 | 3,900-9,800 | 8,350 | 150 | 110 | 3,300-8,600 |
| Myelocytes, % | 0.0 | 0 | 1.0 | 0.0 | 6.0 | 0 |
| Stab neutrophils,
% | 84.4c |
44.0-72.0c | 6.5 | 0.0 | 36.0 | 0-5.0 |
| Segmented
neutrophils, % | | | 83.5 | 16.0 | 14.0 | 35.0-68.0 |
| Monocytes, % | 8.8 | 0.0-12.0 | 3.0 | 2.0 | 18.0 | 3.7-8.8 |
| Eosinophils, % | 0.7 | 0.0-10.0 | 4.0 | 3.0 | 0.0 | 0-6.4 |
| Basophils, % | 0.2 | 0.0-3.0 | 0.0 | 5.0 | 2.0 | 0.2-1.4 |
| Lymphocytes, % | 5.9 | 18.0-59.0 | 1.5 | 74.0 | 24.0 | 22.2-50.9 |
| Atypical lymphocytes,
% | 0.0 | 0 | 0.5 | 0.0 | 0.0 | 0 |
| Platelet count,
/µl | 143,000 | 130,000-362,000 | 115,000 | 20,000 | 23,000 |
158,000-348,000 |
| LD, U/l | 739 | 121-245 | 963 | 216 | 147 | 124-222 |
| Urea nitrogen,
mg/dl | 23 | 8-22 | 63 | 37 | 22 | 8-20 |
| Creatinine,
mg/dl | 1.4 | 0.61-1.04 | 2.19 | 0.88 | 1.51 | 0.65-1.07 |
| CRP, mg/dl | 26.0 | 0-0.30 | 26.35 | 4.34 | 30.69 | 0-0.14 |
| sIL-2R, U/ml | 15,800 | 121-613 | 37,970 | ND | ND | 121-613 |
Upon admission to Osaka Medical and Pharmaceutical
University, the condition of the patient was worse than reported,
with disturbances in consciousness [Glasgow Coma Scale
E3V4M5(28)], general malaise and
tachypnea with respiratory rate of 28 breaths/min, but no fever at
36.4˚C. Immediately after admission, the patient was oliguric with
a urine output of ~10 ml/h; however, with intermittent
administration of furosemide, the daily urine output was maintained
at >1,000 ml thereafter. The patient also exhibited hypoxemia
with an oxygen saturation of 92%, for which oxygen was administered
up to 10 l with a reservoir mask, keeping the oxygen saturation
>94%. A physical examination revealed no lymph node enlargement,
although a small red papule, clinically diagnosed as a cherry
angioma, was present on the trunk of the patient. Laboratory tests
showed anemia and thrombocytopenia, with a white blood cell count
within the normal range, accompanied by atypical lymphocytes
(0.5%), whose clonality was undetectable (Table I). There were also elevations in
sIL-2R, LD, CRP, urea nitrogen and serum creatinine (Table I). As for infectious diseases,
T-SPOT.TB test was negative, Aspergillus antigen was 0.0 (reference
range, <0.5), and CMV-C7HRP was negative, indicating that
tuberculosis, Aspergillus and cytomegalovirus were not detected,
respectively. Elevated levels of sIL-2R and LD suggested malignant
lymphoma; however, repeat CT revealed no enlarged lymph nodes
throughout the body (Fig. S1A).
Based on these findings, the differential diagnoses included
malignant diseases such as IVLBCL or penile malignancy, and
infectious diseases such as tuberculosis. Of the aforementioned
differential diagnoses, IVLBCL was the most suspected, because no
causative infection or primary penile malignancy was detected in
the laboratory data and imaging findings. Biopsies were performed,
with specimens obtained from the cherry angioma and penile skin,
and a bone marrow aspiration was also carried out. Considering the
poor general condition of the patient, a random skin biopsy was not
performed.
For the histological analysis, the biopsied
specimens were fixed in 10% buffered formalin for 24 h at room
temperature (RT) and embedded in paraffin. Sections of 4
µm-thickness from the paraffin block were stained with hematoxylin
for 5 min and eosin for 1 min at RT. The stained sections were
examined under a light microscope (BX53; Olympus Corporation).
Histopathological examination of the skin biopsy from the cherry
angioma with hematoxylin and eosin staining revealed a collection
of dilated capillary vessels in the upper dermis, a typical feature
of cherry angiomas (Fig. 1A and
B). Large lymphoid cells with
irregularly shaped nuclei and nucleoli filled the dilated vessels,
and apoptotic bodies were scattered. No extravascular invasion of
lymphoid cells was observed, nor were large lymphoid cells observed
in the vessels of the dermis or the subcutis of the biopsy
specimen. For further analysis, immunohistochemistry (IHC) was
carried out. Briefly, 4 µm-thick sections obtained from the
paraffin block were stained with primary antibodies shown in
Table SI. For IHC, automated
immunostaining devices, VENTANA BenchMark ULTRA (Roche) and
Histostainer 48A (Nichirei), were used, and the procedures
including antigen retrieval, blocking, washing and detection using
secondary antibodies followed the manufacturer's instructions
(Table SI). Briefly, before
staining, to block endogenous peroxidases, the IHC device-dedicated
reagent (ultra View DAB universal; Roche Tissue Diagnostics) was
used in B-cell lymphoma 6 (Bcl-6), cluster of differentiation 10
(CD10), CD20, CD3, CD30, CD5, CD79a, and multiple myeloma oncogene
1 (MUM1) staining, and standard hydrogen peroxide (3%) was used in
CD31 staining; their temperature/duration were 36˚C/4 min and RT/5
min, respectively. In the deparaffinization process, EZ prep (Roche
Tissue Diagnostics) was used for Bcl-6, CD10, CD20, CD3, CD30, CD5,
CD79a and MUM1 staining, and standard xylene and alcohol series for
CD31 staining. The stained sections were observed under a light
microscope (BX53, Olympus).
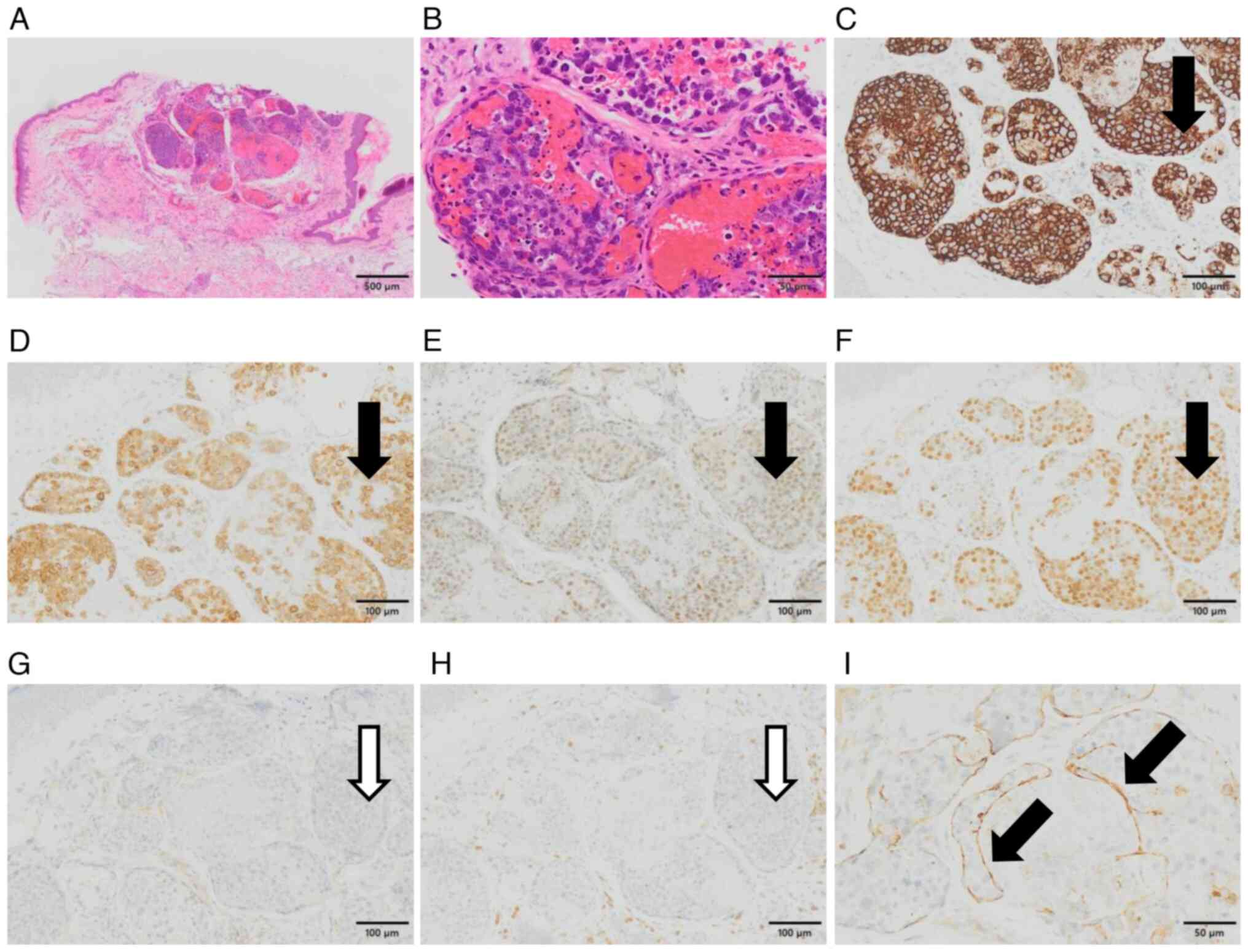 | Figure 1Histopathological and
immunohistochemical features. (A) A collection of the dilated
capillary vessels in the upper dermis, filled with lymphoid cells.
No extravascular invasion of lymphoid cells was observed
(hematoxylin and eosin staining; magnification, x4). (B)
Large-sized lymphoid cells with irregular nuclei and nucleoli and
apoptosis were scattered (hematoxylin and eosin staining;
magnification, x40). Immunohistochemically, the lymphoid cells were
positive for (C) CD20, (D) CD79a, (E) Bcl-6 and (F) MUM1
(magnification, x20). The black arrows indicated areas that were
typically positive for lymphoma cells. The atypical cells were
negative for (G) CD10 and (H) CD3 (magnification, x20). The white
arrows indicated areas that were typically negative for them. (I)
CD31 was expressed surrounding the lymphoma cell cluster (black
arrows), indicating its intravascular localization (magnification,
x40). Bcl-6, B-cell lymphoma 6; CD, cluster of differentiation;
MUM1, multiple myeloma oncogene 1. |
IHC revealed that the large lymphoid cells lodged in
the cherry angioma expressed B cell-associated antigens, such as
CD20, CD79a, MUM1 and Bcl-6 (Fig.
1C-F). However, the lymphoid cells did not express CD3, CD5,
CD10 or CD30 (Figs. 1G and
H, S1B and 1C). Additionally, the atypical cells
were covered with CD31-positive endothelial cells, confirming
intravascular localization (Fig.
1I). A bone marrow smear was air-dried, fixed with methanol for
20 sec, and stained with undiluted May-Grunwald for 2 min, with
diluted May-Grunwald for 2 min, and then with Giemsa solution for
15 min at RT. The stained smear was observed under a light
microscope (BX53; Olympus Corporation). The smear slide revealed
hemophagocytosis and a few large vacuolated lymphocytes (1.9%)
(Fig. S1D and E). While a histopathological examination
of the penile skin revealed dilated dermal vessels and mild
perivascular infiltration by small lymphocytes (Fig. S1F), no large lymphoid cells were
observed in these vessels.
Based on the aforementioned results, the patient was
histopathologically diagnosed with IVLBCL involving a cherry-shaped
angioma. On the 3rd day of hospitalization, chemotherapy (a regimen
of rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisolone) was initiated on the patient in the context of his
rapid disease progression and worsening general condition, and his
family's request for aggressive treatment. On the 13th day of
hospitalization, after the first cycle of chemotherapy, LD and CRP
levels of the patient decreased to 216 U/l and 4.34 mg/dl,
respectively (Table I); however,
the patient again developed a fever of 40.1˚C. Blood cultures were
positive for Corynebacterium striatum and Staphylococcus
epidermidis on the 15th day of hospitalization. On the 17th day
of hospitalization, a catheter tip culture was positive for S.
epidermidis, and the laboratory data showed prominent
leukopenia and CRP elevation (Table
I). In July 2023, the patient died of sepsis on the 19th day of
hospitalization, but no autopsy was performed.
Discussion
The present study, to the best of our knowledge,
describes the 21st case of IVLBCL involving a cherry angioma (in
English) since Rubin et al (9) first reported a case in 1997. Although
the clinical presentation of IVLBCL is heterogeneous and lacks
specific manifestations, two clinical forms have been recognized:
the classic form (largely present in Western countries) and the
hemophagocytic syndrome-associated form. The latter form is
characterized by multiorgan failure, hepatosplenomegaly and
pancytopenia, and is commonly observed in Asian countries (the
so-called ‘Asian variant’) (1-3).
The patient described in the present study showed
hemophagocytosis upon the analysis of the bone marrow; therefore,
hemophagocytic syndrome-associated IVLBCL was suspected. The
prognosis for this variant was poor, and the ultimate cause of
death in the current case was considered to be sepsis due to
opportunistic infection during chemotherapy. Table II summarizes the
clinicopathological features of the previously reported cases, as
well as the present case. The median age of the patients was 71
years (range, 51-86), and men and women were almost evenly affected
(women: men, 9:10). The geographic regions in which cases were
reported were Japan (11 cases), the United States (7 cases),
Austria (1 case) and France (1 case). No specific trend was
observed in the location of cherry angiomas with IVLBCL, although
lymphoma cells were detected in two or more cherry angiomas in five
patients. Of note, lymphoma cells were detected only in cherry
angiomas and not in random skin biopsies of three patients. It is
worth mentioning that in the patient presented in the current study
(Case 21), IVLBCL was diagnosed only by biopsy of a cherry angioma,
while no random skin biopsies were performed. With regard to the
positive rates per procedure for the diagnosis of IVLBCL according
to the data in Table II, the
positive rate was 67% (18/27) for biopsies of cherry angiomas and
25% (4/16) for random skin biopsies. In cases where both of them
were performed (5 cases), the positive rate was 70% (7/10) for
biopsy of cherry angioma and 25% (4/16) for random skin biopsy.
Despite the small number of reports and possible publication bias,
these findings reaffirm the utility of cherry angioma biopsy in the
diagnosis of IVLBCL and may suggest that it is superior to random
skin biopsy in its diagnostic sensitivity.
 | Table IIClinicopathological features,
diagnostic procedures and outcome of intravascular large B-cell
lymphoma involving in cherry angioma. |
Table II
Clinicopathological features,
diagnostic procedures and outcome of intravascular large B-cell
lymphoma involving in cherry angioma.
| Case no. | Age/Sex | Sampling of cherry
angioma Positive/total no. | Location | Random skin biopsy
Positive/total no. | Location/depth | (Refs.) |
|---|
| 1 | 66/M | 2/multiple | Chest, back | NA | | (9) |
| 2 | 52/M | 2/3 | Trunk | NA | | (10) |
| 3 | 82/M | 1/2 | Chest | NA | | (11) |
| 4 | 81/F | 3/6 | Upper arm,
trunk | NA | | (12) |
| 5 | 67/M | 1/NA | Abdomen | NA | | (13) |
| 6 | 64/F | 1/1 | Scalp | NA | | (14) |
| 7 | 55/F | 1/1 | Shoulder | NA | | (14) |
| 8 | 66/F | at least 1/NA | Thorax | NA | | (15) |
| 9 | 78/F | 1/3 | Thorax | 0/3 | Upper arm, abdomen,
upper thigh/NA | (16) |
| 10 | 79/F | 1/1 | Thigh | 0/3 | Upper arm, abdomen,
thigh/NA | (17) |
| 11 | 86/M | 1/1 | Abdomen | 3/4 | Abdomen,
thigh/subcutis | (18) |
| 12 | 74/F | at least 1/NA | Trunk | NA | | (19) |
| 13 | 76/M | 2/2 | Precordial
region | 0/3 | Forearm, abdomen,
thigh/subcutis | (20) |
| 14 | 51/M | 2/3 | Precordial region,
upper arm | 1/3 | Forearm, abdomen,
thigh/subcutis | (20) |
| 15 | 63/F | at least 1/NA | NA | NA | | (21) |
| 16 | 76/F | 1/1 | Neck | NA | | (22) |
| 17 | NA | at least 1/NA | NA | NA | | (23) |
| 18 | 77/M | at least 1/NA | Abdomen | NA | | (24) |
| 19 | 68/M | 1/2 | NA | NA | | (25) |
| 20 | NA | at least 1/NA | NA | NA | | (26) |
| 21 | 82/M | 1/1 | Trunk | Not performed | | |
Although the usefulness of random skin biopsies in
the diagnosis of IVLBCL has traditionally been well recognized
(4-7),
random skin biopsies are not necessarily the optimal strategy for
the diagnosis of IVLBCL at present. For the concrete method of
random skin biopsies, it is recommended that 3-4 specimens be
obtained from the thigh, abdomen and/or posterior upper arm.
However, these biopsies may increase the risk of unintended
prolonged bleeding, especially in patients with pancytopenia, while
the positive detection rates of random skin biopsies for IVLBCL are
not necessarily high (6,8). Although only 20 patients with IVLBCL
involving cherry angiomas have been reported in the relevant
English literature (Table II),
the strategy of performing skin biopsies targeting lesions, such as
cherry angiomas, may lead to a more accurate and easier diagnosis
of IVLBCL. Currently, a comprehensive clinical diagnostic approach
to IVLBCL has been proposed, in which each cherry angioma should be
considered as a biopsy site along with random skin biopsies
(6). We expect that more clinical
data will be collected in the future for the diagnosis of IVLBCL
using biopsies targeting specific lesions, including cherry
angiomas.
Although the mechanism of retention of lymphoma
cells within cherry angiomas is unclear, cherry angiomas may
represent areas of lower blood flow, leading to the entrapment and
subsequent occlusion of lymphoma cells in the vessels of the cherry
angiomas (11). There may be a
molecular mechanism linking the growth and localization patterns of
IVLBCL to the characteristics of cherry angiomas, which further
in vivo and in vitro studies may be able to
elucidate. The mechanism of selective intravascular growth and
localization of IVLBCL is also unclear; however, it has been
suggested that CD44 expression in lymphoma cells and the lack of
lymphoma cells expressing CD54 (intercellular adhesion molecule-1)
may be related to the peculiar growth and localization patterns of
IVLBCL (17).
In conclusion, the present study described the 21st
reported case of IVLBCL involving a cherry angioma. Considering the
poor general condition of the patient, random skin biopsies were
not performed; however, IVLBCL was successfully diagnosed by a skin
biopsy of the cherry angioma. Although random skin biopsies are
recognized as a useful diagnostic tool for IVLBCL, the usefulness
of skin biopsies from cherry angiomas in patients with suspected
IVLBCL should be further explored, due to the overlap in average
patient age and predilection for these diseases.
Supplementary Material
CT and pathological findings. (A)
Image of CT scan showed no lymphadenopathy or mass lesion.
Immunohistochemically, the lymphoid cells were negative for (B) CD5
and (C) CD30 (magnification, x20). The white arrows indicated areas
that were typically negative for them. (D) Hemophagocytosis in the
bone marrow smear, indicated by black arrow (May Grunwald-Giemsa
staining; magnification, x40). (E) Vacuole-like inclusions of the
lymphocyte in the bone marrow smear, indicated by black arrow (May
Grunwald-Giemsa staining; magnification, x40). (F) No atypical
lymphoid cells were found in the capillary vessels of the penile
skin (hematoxylin and eosin staining; magnification, x10). CD,
cluster of differentiation; CT, computed tomography.
Reagents and conditions for
immunohistochemistry.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YF, MI and YH conceived and designed the study. YF,
MI, EY, KS, SS, TH, TS, HK and SM collected and analyzed the data.
YF and YH confirm the authenticity of all the raw data. YF, MI and
YH drafted the manuscript and figures. All of the authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
principles of the Declaration of Helsinki. All data were
anonymized. Consent for participation in the present study was
obtained from the family of the patient, as the patient was unable
to express his intentions owing to a decreased level of
consciousness.
Patient consent for publication
Consent for the publication of the present case
report was obtained from the family of the patient, as the patient
was unable to express his intentions due to a decreased level of
consciousness.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakamura S, Ponzoni M and Campo E:
Intravascular large B-cell lymphoma. In: WHO classification of
tumours of haematopoietic and lymphoid tissues. Revised 4th
edition. IARC, Lyon, pp317-318, 2017.
|
|
2
|
Shimada K and Kiyoi H: Current progress
and future perspectives of research on intravascular large B-cell
lymphoma. Cancer Sci. 112:3953–3961. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ponzoni M, Campo E and Nakamura S:
Intravascular large B-cell lymphoma: A chameleon with multiple
faces and many masks. Blood. 132:1561–1567. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsue K, Asada N, Odawara J, Aoki T,
Kimura S, Iwama K, Fujiwara H, Yamakura M and Takeuchi M: Random
skin biopsy and bone marrow biopsy for diagnosis of intravascular
large B cell lymphoma. Ann Hematol. 90:417–421. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matsue K, Abe Y, Kitadate A, Miura D,
Narita K, Kobayashi H, Takeuchi M, Enzan N, Tanaka A and Takeuchi
K: Sensitivity and specificity of incisional random skin biopsy for
diagnosis of intravascular large B-cell lymphoma. Blood.
133:1257–1259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
MacGillivary ML and Purdy KS:
Recommendations for an approach to random skin biopsy in the
diagnosis of intravascular B-cell lymphoma. J Cutan Med Surg.
27:44–50. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SR, Ko CJ, Nelson CA, Ramachandran S
and Gehlhausen JR: Random skin biopsies for diagnosis of
intravascular large B-cell lymphoma: Retrospective analysis of 31
biopsies from a US dermatology inpatient consultative service with
literature review. J Am Acad Dermatol. 88:714–716. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takigawa M, Yamasaki O, Nomura H, Miyake
T, Yanai H and Morizane S: Probability scoring system of
intravascular large B-cell lymphoma for the application of random
skin biopsy: A retrospective cohort study. JAAD Int. 27:146–152.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rubin MA, Cossman J, Freter CE and Azumi
N: Intravascular large cell lymphoma coexisting within hemangiomas
of the skin. Am J Surg Pathol. 21:860–864. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kobayashi T, Munakata S, Sugiura H,
Koizumi M, Sumida M, Murata K and Shinkai H: Angiotropic lymphoma:
Proliferation of B cells in the capillaries of cutaneous angiomas.
Br J Dermatol. 143:162–164. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Satoh S, Yamazaki M, Yahikozawa H,
Ichikawa N, Saito H, Hanyuu N, Hata S and Hachyou M: Intravascular
large B cell lymphoma diagnosed by senile angioma biopsy. Intern
Med. 42:117–120. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cerroni L, Zalaudek I and Kerl H:
Intravascular large B-cell lymphoma colonizing cutaneous
hemangiomas. Dermatology. 209:132–134. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Motegi S, Tamura A, Takeuchi Y and
Ishikawa O: Senile angioma-like eruption: A skin manifestation of
intravascular large B cell lymphoma. Dermatology. 209:135–137.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nixon BK, Kussick SJ, Carlon MJ and Rubin
BP: Intravascular large B-cell lymphoma involving hemangiomas: An
unusual presentation of a rare neoplasm. Mod Pathol. 18:1121–1126.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakamura Y, Nakamagoe K, Kawachi Y, Hosaka
A, Mukai H, Chiba S, Otsuka F and Tamaoka A: Intravascular large B
cell lymphoma with neurological symptoms diagnosed on the basis of
a senile angioma-like eruption. BMJ Case Rep.
2009(1297)2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ishida M, Hotta M, Hodohara K and Okabe H:
A case of intravascular large B-cell lymphoma colonizing in senile
hemangioma. J Cutan Pathol. 38:251–253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ishida M, Hodohara K, Yoshida T and Okabe
H: Intravascular large B-cell lymphoma colonizing in senile
hemangioma: A case report and proposal of possible diagnostic
strategy for intravascular lymphoma. Pathol Int. 61:555–557.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adachi Y, Kosami K, Mizuta N, Ito M,
Matsuoka Y, Kanata M, Akiyama H, Murao T, Li M, Ieki R and Ikehara
S: Benefits of skin biopsy of senile hemangioma in intravascular
large B-cell lymphoma: A case report and review of the literature.
Oncol Lett. 7:2003–2006. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Katayama K, Tomoda K, Ohya T, Asada H,
Ohbayashi C and Kimura H: Ground-glass opacities and a solitary
nodule on chest in intravascular large B-cell lymphoma. Respirol
Case Rep. 3:108–111. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Sakurai T, Wakida K, Takahashi T, Ishida
K, Iwata H and Nishida H: Usefulness of senile hemangioma biopsy
for diagnosis of intravascular large B-cell lymphoma: A report of
two cases and a literature review. J Neurol Sci. 373:52–54.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Genin V, Enfrein A, Lecouffe-Desprets M,
Gallas P, Bossard C, Moreau A, Ansquer C, Hamidou M, Agard C and
Neel A: Hot lungs, bitter cherry: Intravascular lymphoma. QJM.
111:53–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Weingarten M, Weingarten M, Sidhu H and
Sidhu JS: A poisoned cherry: Migratory cutaneous intravascular
large B-cell lymphoma with subsequent systemic nodal lymphoma. JAAD
Case Rep. 6:1336–1338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rozenbaum D, Tung J, Xue Y, Hoang MP and
Kroshinsky D: Skin biopsy in the diagnosis of intravascular
lymphoma: A retrospective diagnostic accuracy study. J Am Acad
Dermatol. 85:665–670. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cao DY, Zhou J, Dernell C, Chaney K and
Wanat KA: Red papules associated with progressive functional
decline. JAAD Case Rep. 40:23–26. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao CW, Fan TH, Denize T, Coraini A,
Kraft A, Kumar AM, Gao LG, Lorenzo ME, Duncan LM, Faye EC and Lin
DJ: Intravascular lymphoma as a cause of recurrent stroke-case
report and review of the literature. Neurohospitalist. 13:419–424.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Enzan N, Kitadate A and Kono M: Optimizing
randome skin biopsies: A review of techniques and indications for
intravascular large B-cell lymphoma. Int J Hematol. 119:619–625.
2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Betz-Stablein B, Koh U, Edwards HA,
McInerney-Leo A, Janda M and Soyer HP: Anatomic distribution of
cherry angiomas in the general population. Dermatology. 238:18–26.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Teasdale G, Maas A, Lecky F, Manley G,
Stocchetti N and Murray G: The glasgow coma scale at 40 years:
Standing the test of time. Lancet Neurol. 13:844–854.
2014.PubMed/NCBI View Article : Google Scholar
|















