Introduction
Cervical cancer is the second most common cancer
globally, with more than 275,000 deaths and 530,200 new cases in
2010(1). Approximately 13,000 new
cases of cervical cancer were diagnosed in Japan, and an estimated
3,500 women died from this disease (2). Methods for preventing cervical
cancer, such as administration of the human papillomavirus (HPV)
vaccine and cervical cancer screening with cytology, have
developed, in contrast to those for preventing other cancers.
However, these measures are not effective in Japan. In June 2013,
the HPV vaccine was withdrawn in Japan because of several reports
of adverse effects, such as the complex regional pain syndrome,
despite the launch of a program encouraging vaccination. Many
junior high school students and their parents were concerned about
this syndrome, resulting in only a few new vaccinations (0.97%)
compared with those prior to these news reports (3,4).
Monitoring of cervical cancer was also discontinued, leading to a
cancer screening rate of only 28.3% in 2016(5). Thus, the prevention of cervical
cancer in Japan has become difficult, and therefore, the number of
patients with early cancer will continue to increase.
Amid the increase in cases with early cervical
cancer, the demand for minimally invasive surgery (MIS) such as
laparoscopic radical hysterectomy (LRH) and robot-assisted radical
hysterectomy (RARH) is expected to increase. In Japan, LRH and RARH
were recognized as advanced medical treatments in May and August
2016, respectively. However, MIS suddenly developed an unfavorable
impression because of the sensational report of the Laparoscopic
Approach to Cervical Cancer (LACC) trial. This was the first trial
of early-stage cervical cancer (ECC) comparing MIS and open radical
hysterectomy (ORH) (6). According
to the results of this trial, the negative impression of MIS could
not be prevented. However, currently, the unsuitability of MIS for
radical hysterectomy has little scientific basis. Therefore, this
study aimed to compare the surgical outcomes and prognosis of MIS
(including RARH and LRH) with ORH performed at a single center,
determine the factors related to disease recurrence, such as tumor
size and International Federation of Gynecology and Obstetrics
(FIGO) 2008 stage, and investigate the specific recurrence pattern
of lesions after MIS and the ideal indications of MIS for ECC.
Materials and methods
Patient and tumor characteristics
A total of 78 patients underwent surgery for
cervical cancer between December 2008 and January 2019 at the
Obstetrics and Gynecology Hospital of Shimane University, Shimane,
Japan. Of these, 14 patients underwent LRH, 21 underwent RARH, and
43 underwent ORH (ORH: December 2008 to January 2019, LRH: April
2015 to January 2019, and RARH: July 2015 to January 2019). Radical
hysterectomy was performed in patients with FIGO 2008 stage
IA1-IIB. Nerve-sparing techniques were attempted in IA2, IB1, and
IIA1 cases. The indications for the three methods of hysterectomy
were determined based on the criteria of accommodation for cervical
cancer in Japan. LRH was performed for stage IA2-IIA, RARH for
stage IA2-IIB, and ORH for stage IA2-IIB. During the period of
performance of this study, there were no criteria to clarify the
effect of cervical tumor size, as determined by endoscopic surgery,
on the choice of method of hysterectomy. Therefore, in this study,
LRH or RARH was selected regardless of tumor diameter, except for
patients older than 75 years and with severe comorbidities.
We analyzed the following parameters in our study:
perioperative variables such as age, FIGO 2008 stage, histology,
tumor size, lymphovascular space invasion (LVSI), and lymph node
metastasis. The surgical outcomes included hospitalization
duration, operative duration, estimated blood loss, number of
dissected lymph nodes, transfusion rate, conversion to laparotomy,
and intraoperative and postoperative complications. The region of
recurrence and the period until recurrence were also assessed.
Operative duration was defined as the duration between incision and
wound closure. Operative duration was defined as console duration
only in RARH. Estimated blood loss was the sum of volume of
suctioned fluids, weighted gauze minus dry gauze, and other fluids
at the end of surgery.
Surgical procedure. Equipment system
and electric device
We used the 1588 AIM camera system (Stryker) for LRH
and the da Vinci S or Xi Surgical System (Intuitive Surgical Inc.)
for RARH. General anesthesia was induced via endotracheal
intubation with the patient in a lithotomy-Trendelenburg position.
The details of the surgical technique have been described in
previous studies. The uterine artery was cut, and the vessels over
the ureter were dissected. The bilateral anterior and posterior
vesicouterine ligaments were divided and incised. Briefly, for LRH,
a 12 mm trocar was inserted through the umbilicus. Two 5 mm lateral
trocars were inserted symmetrically 6 cm from the umbilicus below
the horizontal line of the umbilicus. Two other 12 mm trocars were
inserted bilaterally at the outer third of the iliac spine. For
RARH, 5 trocars were used. A 12 mm trocar was placed 3 cm from the
umbilicus for the camera. Two 8 mm trocars were placed bilaterally
8 cm from the umbilicus for the three robotic arms. In addition, a
12 mm trocar was placed in the right upper quadrant for
assistance.
For LRH, a Harmonic Ace+® device
(Ethicon, Cincinnati, Ohio) was used, whereas for RARH, a pair of
monopolar scissors (Intuitive Surgical Inc.) and fenestrated
bipolar forceps (Intuitive Surgical Inc.) were used. Sometimes,
LigaSure™ (Coviden) was used. Pelvic lymphadenectomy was
performed and the ureter was separated from the lateral
peritoneum.
Method of surgery (lymphadenectomy,
nerve-sparing, or vaginal wall incision). The surgical steps
were performed as follows: i) pelvic lymphadenectomy, ii)
dissection of the uterine artery and deep uterine veins, iii)
isolation of the ureter, iv) resection of the round ligament,
cardinal ligament, and uterosacral ligament of the uterus, v) nerve
sparing technique: identification of the nerve fibers (bladder
branches) and their detachment from the vaginal wall, vi) radical
separation of the nerve fibers attached to the pelvic side wall,
with preservation of all parasympathetic fibers, vii) separation of
the parametrium and paracolpium from the uterus and vagina, viii)
resection of the anterior and posterior parts of the vesico-vaginal
ligament, ix) Incision of the vaginal wall using the
transperitoneal approach, and x) repair of the vaginal wound with
interrupted sutures.
A nerve-sparing approach was performed for cases
with stage IA2-IIA lesions. In all cases, the parametrial tissue
was incised 1 to 2 cm from the margin or to one fourth to one third
of the vagina for type C radical hysterectomy.
In cases in which LRH and RARH were performed, we
used the uterine manipulator. A vaginal cuff was not made in all
cases. We removed isolated lymph nodes thorough the abdominal
trocar in a bag or using a trocar sleeve.
Survival duration
Survival duration was calculated from the date of
initial surgery to the date of last follow-up for patients who were
alive or to the date of death for patients who died with evidence
of cervical cancer. Recurrence duration was calculated from the
date of first treatment to the date of last follow-up for patients
who were diagnosed with recurrent cervical cancer.
Statistical analysis
Statistical calculations were performed using
Statistical Package for Social Sciences 23.0 (SPSS Inc.). One-way
analysis of variance followed by Tukey's post hoc test, and
Kruskal-Wallis test followed by Dunn's post hoc test were used for
parametric and non-parametric variables, respectively. Differences
between proportions were compared using Fisher's exact test or
χ2 test. Differences between ORH and MIS were evaluated
using Fisher's exact test. Among patients who underwent radical
hysterectomy and were followed up for more than 3 years,
progression-free survival (PFS) and overall survival (OS) were
calculated using the Kaplan-Meier method with the log-rank test. A
multivariate logistic regression model was used to evaluate the
association between outcome and exposure. The hazard ratio was
calculated for binary data with a confidence interval of 95%.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient and tumor characteristics
Patient demographics are shown in Table I. We determined that 37.2, 71.4,
and 52.4% of patients who underwent ORH, LRH, and RARH,
respectively, had FIGO 2008 stage IB1 disease. Additionally, 32.6%
of patients in the ORH and 20.0% of patients in RARH group had FIGO
2008 stage IIB disease. Regarding the histological analysis of
cancerous tissue, adenocarcinoma was evident in 19.6, 28.6, and
50.0% of patients in the ORH, LRH, and RARH groups, respectively.
The mean tumor size was 44.4 mm in the ORH, 22.5 mm in the LRH, and
29.7 mm in the RARH groups. There were statistically significant
differences in these factors between ORH and LRH, and ORH and RARH
groups. In the ORH and RARH groups, deep vascular infiltration of
the cervical stroma was evident in approximately 60.0-70.0% of
patients, compared with 28.6% of patients in the LRH group.
Metastasis of pelvic lymph nodes was found in 34.9 and 23.8% of
patients in the ORH and RARH groups, respectively. In the LRH
group, no patient had metastasis to the pelvic lymph nodes. There
was a statistically significant difference in age between the ORH
and LRH (P=0.026) and in tumor size between the ORH and MIS
(LRH+RARH) groups. Other factors were not significantly different
between the groups.
 | Table IPatients' characteristics. |
Table I
Patients' characteristics.
| Variable | RH (n=43) | LRH (n=14) | RARH (n=21) | P(ORH/ LRH) | P(ORH/ RARH) | P(LRH/ RARH) |
|---|
| Age, years | 56.8 | 47.4 | 51 | 0.026 | 0.098 | 0.373 |
| BMI,
kg/m2 | 22.4 | 21 | 21.6 | 0.123 | 0.248 | 0.594 |
| FIGO stage, n
(%) | | | | | | |
|
IA1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | | | |
|
IA2 | 1 (2.3) | 1 (7.1) | 0 (0.0) | | | |
|
IB1 | 16 (37.2) | 10 (71.4) | 11 (52.4) | | | |
|
IB2 | 7 (16.3) | 1 (7.1) | 4 (19.0) | | | |
|
IIA1 | 2 (4.7) | 1 (7.1) | 1 (4.8) | | | |
|
IIA2 | 3 (7.0) | 1 (7.1) | 0 (0.0) | | | |
|
IIB | 14 (32.6) | 0 (0.0) | 5 (23.8) | | | |
| Histology, n (%) | | | | | | |
|
Adenocarcinoma | 8 (18.6) | 4 (28.6) | 9 (50.0) | | | |
|
Adenosquamous
cell carcinoma | 3 (7.0) | 0 (0.0) | 1 (0.0) | | | |
|
Squamous
cell carcinoma | 32 (74.4) | 10 (71.4) | 11 (50.0) | | | |
| Tumor size, n
(%) | | | | | | |
|
<4
cm | 17 (39.5) | 13 (92.9) | 13 (61.9) | | | |
|
≥4 cm | 26 (60.5) | 1 (7.1) | 8 (38.1) | | | |
| Tumor size, cm | 44.4 | 22.5 | 29.7 | 0.001 | 0.008 | 0.174 |
| Infiltration depth of
cervical tumor, n (%) | | | | | | |
|
No
infiltration | 2 (4.7) | 4 (28.6) | 2 (9.5) | | | |
|
Superficial | 9 (20.9) | 5 (35.7) | 6 (28.6) | | | |
|
Deep
muscular | 31 (72.1) | 4 (28.6) | 13 (61.9) | | | |
|
N/A | 1 (2.3) | 1 (7.1) | 0 | | | |
| Metastasis, n
(%) | | | | | | |
|
Pelvic lymph
node | 15 (34.9) | 0 (0.0) | 5 (23.8) | | | |
|
Lymphovascular
space invasion n(%) | 31 () | 7 (50.0) | 13 () | | | |
|
N/A | 4 () | 1 (7.1) | 0 | | | |
Surgical outcomes
As shown in Table
II, preparation duration, operative duration, estimated blood
loss, number of dissected lymph nodes, hospitalization duration,
and amount of transfused blood were compared between groups. The
mean operative duration for RARH was significantly longer than that
for ORH and LRH [(PORH/RARH) <0.0001 and P(LRH/RARH)=0.014]. The
mean estimated blood loss was significantly less in the MIS groups
[P(ORH/LRH) <0.0001 and P(ORH/RARH) <0.0001]. The mean
hospitalization duration was also significantly less in the LRH
group [P(ORH/LRH) <0.0001]. No statistically significant
differences were found in the number of dissected lymph nodes
between the groups. Intraoperative complications were observed in
all groups. In the ORH group, 2 cases of bladder injury occurred.
In the LRH group, one case of bladder injury and one case of vessel
injury occurred. These injuries were repaired immediately during
surgery. In the RARH group, one case of ureteral injury and one
case of rectal injury occurred. Of the 35 cases, no case required
conversion from laparoscopy to laparotomy. Further, no case in the
RARH group needed a switch to conventional laparoscopy. All
patients had an uncomplicated postoperative course.
 | Table IISurgical outcomes and operating
findings. |
Table II
Surgical outcomes and operating
findings.
| Variable | ORH (n=43) | LRH (n=14) | RARH (n=21) | P(ORH/ LRH) | P(ORH/ RARH) | P(LRH/ RARH) |
|---|
| Operative time,
min | 330.2±75.3 | 353.5±52.5 | 435.4±90.6 | 0.113 | <0.0001 | 0.014 |
| Estimated blood
loss, ml | 1,212.2±820.8 | 250.7±272.0 | 241.2±237.6 | <0.0001 | <0.0001 | 0.913 |
| Hospitalization,
days | 25.3±22.8 | 10.8±3.0 | 16.64±17.2 | <0.0001 | 0.128 | 0.225 |
| Blood transfusion,
n (%) | 13 (28.3) | 1 (7.1) | 2 (10.0) | | | |
| Lymph nodes
counted, n | 33.5±12.5 | 33.4±9.5 | 42.5±16.3 | 0.517 | 0.017 | 0.184 |
| Conversion to
laparotomy, n (%) | | 0 (0.0) | 0 (0.0) | | | |
| Follow-up visit
result, n(%) | | | | | | |
|
No
relapse | 36 (83.7) | 12 (85.7) | 14 (66.7) | | | |
|
Relapse | 7 (16.3) | 2 (14.3) | 7 (33.3) | | | |
|
Death | 5 (11.6) | 0 (0.0) | 2 (9.5) | | | |
| Intra- or
post-operation complications, n (%) | 2 (4.7) | 2 (14.3) | 2 (9.5) | | | |
Pattern of recurrence
The detailed pattern of recurrence for each
procedure is shown in Table III
and Fig. 1. Patients in the ORH
group had several patterns of disease recurrence. Patients in the
LRH group treated without adjuvant therapy had local recurrence. In
the RARH group, intraperitoneal dissemination was found in two
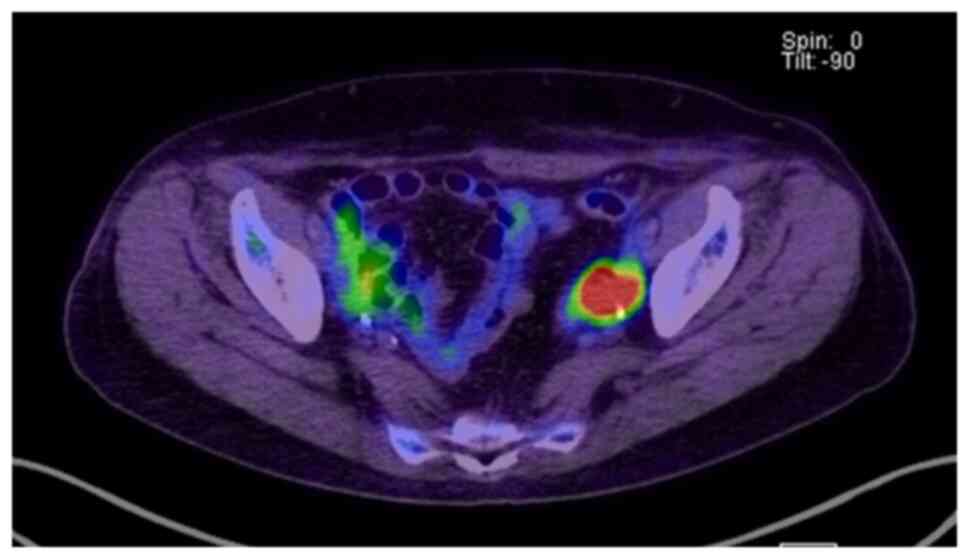
cases, which could be a specific pattern in RARH (Fig. 1). These two patients had large
tumors. In case 11, disseminated lesions in the peritoneal cavity
and port site were observed. In case 12, a disseminated lesion on
the surface of the left iliopsoas muscle was observed (Fig. 1). However, it should be noted that
no patients with stage IB1 disease who underwent RARH exhibited
recurrence (Table IV).
 | Table IIIPattern of recurrence in each
surgical procedure. |
Table III
Pattern of recurrence in each
surgical procedure.
| Case | Surgery | Age, years | FIGO stage | pT | Tumor size, mm | Histology | LN metastasis | LVSI | Infiltration on
depth of cervical tumor, % | Adjuvant
therapy | Recurrence
region | Recurrence
interval, months |
|---|
| 1 | ORH | 75 | ⅡA1 | pT2a1 | 9 | Adeno | (+) | (+) | 70 | RT | Vaginal stump,
pelvic lymph node | 9 |
| 2 | ORH | 34 | ⅠB1 | pT1b1 | 25 | Adeno | (+) | (+) | 80 | CCRT | Paraaortic LN,
liver, meninges | 3 |
| 3 | ORH | 81 | ⅠB1 | pT1b2 | 45 | SCC | (-) | (+) | 40 | RT | Stump, pelvic lymph
node | 12 |
| 4 | ORH | 74 | ⅡB | pT2b | 85 | Adeno-
squamous | (+) | (+) | 100 | CCRT | Pelvic lymph
node | 42 |
| 5 | ORH | 69 | ⅡB | pT2b | 65 | SCC | (+) | (+) | 100 | CCRT | Stump, pelvic lymph
node | 8 |
| 6 | ORH | 52 | ⅡB | pT2b | 63 | SCC | (-) | (+) | 100 | CCRT | Pelvic lymph
node | 3 |
| 7 | ORH | 77 | ⅡA2 | pT2a2 | 50 | Adeno | (+) | (+) | 69 | (-) rejected | Pelvic lymph
node | 2 |
| 8 | LRH | 64 | ⅠB1 | pT1b1 | 30 | SCC | (-) | (+) | 40 | (-) | Stump, pelvic lymph
node | 10 |
| 9 | LRH | 66 | ⅡA1 | pTis | (-) | SCC | (-) | (-) | 0 | (-) | Stump | 17 |
| 10 | RARH | 68 | ⅡA1 | pT1a1 | 2.6 | SCC | (-) | (-) | (-) | (-) | Stump, pelvic lymph
node | 7 |
| 11 | RARH | 40 | ⅠB2 | pT1b2 | 55 | Adeno | (-) | (+) | 90 | CCRT | Intraperitoneal
dissemination | 24 |
| 12 | RARH | 44 | ⅠB2 | pT1b2 | 72 | Adeno-
squamous | (-) | (+) | 90 | CCRT | Surface of left
iliopsoas muscle | 4 |
| 13 | RARH | 60 | ⅡB | pT2b | 42 | Adeno | (+) | (+) | 100 | CCRT | Lung, pelvic lymph
node | 5 |
| 14 | RARH | 59 | ⅡB | pT2b | 45 | SCC | (+) | (+) | 80 | CCRT | Mediastinal lymph
node | 30 |
| 15 | RARH | 61 | ⅡB | pT2b | 45 | SCC | (+) | (+) | 100 | CT | Lung, pelvic lymph
node | 5 |
| 16 | RARH | 61 | ⅡB | pT2b | 25 | SCC | (+) | (+) | 80 | CT | Pelvic lymph
node | 4 |
 | Table IVPatient characteristics in RARH
group. |
Table IV
Patient characteristics in RARH
group.
| Age, years | FIGO stage | pT | Tumor size, mm | Histology | LN metastasis | LVSI | Infiltration depth
of cervical tumor, % | Adjuvant
therapy | Recur- rence | Site of
recurrence |
|---|
| 35 | ⅠB1 | ⅠB1 | 37 | Adeno | (-) | (+) | 80 | CCRT | (-) | (-) |
| 43 | ⅠB1 | ⅠB1 | 23 | Adeno | (-) | (+) | 45 | CCRT | (-) | (-) |
| 56 | ⅠB1 | ⅠB1 | 12 | Adeno | (-) | (-) | 20 | CCRT | (-) | (-) |
| 49 | ⅠB1 | ⅠB1 | 4 | Adeno | (-) | (-) | 10 | (-) | (-) | (-) |
| 57 | ⅠB1 | ⅠB1 | 8 | SCC | (-) | (-) | 0 | (-) | (-) | (-) |
| 50 | ⅠB1 | ⅠB1 | 4 | Adeno | (-) | (-) | 0 | (-) | (-) | (-) |
| 53 | ⅠB1 | ⅠB1 | 20 | Adeno | (-) | (+) | 55 | CCRT | (-) | (-) |
| 43 | ⅠB1 | ⅠB1 | 35 | Adeno | (-) | (+) | 25 | CCRT | (-) | (-) |
| 39 | ⅠB1 | ⅠB1 | 10 | Adeno | (-) | (-) | 10 | CCRT | (-) | (-) |
| 54 | ⅠB1 | ⅠB1 | 15 | SCC | (-) | (+) | 55 | CCRT | (-) | (-) |
| 37 | ⅠB1 | ⅠB1 | 18 | SCC | (-) | (-) | 100 | CCRT | (-) | (-) |
| 40 | ⅠB2 | ⅠB2 | 55 | Adeno | (-) | (+) | 90 | CCRT | (+) | Surface of left
iliopsoas muscle |
| 44 | ⅠB2 | ⅠB2 | 72 | Adeno | (-) | (+) | 90 | CCRT | (+) | Peritoneal
dissemination |
| 55 | ⅠB2 | ⅠB2 | 43 | Adeno | (-) | (-) | 25 | CCRT | (-) | (-) |
| 32 | ⅠB2 | ⅠB2 | 45 | SCC | (-) | (+) | 95 | CCRT | (-) | (-) |
| 68 | ⅡA1 | ⅠA1 | 22 | SCC | (-) | (-) | 20 | (-) | (+) | Stump, pelvic lymph
node |
| 59 | ⅡB | ⅡB | 45 | SCC | (+) | (+) | 80 | CCRT | (+) | Mediastinal lymph
node |
| 60 | ⅡB | ⅡB | 42 | Adeno | (+) | (+) | 100 | CCRT | (+) | Lung, pelvic lymph
node |
| 61 | ⅡB | ⅡB | 45 | SCC | (+) | (+) | 100 | TC | (+) | Lung, pelvic lymph
node |
| 61 | ⅡB | ⅡB | 25 | SCC | (+) | (+) | 80 | TC | (+) | Pelvic lymph
node |
| 74 | ⅡB | ⅡB | 43 | SCC | (+) | (+) | 81 | RT (rejected
CCRT) | (-) | (-) |
Oncologic outcomes
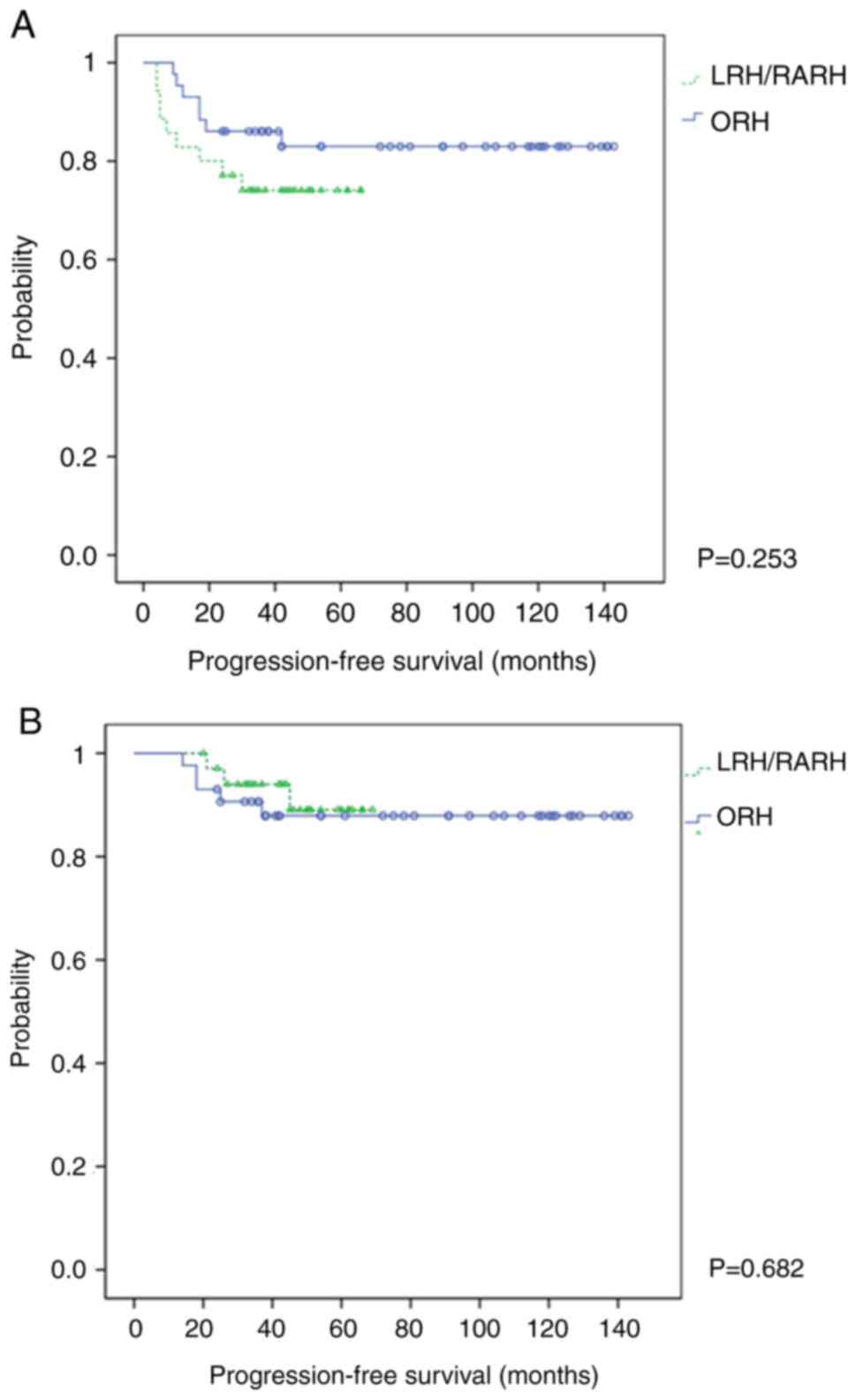
PFS and OS were not significantly different between
the surgical procedures (Figs. 2
and 3). The 3-year PFS was 74.3%
in the MIS group and 83.7% in the ORH group (log-rank P=0.253)
(Fig. 2A). The 3-year OS was 91.4%
in the MIS group and 88.4% in the ORH group (log-rank P=0.682)
(Fig. 2B). More specifically, the
3-year PFS was 85.7, 66.7, and 83.7% in the LRH, RARH, and ORH
groups, respectively [log-rank P(ORH/LRH)=0.861, P(ORH/RARH)=
0.077, and P(LRH/RARH)=0.192] (Fig.
3A). The 3-year OS was 100.0, 85.7, and 88.4% in the LRH, RARH,
and ORH groups, respectively [log-rank P(ORH/LRH)=0.189,
P(ORH/RARH)= 0.671, and P(LRH/RARH)=0.081] (Fig. 3B). There was no statistically
significant difference in PFS or OS between surgical procedures;
however, the survival tended to be shorter with MIS. Furthermore,
in patients with stage IB1 disease, the prognosis of MIS was not
inferior to that of ORH (Fig. S1A
and B).
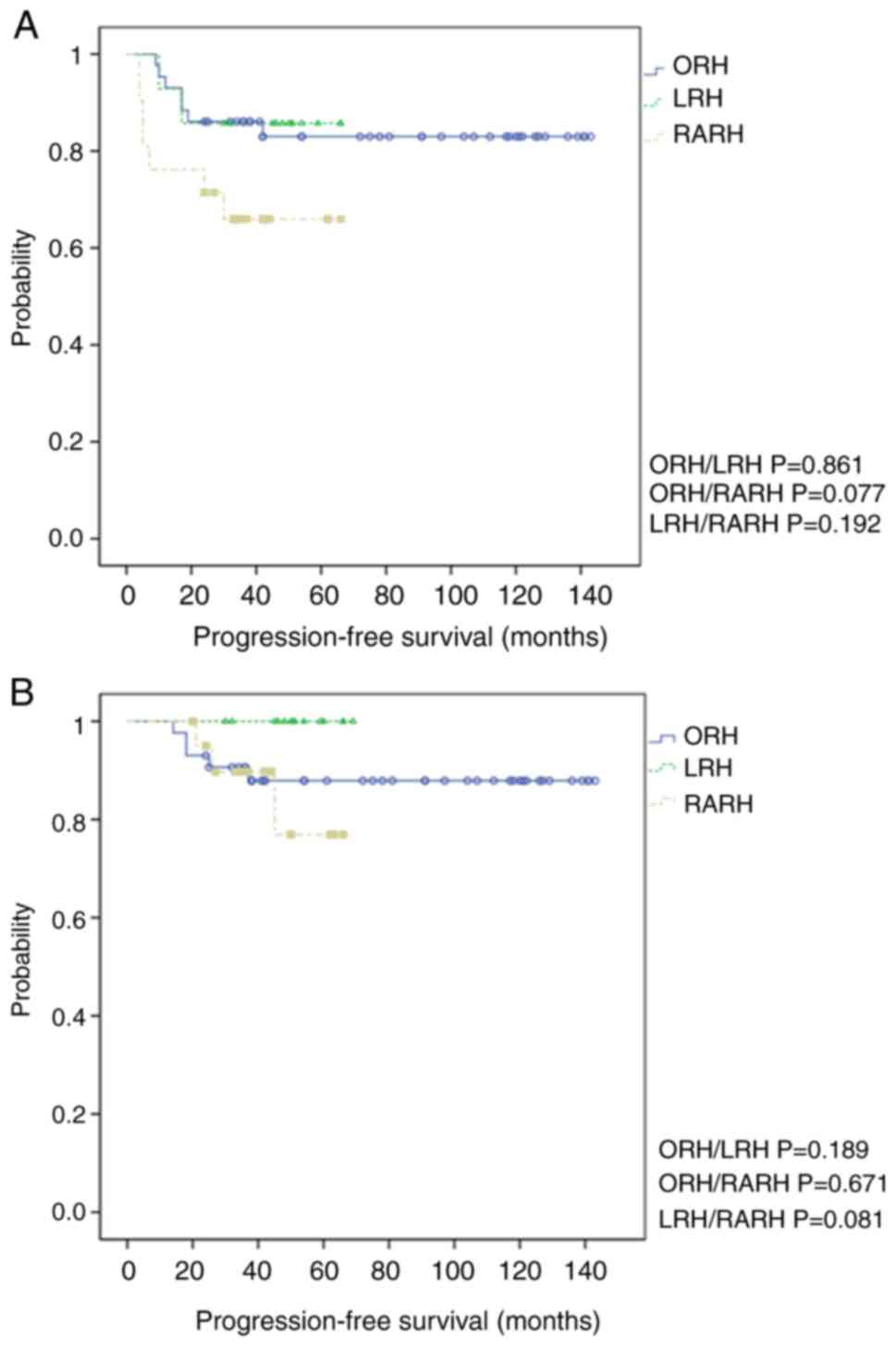 | Figure 3Kaplan-Meier survival analysis
demonstrating the difference in 3-year recurrence between ORH, LRH,
and RARH cohorts. (A) The 3-year PFS of 85.7, 66.7, and 83.7% in
patients treated using LRH, RARH, and ORH [log-rank
P(ORH/LRH)=0.861, P(ORH/RARH)=0.077, and P(LRH/RARH)=0.192,
respectively]. (B) The 3-year OS of 100.0, 85.7, and 88.4% in
patients treated using LRH, RARH, and ORH [log-rank
P(ORH/LRH)=0.189, P(ORH/RARH)=0.671, and P(LRH/RARH)=0.081,
respectively]. PFS, progression-free survival; OS, overall
survival; RARH, robot-assisted radical hysterectomy; LRH,
laparoscopic radical hysterectomy; ORH, open radical hysterectomy;
MIS, minimally invasive surgery. |
Cox univariate and multivariate regression analyses
were performed for PFS and OS (Table
VA and B) including known risk
factors for survival such as histology, FIGO 2008 stage, lymph node
metastasis, LVSI, and depth of invasion and surgical factors such
as operative duration and estimated blood loss. Univariate analysis
revealed that elderly patients, those with lymph node metastasis,
and those with higher than stage IB2 disease were more prone to
disease recurrence. Long operative duration was also related to
disease recurrence. Multivariate analysis revealed that age, FIGO
2008 stage, depth of invasion, and operative duration were risk
factors for disease recurrence. Furthermore, MIS was not proven to
be a factor for shortened PFS. The relationship between OS and
recurrence factors was similar to that between PFS and recurrence
factors (Table VB).
 | Table VUnivariate and multivariate analysis
of survival using a Cox proportional hazards model in patients with
cervical carcinoma. |
Table V
Univariate and multivariate analysis
of survival using a Cox proportional hazards model in patients with
cervical carcinoma.
| A, Progression-free
survival |
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Patients
(n=78) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | | | | | |
|
<60 | 49 | 0.157 | 0.050-0.487 | 0.001 | 0.105 | 0.023-0.477 | 0.004 |
|
≥60 | 29 | ref. | | ref. | | | |
| FIGO stage | | | | | | | |
|
≤ⅠB1 | 39 | 0.117 | 0.027-0.518 | 0.005 | 0.02 | 0.001-0.346 | 0.007 |
|
≥ⅠB2 | 39 | ref. | | ref. | | | |
| Pelvic lymph node
metastasis | | | | | | | |
|
Negative | 58 | 0.284 | 0.106-0.758 | 0.012 | 0.182 | 0.030-1.119 | 0.066 |
|
Positive | 20 | ref. | | ref. | | | |
| Pathological
subtype | | | | | | | |
|
SCC | 53 | 0.863 | 0.314-2.374 | 0.775 | 0.494 | 0.148-1.648 | 0.251 |
|
Non-SCC | 25 | ref. | | ref. | | | |
| LVSI
metastasis | | | | | | | |
|
No | 22 | 0.324 | 0.073-1.436 | 0.138 | 0.105 | 0.007-1.706 | 0.113 |
|
Yes | 51 | ref. | | ref. | | | |
| Infiltration depth
of cervical tumor, % | | | | | | | |
|
<50 | 28 | 0.466 | 0.150-1.445 | ref. | | | |
|
≤50 | 48 | ref. | | 0.186 | 35.723 | 1.846-691.388 | 0.018 |
| Operation time,
min | | | | | | | |
|
<400 | 56 | 0.25 | 0.093-0.672 | 0.006 | 0.059 | 0.010-0.344 | 0.002 |
|
≥400 | 22 | ref. | | ref. | | | |
| Blood loss, ml | | | | | | | |
|
<500 | 37 | ref. | | ref. | | | |
|
≥500 | 41 | 2.182 | 0.792-6.010 | 0.131 | 3.598 | 0.496-26.094 | 0.205 |
| Operation | | | | | | | |
|
ORH | 43 | 0.568 | 0.211-1.525 | 0.262 | ref. | | |
|
LRH/RARH | 35 | ref. | | | 1.305 | 0.122-13.948 | 0.826 |
| B, Overall
survival |
| | Univariate
analysis | Multivariate
analysis |
| Factor | Patients
(n=78) | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age, years | | | | | | | |
|
<60 | 49 | 0.173 | 0.035-0.859 | 0.032 | 0.402 | 0.065-2.477 | 0.326 |
|
≥60 | 29 | ref. | | | ref. | | |
| FIGO stage | | | | | | | |
|
≤ⅠB1 | 39 | 0.122 | 0.0015-0.998 | 0.05 | 0.063 | 0.002-2.128 | 0.123 |
|
≥ⅠB2 | 39 | ref. | | | ref. | | |
| Pelvic lymph node
metastasis | | | | | | | |
|
Negative | 58 | 0.175 | 0.042-0.736 | 0.017 | 0.166 | 0.018-1.489 | 0.109 |
|
Positive | 20 | ref. | | | ref. | | |
| Pathological
subtype | | | | | | | |
|
SCC | 53 | 0.336 | 0.126-2.301 | 0.505 | 0.416 | 0.062-2.807 | 0.368 |
|
Non-SCC | 25 | ref. | | | ref. | | |
| LVSI
metastasis | | | | | | | |
|
No | 22 | 0.027 | 0.000-20.578 | 0.285 | NA | NA | NA |
|
Yes | 51 | ref. | | | | | |
| Infiltration depth
of cervical tumor, % | | | | | | | |
|
<50 | 28 | 0.198 | 0.024-1.615 | 0.131 | ref. | | |
|
≥50 | 48 | ref. | | | 15.008 | 0.283-795.880 | 0.181 |
| Operation time,
min | | | | | | | |
|
<400 | 56 | 0.392 | 0.098-1.568 | 0.186 | 0.049 | 0.003-0.856 | 0.039 |
|
≥400 | 22 | ref. | | | ref. | | |
| Blood loss, ml | | | | | | | |
|
<500 | 37 | ref. | | | ref. | | |
|
≥500 | 41 | 2.07 | 0.494-8.681 | 0.32 | 9.531 | 0.663-137.083 | 0.097 |
| Operation | | | | | | | |
|
ORH | 43 | ref. | | | ref. | | |
|
LRH/RARH | 35 | 1.384 | 0.331-5.796 | 0.656 | 31.943 | 0.465-2193.066 | 0.108 |
Discussion
We analyzed the following parameters in our study:
perioperative variables such as age, FIGO stage 2008, histology,
tumor size, depth of invasion, and metastasis. Intraoperative
factors were operative duration, estimated blood loss, blood
transfusion, number of dissected lymph nodes, and conversion to
laparotomy. Postoperative variables included complications,
hospitalization duration, and follow-up results. The results of
this study suggest that RARH and LRH are superior to ORH with
regard to surgical outcomes and may be safe and feasible
alternatives to ORH. The operative duration was acceptable.
However, disease recurrence was found to be associated with FIGO
2008 stage-IB2 in the RARH group (Table IV). Further, a long operative
duration tended to lead to disease recurrence (Table VA).
We observed two major findings in this study. First,
this single-center retrospective study indicated that the risk of
disease recurrence was highly associated with the diameter of the
tumor and FIGO 2008 stage. Locally advanced cases, such as those
with stage IB2 and stage IIB disease, were prone for recurrence in
every group. However, no recurrence was observed in patients with
stage IB1 cervical cancer in the RARH group. These facts indicate
that one reason of recurrence is cancer cell spillage, which would
influence the decision to perform radical hysterectomy. RARH is an
appropriate surgical approach for patients with cervical cancers
having unfavorable outcomes because of large tumor size and/or
parametrium invasion. Furthermore, patients with large tumors in
the RARH group had specific sites of intraperitoneal recurrence
(Fig. 1). We should reassess the
ideal indications for each surgery and discuss the significance of
the relationship between the difficulty of surgery and disease
recurrence.
According to the results of previous studies, MIS is
more favorable for the treatment of cervical cancer than ORH.
Because of the recent progress in gynecologic laparoscopic
technology, LRH has become a preferred surgical method worldwide
(7). In addition, since the Food
and Drug Administration approved the Da Vinci surgical system for
gynecologic procedures in 2007, RARH has gained popularity for the
treatment of invasive cervical cancer (8). RARH has been accepted for the
treatment of ECC (9,10). RARH has also been associated with
better surgical outcomes (11).
However, the results of the LACC trial (6) affected the application of RARH in
Japan. Institutions as well as the front office of the Japanese
Society of Obstetrics and Gynecology redefined the indications for
MIS and procedures for prevention of local disease recurrence, such
as creating a vaginal cuff and complete removal of tumors and lymph
nodes. After the report of the LACC trial, the number of studies
questioning the use of MIS increased (12-14).
The results of the LACC trial might have been reliable, but every
report published following the LACC trial cannot be considered
reliable. Several of these reports were published to demonstrate
the disadvantages of MIS; however, many questions remain
unanswered. Recently, several reports have shown that MIS is an
acceptable surgical procedure for small tumors (15-18).
Wiebrend and Tjalma (19) also
questioned the tendency to avoid MIS in every ECC case. They argued
that medical professionals can justify this approach and that
surgery should be tailored and performed according to individual
patient needs and demands. In the current study, we observed that
disease recurrence can be prevented in small tumors such as stage
IB1 tumors even with the use of a uterine manipulator and without a
vaginal cuff for all cases. This shows that tumor diameter is a
more significant factor affecting disease recurrence after MIS than
the surgical procedure itself. Of course, cancer cell spillage
should be minimized, but not using a uterine manipulator or making
a vaginal cuff for small tumors does not ensure prevention of
cancer cell spillage. This is because a uterine manipulator ensures
a safe operation.
We should reassess the ideal indications for each
surgery. It is more important to decide whether to choose MIS or
open laparotomy for large tumors.
The strength of our study is the fact that it is a
retrospective study performed at a single institution, which
unified several treatment decisions, such as selecting the
treatment modality, surgical procedure, and post-treatment
follow-up. Because of the uniformity in treatment methods, patient
prognosis is expected to be more accurate.
However, there are some limitations to our study.
First, as mentioned earlier, this study was conducted in a single
center; therefore, the number of cases was not sufficient for
several statistical analyses. A large number of cases are needed
for an accurate analysis. Second, it was difficult to standardize
the use of adjuvant therapy. Some cases could not be treated with
adjuvant therapy because of the patients' wishes. Two cases that
exhibited local recurrence in the LRH group required radiotherapy
after surgery because of vaginal invasion or LVSI. If they were
treated properly, local recurrence would be prevented. Third, this
study applied an approach based on country-specific
recommendations; therefore, the findings cannot be extrapolated to
women with cervical cancer worldwide. Until now, patients with ECC
were undergoing radical hysterectomy and having favorable outcomes
in Japan, though these patients would have been treated with
concurrent chemoradiotherapy in other countries. The surgical
approach for large tumors may not be acceptable in other countries.
Forth, in the present study, the choice of surgical method of
hysterectomy was not dependent on tumor diameter. However, larger
tumors were mostly included in the ORH group, which may have
affected the prognostic evaluation. A stratification method could
have been considered to avoid such a bias, but the small number of
cases did not allow for prognostic analysis in the group with
larger tumors; we will address this in a future study. Instead,
prognostic analysis was performed using the Kaplan-Meier method for
the group with smaller tumors, such as stage IB1 tumors, and showed
no significant difference in prognosis depending on the type of
hysterectomy performed (Fig. S1A
and B).
Finally, all patients treated with MIS would benefit
from no recurrence and minimal invasiveness. Laparotomy should be
avoided for patients unless the clinician suspects a possibility of
recurrence. At the same time, procedures used worldwide to prevent
cancer cell spillage should be shared and learnt. Through this
research and similar studies that have been reported in recent
years, it can be concluded that preventing cancer cell spillage and
operating small tumors using MIS is probably safe and will be
associated with a better prognosis. Therefore, we must acquire more
knowledge about surgical assessment for MIS by conducting more
studies, including a large clinical study with new candidates for
surgery and new methods of prevention for disease recurrence. These
methods can be useful for obtaining better outcomes for these
patients.
This study demonstrated that MIS provided good
short-term surgical outcomes. Kaplan-Meier analyses showed no
significant difference in prognosis between MIS and open surgery;
however, there were some specific recurrence patterns in patients
after MIS. We suggest that MIS is a safe and useful procedure if
proper case selection is ensured.
Supplementary Material
Kaplan-Meier survival analysis
demonstrating the difference in 3-year recurrence between ORH, LRH,
and RARH cohorts with FIGO 2008 stage IB1 disease. (A) The 3-year
PFS of 90.9, 100.0, and 94.1% in the LRH, RARH, and ORH cohorts
[log-rank P(ORH/LRH)=0.730, P(ORH/RARH)=0.421, and
P(LRH/RARH)=0.317, respectively]. (B) The 3-year OS of 100.0,
100.0, and 94.1% in the LRH, RARH, and ORH cohorts [log-rank
P(ORH/LRH)=0.421, P(ORH/RARH)=0.421, and P(LRH/RARH)=NA,
respectively]. PFS, progression-free survival; OS, overall
survival; ORH, open radical hysterectomy; LRH, laparoscopic radical
hysterectomy; RARH, robot-assisted radical hysterectomy; FIGO,
International Federation of Gynecology and Obstetrics.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MI designed the study, diagnosed the patients,
collected and analyzed clinical data, and drafted the manuscript.
KN designed the study, collected and analyzed clinical data, gave
advice and drafted the manuscript, as well as performing the final
revision. TI collected and analyzed clinical data. HY collected and
analyzed clinical data. SS collected and analyzed clinical data. SR
drafted the manuscript and analyzed clinical data. SK designed the
study, advised on manuscript preparation and revised the final
manuscript. MI and SR confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study protocol was approved by the
Institutional Ethics and Research Review Board at Shimane
University (IRB No. 20191120-1; Izumo, Japan). The need for
individual patient consent for this retrospective analysis was
waived. Patients could opt out at any moment from the study using
the opt-out option on the hospital website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H: Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the monitoring of cancer
incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hanley SJ, Yoshioka E, Ito Y and Kishi R:
HPV vaccination crisis in Japan. Lancet. 385(2571)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yagi A, Ueda Y, Egawa-Takata T, Tanaka Y,
Nakae R, Morimoto A, Terai Y, Ohmichi M, Ichimura T, Sumi T, et al:
Realistic fear of cervical cancer risk in Japan depending on birth
year. Hum Vaccin Immunother. 13:1700–1704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cancer Registry and Statistics Cancer
Information Service, National Cancer Center, Japan. Available from:
https://ganjoho.jp/reg_stat/statistics/dl_screening/index.html#a16.
|
|
6
|
Ramirez PT, Frumovitz M, Pareja R, Lopez
A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, et al:
Minimally invasive versus abdominal radical hysterectomy for
cervical cancer. N Engl J Med. 379:1895–1904. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Malzoni M, Tinelli R, Cosentino F, Fusco A
and Malzoni C: Total laparoscopic radical hysterectomy versus
abdominal radical hysterectomy with lymphadenectomy in patients
with early cervical cancer: Our experience. Ann Surg Oncol.
16:1316–1323. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nezhat FR, Datta MS, Liu C, Chuang L and
Zakashansky K: Robotic radical hysterectomy versus total
laparoscopic radical hysterectomy with pelvic lymphadenectomy for
treatment of early cervical cancer. JSLS. 12:227–237.
2008.PubMed/NCBI
|
|
9
|
Hao X, Han S and Wang Y: Comparison of
conventional laparoscopy and robotic radical hysterectomy for
early-stage cervical cancer: A meta-analysis. J Cancer Res Ther. 11
(Suppl 1):C258–C264. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ko EM, Muto MG, Berkowitz RS and Feltmate
CM: Robotic versus open radical hysterectomy: A comparative study
at a single institution. Gynecol Oncol. 111:425–430.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Magrina JF, Kho RM, Weaver AL, Montero RP
and Magtibay PM: Robotic radical hysterectomy: Comparison with
laparoscopy and laparotomy. Gynecol Oncol. 109:86–91.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nitecki R, Ramirez PT, Frumovitz M, Krause
KJ, Tergas AI, Wright JD, Rauh-Hain JA and Melamed A: Survival
after minimally invasive vs open radical hysterectomy for
early-stage cervical cancer: A systematic review and meta-analysis.
JAMA Oncol. 6:1019–1027. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Y, Li B, Ren F, Song Z, Ouyang L and
Liu K: Survival after minimally invasive vs. open radical
hysterectomy for cervical cancer: A meta-analysis. Front Oncol.
10(1236)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dai D, Huang H, Feng Y, Wan T, Liu Z, Tong
C and Liu J: Minimally invasive surgery vs laparotomy for early
stage cervical cancer: A propensity score-matched cohort study.
Cancer Med. 9:9236–9245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alfonzo E, Wallin E, Ekdahl L, Staf C,
Rådestad AF, Reynisson P, Stålberg K, Falconer H, Persson J and
Dahm-Kähler P: No survival difference between robotic and open
radical hysterectomy for women with early-stage cervical cancer:
Results from a nationwide population-based cohort study. Eur J
Cancer. 116:169–177. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang M, Dai W, Yuexiu S, Shi Y, Li X,
Jiang K, Shen J and Ying L: Comparison of minimally invasive versus
abdominal radical hysterectomy for early-stage cervical cancer: An
updated meta-analysis. Front Oncol. 11(762921)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Doo DW, Kirkland CT, Griswold LH, McGwin
G, Huh WK, Leath CA III and Kim KH: Comparative outcomes between
robotic and abdominal radical hysterectomy for IB1 cervical cancer:
Results from a single high volume institution. Gynecol Oncol.
153:242–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiva L, Zanagnolo V, Querleu D,
Martin-Calvo N, Arévalo-Serrano J, Căpîlna ME, Fagotti A,
Kucukmetin A, Mom C, Chakalova G, et al: SUCCOR Study Group: SUCCOR
study: An international European cohort observational study
comparing minimally invasive surgery versus open abdominal radical
hysterectomy in patients with stage IB1 cervical cancer. Int J
Gynecol Cancer. 30:1269–1277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wiebren A and Tjalma A: The survival after
a radical hysterectomy for cervical cancer by open surgery is
significantly better then after minimal invasive surgery: Evidence
beats gut feeling! Eur J Obstet Gynecol Reprod Biol. 229:195–197.
2018.PubMed/NCBI View Article : Google Scholar
|