Introduction
PHESGO®, a fixed-dose combination of
pertuzumab and trastuzumab for subcutaneous injection (1-5),
is used in Japan for treating human epidermal growth factor
receptor type 2 (HER2)-positive breast cancer and advanced or
recurrent unresectable colorectal cancer that has progressed after
chemotherapy (6). As of March 31,
2023, it has been approved for the treatment of early-stage and
metastatic breast cancer in more than 100 countries and regions
worldwide, including six countries in Europe and the United States
(the United States, the United Kingdom, Germany, France, Canada,
and Australia). Previously, these drugs were only available via
intravenous infusion. However, the introduction of PHESGO has made
subcutaneous injection an option. PHESGO differs from conventional
intravenous formulations in several ways. First, it changes the
route of administration. Second, it offers a fixed-dose
formulation, irrespective of body weight: 15 ml (1,200 mg
pertuzumab, 600 mg trastuzumab) for the initial dose and 10 ml (600
mg pertuzumab, 600 mg trastuzumab) for maintenance and subsequent
doses. This contrasts with conventional intravenous trastuzumab
formulations, which have a fixed-dose per unit of body weight.
Third, PHESGO significantly reduces administration time. While
intravenous pertuzumab and trastuzumab typically take 60 and 90 min
respectively, PHESGO is administered in 8 min for the initial dose
and 5 min for subsequent doses. Shorter dosing times at the same
dose can increase the risk of infusion reactions (IRs) (7). However, subcutaneous injection is
absorbed more slowly than intravenous injection, potentially
reducing IR incidence. Despite reports of IRs with trastuzumab
(8-11),
and overseas use of PHESGO (12),
there are no reports from actual clinical settings in Japan, making
safety information scarce. PHESGO appears to have many advantages
over existing intravenous formulations, such as reduced
administration time and no need for weight-based dose adjustment.
However, patients may switch back to the intravenous formulation
after switching to PHESGO. Therefore, investigating IR information
on PHESGO and the safety situation associated with switching could
help select a formulation that meets patient requirements. In this
study, we investigated safety information, particularly IR
information, and patient preference regarding the drug when side
effects occurred in patients with HER2-positive breast and
colorectal cancer. In addition, the clinical trials aimed to
accumulate safety data for patients with breast cancer, as
information for patients with colorectal cancer is extremely
limited.
Patients and methods
Patients and study design
The medical records of colorectal or breast cancer
patients who received PHESGO at the Saitama Cancer Center (Saitama,
Japan) between January and March 2024. All patients with colorectal
or breast cancer who received PHESGO were included in the study,
with no exclusion criteria. This study was approved by the
institutional review board of Saitama Cancer Center (approval no.
1816; approved May 8, 2024). It was confirmed at the time of sample
collection that comprehensive consent had been obtained. We
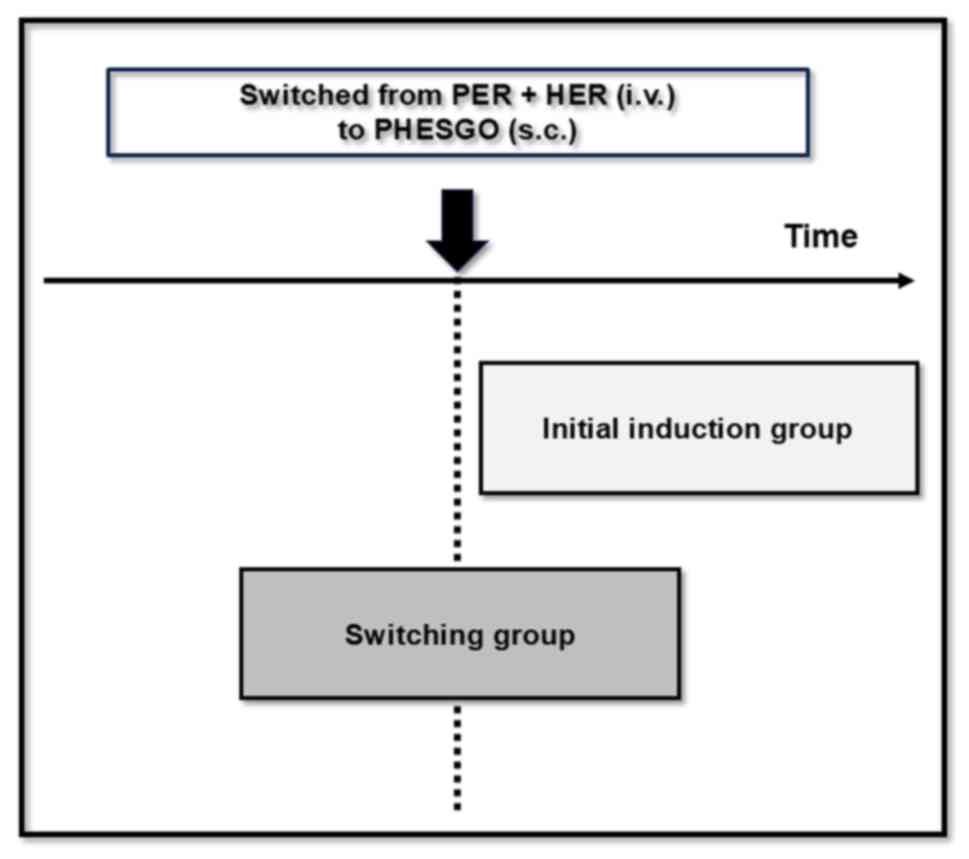
examined the IRs in the group that switched from pertuzumab +
trastuzumab intravenous formulations to PHESGO and the group that
used PHESGO from the start (Fig.
1).
Treatment
Patients treated with PHESGO alone or in combination
with docetaxel (DTX) or paclitaxel (PTX).
Survey items
Baseline patient characteristics, such as the number
of males and females, median age, and age range, sex, weight, BMI,
HER2 score, combination regimen, cancer stages, dosing time,
injection site reactions, and IR expression status, were recorded.
IRs were defined as the occurrence of symptoms such as fever,
chills, hypersensitivity, psychogenic reactions, pruritus,
urticaria, diarrhea, systemic disorders, or immune system disorders
on the day of administration or the day after. IR presence and
symptom details were extracted from electronic medical records. IRs
of Grade 1 or higher were evaluated based on the Common Terminology
Criteria for Adverse Events version 5.0 (CTCAE. Ver. 5.0).
The study also investigated whether PHESGO was used
as initial induction or as a switch from the intravenous
formulation, to determine the difference in the frequency of IRs
between these two groups. Additionally, the study examined the
reasons for patients switching from PHESGO to the intravenous
formulation.
Statistical analysis
Statistical analysis was performed using JMP17 Pro
(SAS Institute, Cary, NC, USA). The Fisher's exact test was used to
compare patient characteristics and the expression of IR in the
initial induction group and the switching group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline clinical characteristics
The study encompassed 51 patients (2 males and 49
females; median age 56 years, range 33-84 years), with 16 in the
initial induction group and 35 in the switching group (Table I).
 | Table IPatients' baseline
characteristics. |
Table I
Patients' baseline
characteristics.
| Characteristic | Initial induction
group (n=16) | Switching group
(n=35) |
|---|
| Age, years | | |
|
Mean
(SD) | 59.8 (12.3) | 57.1 (12.1) |
|
Median
(range) | 56.0 (43-84) | 58.0 (33-77) |
| Weight, kg | | |
|
Mean
(SD) | 59.6 (11.3) | 54.6 (10.1) |
|
Median
(range) | 56.3 (46.0-87.0) | 51.1 (35.3-78.6) |
| BMI | | |
|
Mean
(SD) | 23.9 (3.6) | 22.6 (3.5) |
|
Median
(range) | 23.7 (19.1-30.6) | 22.5 (15.4-30.9) |
| Cancer type | | |
|
Breast
cancer | 12 | 35 |
|
Stage | | |
|
IA | 0 | 4 |
|
IB | 1 | 0 |
|
IIA | 4 | 6 |
|
IIB | 5 | 3 |
|
IIIA | 1 | 4 |
|
Ⅳ | 1 | 18 |
|
Colorectal
cancer | 4 | 0 |
|
Stage | | |
|
Ⅳ | 4 | 0 |
| HER2 score | | |
|
2+ | 6 | 3 |
|
3+ | 10 | 32 |
| Combination
regimen | | |
|
DTX | 11 | 6 |
|
PTX | 0 | 1 |
|
None | 5 | 28 |
IR expression
IRs of Grade 1 or higher were observed in four
patients (25%) in the initial induction group, while no patients
(0%) in the switching group experienced IRs (Table II). This significant difference in
IR occurrence between the two groups was statistically significant
(P=0.0073). IRs were characterized by symptoms such as fever,
chill, and diarrhea, which manifested on the day of treatment or
the following day. All patients who developed IRs were in the
initial induction group, including patients with colorectal cancer
(Table III). Five of the 51
patients experienced skin and subcutaneous tissue disorders, all of
whom were in the switching group and had breast cancer. Three of
these patients continued on a slower dose rate, while two switched
from PHESGO to the intravenous formulation of pertuzumab and
trastuzumab (Table IV). All
patients experienced pain at the administration site.
 | Table IIIncidence of infusion reactions. |
Table II
Incidence of infusion reactions.
| Infusion
reaction | Initial induction
group (n=16) | Switching group
(n=35) | P-value |
|---|
| Yes | 4 (25%) | 0 (0%) | 0.0073 |
| No | 12 (75%) | 35 (100%) | |
 | Table IIIDetailed information. |
Table III
Detailed information.
| Patient no. | Group | Cancer type | Combination
regimen | Adverse events | Grade |
|---|
| 21 | Initial induction
group | Colorectal
cancer | None | Fever, Chill | Ⅱ |
| 22 | Initial induction
group | Colorectal
cancer | None | Fever | Ⅰ |
| 24 | Initial induction
group | Colorectal
cancer | None | Fever | Ⅰ |
| 50 | Initial induction
group | Colorectal
cancer | None | Fever, Diarrhea | Ⅱ |
 | Table IVSide effects excluding infusion
reactions. |
Table IV
Side effects excluding infusion
reactions.
| Patient no. | Group | Cancer type | Side effect | Coping strategy |
|---|
| 3 | Switching group | Breast cancer | Injection site
reaction | Decreased dosing
rate |
| 8 | Switching group | Breast cancer | Injection site
reaction | Decreased dosing
rate |
| 17 | Switching group | Breast cancer | Injection site
reaction | Switch from
subcutaneous to intravenous administration |
| 34 | Switching group | Breast cancer | Injection site
reaction | Decreased dosing
rate |
| 48 | Switching group | Breast cancer | Injection site
reaction | Switch from
subcutaneous to intravenous administration |
Discussion
Pertuzumab and trastuzumab, when administered as
intravenous formulations, are known to cause IR, especially with
the first dose (2,11). This study reveals that IRs occurred
exclusively in the initial induction group (Table II), suggesting that the risk of
IRs is also elevated with subcutaneous formulations upon first
administration. Interestingly, all four cases with IRs involved
patients with colorectal cancer. This suggests that the frequency
of IRs might vary based on the type of cancer. However, given that
all cases were first inductions, it is plausible that the
occurrence of IRs is influenced more by whether the patient was a
first induction rather than the type of cancer. Regardless, due to
the small sample size, further accumulation of cases is necessary
for a more comprehensive understanding. Considering that none of
the cases in the switching group developed IR, we believe the most
significant factor is whether it is the first induction or not. Due
to small sample size limitations, this study did not examine cancer
types, and this is an issue for future research direction.
One factor contributing to IRs with intravenous
trastuzumab is the high dose per unit time. Specifically, the
biweekly dose of 4 mg/kg and the triweekly dose of 6 mg/kg are more
likely to cause IRs than the weekly dose of 2 mg/kg (7). IRs are also more likely to occur when
the dose per unit time is higher for 30-min doses than for 90-min
doses, suggesting that the first dose should be administered over a
longer duration. This study found that IRs occurred in 4 (25%)
patients treated with PHESGO (Table
II), a rate comparable to that reported for intravenous
formulations of pertuzumab and trastuzumab (9,11,13).
The dose of trastuzumab in PHESGO is 600 mg, equivalent to a body
weight of 75 kg, compared to the loading dose of 8 mg/kg for the
intravenous formulation. Similarly, the dose of pertuzumab in
PHESGO is higher than in the intravenous formulation
PERJETA® (loading dose: 840 mg, maintenance dose: 420
mg). Despite these higher doses and the simultaneous administration
of two drugs in PHESGO, the frequency of IRs is similar to that of
the intravenous formulation. This suggests that IRs may be less
likely to occur with PHESGO than with two drugs in the intravenous
formulation. This phenomenon is thought to be due to the nature of
the subcutaneous route of administration. Absorption from the
subcutis into the body occurs in a rate-limiting step, suppressing
a rapid rise in blood concentration, even if the drug is
administered at a faster rate or in a larger volume. However, 2 out
of 51 patients switched to the intravenous formulation after
receiving PHESGO. The main reason for this switch was site
reactions (Table IV), suggesting
that the potential disadvantages of skin and subcutaneous tissue
damage from injections might outweigh the benefits of shorter
administration times and reduced risk of IRs. Pain perception
varies among individuals and can be influenced by the
administration technique, leading to several formulation switches
due to injection site reactions. Some patients, who have been on
maintenance therapy for several years, might have switched to
PHESGO once and then returned to the intravenous formulation due to
familiarity with the previous method of administration. In this
study, all patients who switched from PHESGO to the intravenous
formulation had previously been treated with the intravenous
formulation. Initially, the study focused solely on patients who
were treated with PHESGO. However, it is likely that a small subset
of these patients were given the option to switch to PHESGO but
chose to continue with their conventional treatment. This is not
necessarily a drawback; instead, it underscores the importance of
having a variety of treatment options. The factors that patients'
consider when choosing a treatment can vary widely, including the
time and location of administration, the method of administration,
the cost, and their expectations or concerns about new medications.
Patients' satisfaction plays a crucial role in the continuation of
treatment. For instance, shorter administration times can lead to
reduced hospital stays, potentially enhancing patient satisfaction
(14,15). In a previous study of a crossover
trial comparing subcutaneous and intravenous formulations of
pertuzumab + trastuzumab, the subcutaneous formulation was
overwhelmingly preferred by a larger percentage of patients
(4). The main reason for this
preference was the shorter time required at the clinic. However, a
higher percentage of patients reported preferring the intravenous
formulation because of a lower level of injection site pain and a
more comfortable experience during administration. In this study,
two patients switched from subcutaneous to intravenous formulations
(Table IV), suggesting that, as
in previous reports, pain during administration is a factor in the
choice of formulation. The method of administration is also a key
factor in treatment adherence, as it empowers patients to
participate in their own treatment decisions. The introduction of
PHESGO is significant in this context. It is crucial that patients
are confident in the treatment's effectiveness and are comfortable
with its administration.
The study's limitations encompass a brief research
period and a limited number of cases. There were a few instances in
this study where patients with a history of intravenous treatment
transitioned to PHESGO, and then reverted back to intravenous
treatment (Table IV). However, no
patients who initiated treatment with PHESGO switched to
intravenous treatment, though such a transition is anticipated in
the future. Conversely, the study's strength lies in its novelty.
To date, the only report on PHESGO involving a switch from an
intravenous formulation in Japanese patients is the FeDeriCa study,
which involved 20 Japanese participants. There is undeniably a lack
of clinical information regarding the switch between formulations.
Actual clinical data are essential for making informed decisions
about formulation selection and switching (5). It is the first to present IR data on
PHESGO and its practical application, with a particular focus on
the safety of transitioning to PHESGO, within a Japanese context.
Although, we believe it is important to accumulate more information
in the future because of its novelty and preliminary results. We
intend to extend the study and longer prospective studies with a
larger cohort as a future direction.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TA conceived the study. TA, AS, TS, HK and DO
designed the experiments. TA, AS, DO, KM, TS, MH, MS, DT and TN
analyzed the data. TA and AS wrote the paper. All authors confirm
the authenticity of all the raw data, provided intellectual input,
and were responsible for the contents of the paper, including the
data, analysis and interpretation. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Saitama Cancer Center (approval no. 1816; approved
May 8, 2024). As this was a retrospective observational study,
consent was not obtained from individual patients. An information
disclosure document about this study was created and published for
the patients, guaranteeing the opportunity for patients to refuse
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kirschbrown WP, Wynne C, Kågedal M, Wada
R, Li H, Wang B, Nijem I, Crnjevic TB, Gasser H, Heeson S, et al:
Development of a subcutaneous fixed-dose combination of pertuzumab
and trastuzumab: Results from the phase Ib dose-finding study. J
Clin Pharmacol. 59:702–716. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Swain SM, Miles D, Kim SB, Im YH, Im SA,
Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al:
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic
breast cancer (CLEOPATRA): End-of-study results from a
double-blind, randomised, placebo-controlled, phase 3 study. Lancet
Oncol. 21:519–530. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
DuMond B, Patel V, Gross A, Fung A and
Weber S: Fixed-dose combination of pertuzumab and trastuzumab for
subcutaneous injection in patients with HER2-positive breast
cancer: A multidisciplinary approach. J Oncol Pharm Pract.
27:1214–1221. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Shaughnessy J, Sousa S, Cruz J,
Fallowfield L, Auvinen P, Pulido C, Cvetanovic A, Wilks S, Ribeiro
L, Burotto M, et al: Preference for the fixed-dose combination of
pertuzumab and trastuzumab for subcutaneous injection in patients
with HER2-positive early breast cancer (PHranceSCa): A randomised,
open-label phase II study. Eur J Cancer. 152:223–232.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan AR, Im SA, Mattar A, Colomer R,
Stroyakovskii D, Nowecki Z, De Laurentiis M, Pierga JY, Jung KH,
Schem C, et al: Fixed-dose combination of pertuzumab and
trastuzumab for subcutaneous injection plus chemotherapy in
HER2-positive early breast cancer (FeDeriCa): A randomised,
open-label, multicentre, non-inferiority, phase 3 study. Lancet
Oncol. 22:85–97. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sakaeda M, Kotani N, Yoneya T, Zheng Y and
Habara Y: [Pharmacological properties and clinical development
overview of pertuzumab (genetical recombination), trastuzumab
(genetical recombination) and vorhyaluronidase alfa (genetical
recombination) (PHESGO(®) combination for subcutaneous injection
MA, IN)]. Nihon Yakurigaku Zasshi. 159:241–253. 2024.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
7
|
Thompson LM, Eckmann K, Boster BL, Hess
KR, Michaud LB, Esteva FJ, Hortobágyi GN and Barnett CM: Incidence,
risk factors, and management of infusion-related reactions in
breast cancer patients receiving trastuzumab. Oncologist.
19:228–234. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tabuchi Y, Tsujimoto M, Yamamoto K,
Shimizu R, Kosaka T, Sakaguchi K, Dobuchi N, Nishiguchi K and
Shikata K: Risk factors for infusion reactions in patients with
breast cancer administered trastuzumab therapy. Biol Pharm Bull.
46:964–968. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tatsuta R, Sumimoto T, Nakahara R, Tanaka
R and Itoh H: Comparison of treatment safety between brand-name
product and biosimilar of trastuzumab. Gan To Kagaku Ryoho.
48:945–949. 2021.PubMed/NCBI(In Japanese).
|
|
10
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
von Minckwitz G, Procter M, de Azambuja E,
Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N,
Clark E, et al: Adjuvant pertuzumab and trastuzumab in early
HER2-positive breast cancer. N Engl J Med. 377:122–131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Swain SM, Tan AR, Gianni L, Kuemmel S,
Dang CT, Schneeweiss A, O'Shaughnessy J, Liu H, Aguila C, Heeson S,
et al: Incidence and severity of anaphylaxis and hypersensitivity
in trials of intravenous pertuzumab plus trastuzumab or the
fixed-dose combination of pertuzumab and trastuzumab for
subcutaneous injection for HER2-positive breast cancer. Eur J
Cancer. 178:70–81. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stebbing J, Baranau Y, Baryash V, Manikhas
A, Moiseyenko V, Dzagnidze G, Zhavrid E, Boliukh D, Stroyakovskii
D, Pikiel J, et al: CT-P6 compared with reference trastuzumab for
HER2-positive breast cancer: A randomised, double-blind,
active-controlled, phase 3 equivalence trial. Lancet Oncol.
18:917–928. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tokunaga M, Watanabe S and Nakane N:
Survey on outpatient's waiting time and satisfaction level. J Jpn
Soc Health Care Manag. 7:324–328. 2006.
|
|
15
|
Toga-Sato S, Tosaki T, Kondo M, Tsunekawa
S, Kato Y, Nakamura J and Kamiya H: Impact of actual waiting time
and perceived waiting time on treatment satisfaction in patients
receiving outpatient diabetes care. Diabetol Int. 12:293–300.
2021.PubMed/NCBI View Article : Google Scholar
|