Introduction
Lung carcinoma has a poor prognosis, is metastatic
and progresses in an aggressive manner (1,2). The
majority of cases of lung carcinoma are non-small cell lung cancer
(NSCLC), which are caused by a wide range of molecular alterations.
In lung adenocarcinomas, an echinoderm microtubule-associated
protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion has
been detected in 3-7% of cases. Tyrosine kinase inhibitors (TKIs)
have a considerable effect on survival compared to other treatments
in adenocarcinomas positive for EML4-ALK (3). Lung adenocarcinomas can be treated by
targeting ALK. ALK, a transmembrane tyrosine kinase receptor from
the insulin receptor superfamily that is located on the second
chromosome, has been the subject of extensive studies. Following
the discovery of ALK rearrangement, increasingly potent first-,
second- and third-generation ALK TKIs are being developed for
treatment. Patients with metastatic lung adenocarcinomas that are
ALK-positive have a better prognosis and respond well to ALK
inhibitor therapies. ALK inhibitors have been used in
tumor-targeted medicines to achieve progression-free survival and
an improved quality of life (1,4,5). Since the introduction of the
first-generation ALK TKI, crizotinib, more selective
second-generation (brigatinib and alectinib) and third-generation
(lorlatinib) TKIs have been developed; however, resistance
mechanisms complicate treatment. However, there are still numerous
unresolved questions and additional research is warranted (1,4-6).
A second-generation ALK inhibitor known as alectinib
is used in the treatment of metastasized or relapsed NSCLC
(7,8). Thyroid metastasis is a very rare
occurrence, although its incidence increases with autopsy (9). According to autopsy results, breast
cancer, renal cell carcinoma and lung cancer may be the origins of
thyroid metastases. Thyroid metastases have a negative impact on
patient prognosis (10). Patients
with lung cancer metastases to the thyroid were previously treated
with chemotherapy using the drugs, cisplatin and etoposide, or with
chemotherapy and radiotherapy (8).
The present study describes a rare case of lung
cancer in which the metastasis in the thyroid exhibited a complete
response to targeted therapy, while the main lesion responded
poorly.
Case report
A 46-year-old male patient was admitted to the chest
diseases clinic of Çukurova University Balcalı Hospital with
dyspnea and a mass was detected in the left lower lung lobe during
imaging. The patient had mediastinum, thyroid and lung involvement
according to the results of whole-body positron emission
tomography/computed tomography (PET-CT) (as shown below). A thyroid
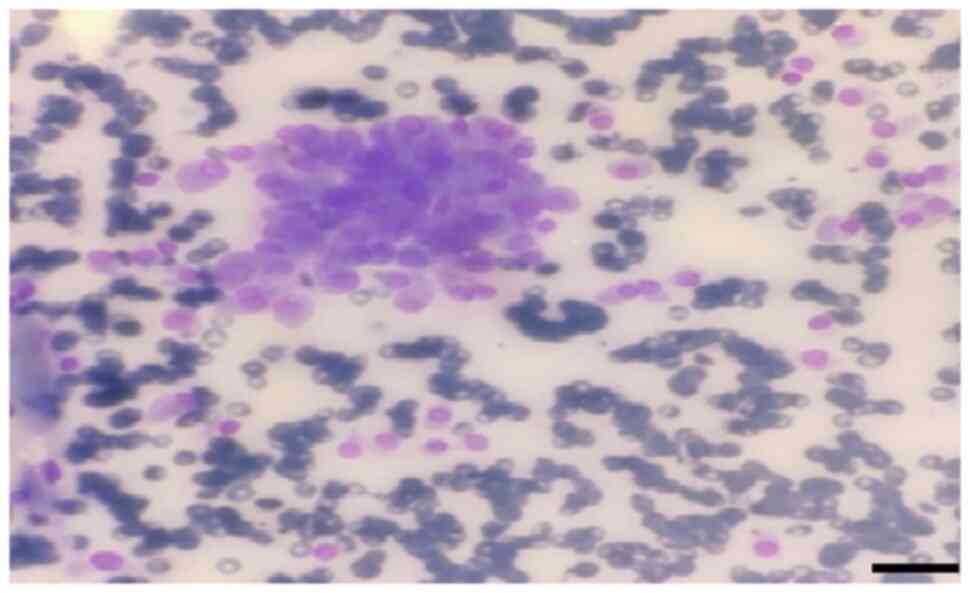
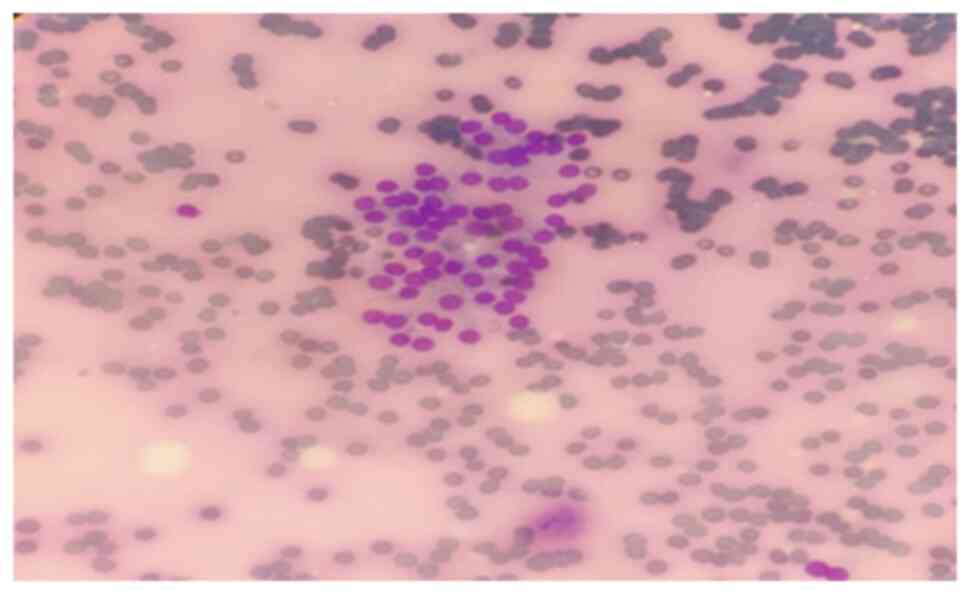
fine needle aspiration biopsy was performed for metastasis
(Fig. 1). Giemsa staining was
performed in the pathology laboratory. The May-Grünwald solution
used was from MilliporeSigma. Thin needle smears for Giemsa
staining were air-dried at 23-25˚C. Approximately 60 cc of
May-Grünwald solution were added to a Coplin jar. The air-dried
smears were immersed in the Coplin jar and left for 10 min. Another
Coplin jar was prepared with ~60 cc of 1% Giemsa solution (1 cc
Giemsa in 9 cc distilled water, for example). The smears were then
incubated in this solution for 10 min. Subsequently, they were
rinsed with tap water. Stained slides were dried using a heat
source emitting mild heat at moderate temperature. Prepared slides
were examined under an Olympus Bx51 light microscope (Olympus
Corporation). The scan results included a spiculated contoured
hypermetabolic soft tissue mass in the central part of the lower
lobe of the left lung consistent with atelectasis, pleural
effusion, intense focal increased metabolic activity in the left
thyroid lobe and hypermetabolic lesions in other regions.
Adenocarcinoma was diagnosed following a biopsy of the lung mass
(data not shown).
The biopsy from the left thyroid lobe was compatible
with adenocarcinoma metastasis. Video-assisted thoracoscopic
surgery, left thoracotomy, lymph node biopsies and pleural biopsy
were performed for the mass in the left lung, with the diagnosis of
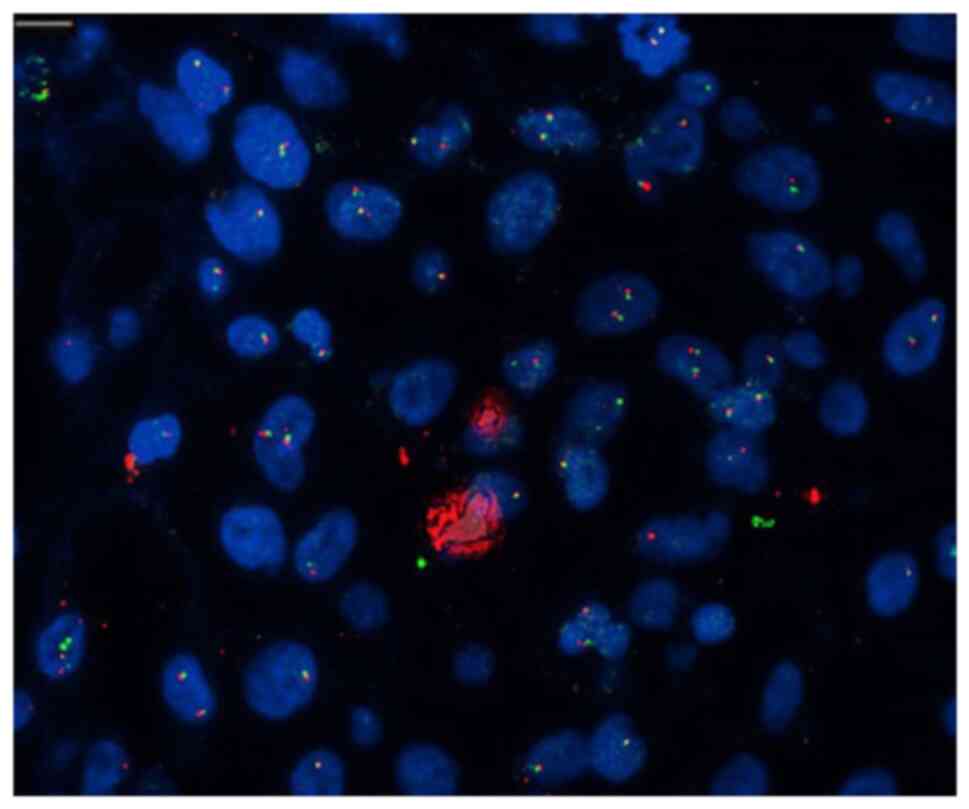
lung adenocarcinoma. EML4/ALK FISH analysis was performed.
Four-micron sections were obtained from paraffin blocks of the case
and mounted on positively charged slides. The sections were
incubated in an oven at 56˚C overnight. They were then soaked in
three separate containers, each containing xylene, for 10 min.
Subsequently, they were dehydrated by being immersed twice in
absolute ethanol for 5 min each time and air-dried. A water bath
was set to 80˚C, and a deparaffinization pre-wash solution was
placed in a heat-resistant covered container. For every five
slides, a separate slide box was prepared, and it contained 15 ml
of distilled water and 150 µl of 1 M HCl. These slide boxes were
placed inside a water bath set at 37˚C. Following removal from the
water bath, the slides were briefly dipped (10-15 sec) in distilled
water at room temperature. Following this, they were immersed twice
for 3 min each in 2X SSC solution. The sections were subsequently
passed through a series of alcohol solutions (70, 85 and 100%) for
3 min each and then allowed to air-dry.
After drying, 10 µl of ALK Break Apart Probe (Abbott
Pharmaceutical Co. Ltd./Vysis ALK Break Apart FISH Probe kit) was
applied to the sections. The slides were then cover-slipped, and
the edges were sealed with rubber. Denaturation was carried out in
a ThermoBrite device at 73˚C for 5 min. Hybridization was performed
for 16 h at 37˚C. The following morning, the sections were
incubated in a washing solution at 73˚C for 3 min, followed by a
2-min incubation at room temperature. After drying, 10 µl of DAPI
was applied to the sections for counterstaining, and cover slips
were placed. Prior to evaluation, the slides were stored at -20˚C
for 1 h. The FISH analysis was conducted using a computer-linked
fluorescent microscope (Olympus BX61; Olympus Corporation). At
least 100 nuclei were counted in each slide, and separation signals
(break apart) were considered present when the distance between red
and green signals was at least twice the estimated signal diameter
or when a single red signal was detected. The presence of
separation signals in more than 15% of tumor cells was considered
positive for the 2p23 translocation. Herein, as 25 out of 100
counted cells exhibited translocation, it was considered positive
(Fig. 2).
The patient commenced treatment with the ALK
inhibitor, alectinib. The clinical course of the patient was quite
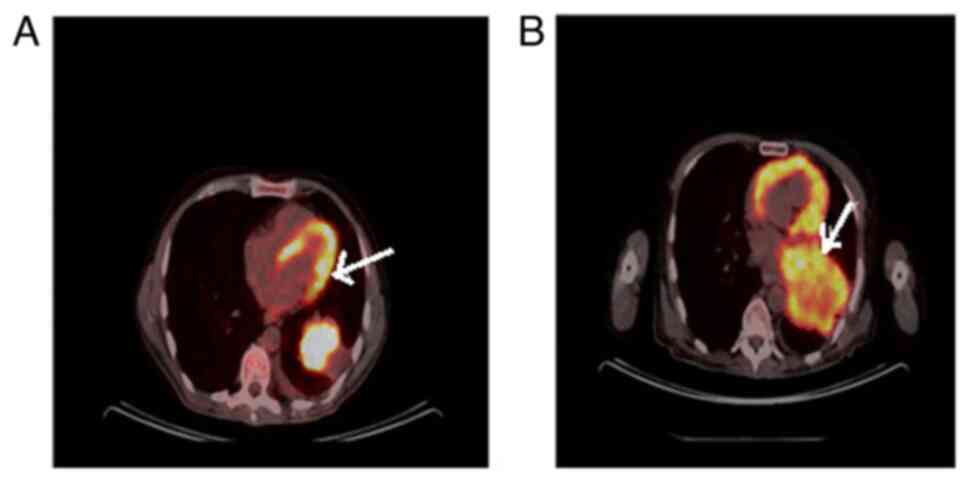
complex. Despite the treatments, the primary lesion in the lungs
progressed rapidly (Fig. 3), while
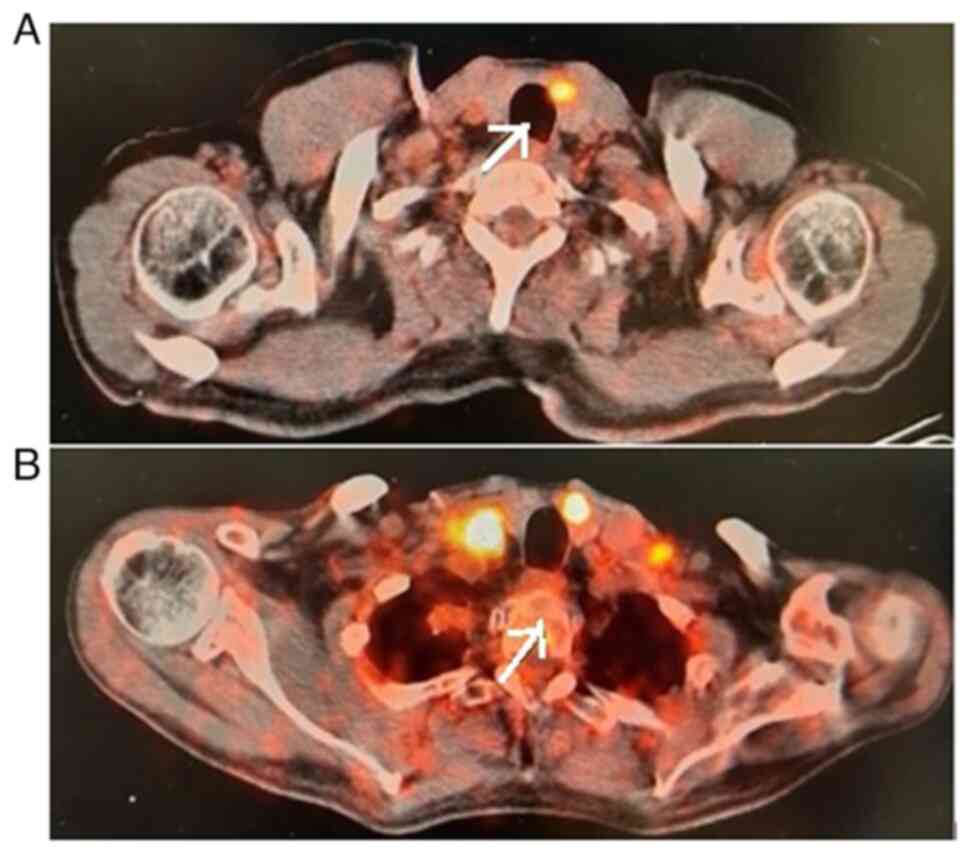
PET-CT imaging revealed the regression of the thyroid gland
(Fig. 4). A re-biopsy from the same
site showed a pathologic complete response in the thyroid (Fig. 5). In the clinical follow-up of the
patient, the lesion in the lung progressed and chemotherapy
(platinum and taxane) was commenced due to a visceral crisis. The
patient's final condition was characterized by respiratory
distress, signs of infection, and worsening blood counts. Finally,
the patient's respiratory distress became severe and he was
admitted to the intensive care unit of Cukurova University
Hospital, where he was intubated, and eventually, succumbed.
Discussion
The incidence of lung cancer has steadily risen due
to the increased life expectancy, smoking status and environmental
factors. NSCLC represents ~85% of lung cancer cases. Lung cancer is
a malignancy that, at the time of diagnosis, has generally already
metastasized. NSCLC metastasis to the thyroid is a very rare
occurrence. Despite the extensive circulatory network of the
thyroid gland, carcinomas do not often spread to the thyroid gland.
Not only ALK-positive NSCLC, but also renal cell carcinomas (~50%)
are the most common primary carcinomas metastasizing to the thyroid
gland (2,11,12). The
EML4-ALK fusion has been reported in 3 to 7% of NSCLC cases.
Patients with metastatic NSCLC that have a driver mutation are
treated with medications that target specific molecular pathways
(13,14). ALK inhibitors have been used in
tumor-targeted therapies to achieve progression-free survival and
an improved quality of life. In the case described herein, tumor
heterogeneity was assumed to be responsible for the poor response
of the main lesion and the complete response of the metastasis in
the thyroid. The small number of ALK-positive clones in the initial
lesion and the ALK-positive clone that metastasizes to the thyroid
may be related to this disorder. Primary thyroid tumors can also
reveal ALK-positive clones, and these clones may similarly have a
stronger propensity for the thyroid. Tumor heterogeneity is a key
factor in the variable outcomes of treatment (15), as in the rare case of thyroid
metastasis described in the present study.
The primary objective of the treatment approach used
for the patient described herein was to assess the response of
EML4-ALK-positive lung cancer to the ALK inhibitor, alectinib. A
marked positive response in the thyroid gland and surrounding lymph
node metastases was observed, as evidenced by PET-CT imaging and
confirmed by a pathological examination, which indicated a
pathological complete response in the thyroid.
However, despite the promising response in the
thyroid gland, the main finding of the treatment was the rapid
progression of the primary lung lesion. Notably, the lung lesion
continued to advance even though there were no signs of lesions in
the thyroid gland. This finding suggests that while alectinib was
effective in controlling metastatic disease in the thyroid, it did
not have the same level of success in treating the primary lung
tumor. Despite advances in target-specific therapies in cases in
the literature even without thyroid metastases (16,17), the
majority of patients with ALK-positive NSCLC treated with TKIs
ultimately experience disease progression due to various mechanisms
of drug resistance (18).
As regards the patient's demise, it is crucial to
clarify that treatment with alectinib was not directly associated
with patient not surviving. Following the progression of the
primary lung lesion and the initiation of chemotherapy (platinum
and taxane) due to a visceral crisis, the patient did not respond
to chemotherapy, leading to her passing away.
In summary, the main finding of the treatment was
the disparate response between the thyroid gland metastases
(positive response to alectinib) and the primary lung lesion (rapid
progression). Furthermore, the patient's demise was primarily
attributed to the progression of the primary lung lesion and the
subsequent failure of chemotherapy to control it.
The percentage of ALK-positive cells may differ in
tumor tissue in the thyroid and lung. The high percentage of
ALK-positive cells in the thyroid tissue may have resulted in a
greater benefit from alectinib therapy. Patients with varying
percentages of ALK-positive cells also have varying alectinib
responses. Patients with higher ALK percentages have better
responses (15). The present study
aimed to draw attention to the fact that the difference in ALK
percentages between patients may also differ in metastatic tissues
of the same patient. In the case described herein, a low ALK
percentage positivity in the lung tissue may explain the weak
response in the lung (19).
Next-generation sequencing (NGS) is occasionally
indicated in oncology in most recent years to identify actionable
targets; however, the methodology itself still requires a
well-identified and sufficient number of tumor cells to perform the
sequencing and an experienced team to perform the analysis. For
technical reasons, the authors of the present study could not
evaluate the ALK cell percentage and further analysis from thyroid
tissue was not possible. Although the sample collected in the
present case was sufficient to diagnose the tumor, it was
insufficient for further examination. In the case described herein,
only detect cancer cells could be detected. In targeted treatments
with precision medicine, both liquid-based and tissue-based
biopsies may need to be performed together in NGS. According to
precision medicine, liquid-based and tissue-based biopsies are
complementary, not an alternative to each other, in offering the
optimal treatment strategy (20).
Tumor heterogeneity is a key factor that may result
in inconsistent treatment outcomes. In order to improve treatment
outcomes, individualized and targeted medicines that take into
consideration the particular molecular characteristics of each
patient's tumor may be recommended. The development of genomic and
proteomic profiling techniques holds promise for the future
identification of particular biological targets and the improvement
of therapeutic outcomes.
There are some limitations to the present study that
should be mentioned. The main limitation of the patient's data was
that the percentage of ALK expression and the presence of other
driver gene mutations were not investigated. In future cases,
further extensive molecular investigations need to be
performed.
New information on targeted treatments is
continually being obtained, which is one of the most notable
achievements of precision medicine. As the data on this subject
increase, further light will be shed on this entity. It is clear
that more advanced diagnostic methods and new strategies are
required. The case in the present study is important in terms of
guiding future studies on this subject and allowing clinicians to
question treatment decisions based on genetic test results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EB was a major contributor to the conception of the
study, as well as to the literature search for related studies. EB,
TT, BG, AHU, DG and SP were involved in the writing of the
manuscript, and in the analysis and interpretation of the patient's
data. EB, TT, AHU and DG were involved in the literature review, in
the design of the study, in the revision of the manuscript and in
the processing of the figures. EB and TT confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient signed an informed consent form after
being informed of its details for the inclusion of her data in the
present case report.
Patient consent for publication
The patient signed an informed consent form agreeing
to the publication of his data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brundage MD, Davies D and Mackillop WJ:
Prognostic factors in non-small cell lung cancer: A decade of
progress. Chest. 122:1037–1057. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rudin CM, Brambilla E, Faivre-Finn C and
Sage J: Small-cell lung cancer. Nat Rev Dis Primers.
7(3)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fukui T, Yatabe Y, Kobayashi Y, Tomizawa
K, Ito S, Hatooka S, Matsuo K and Mitsudomi T: Clinicoradiologic
characteristics of patients with lung adenocarcinoma harboring
EML4-ALK fusion oncogene. Lung Cancer. 77:319–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aydemirli MD, van Eendenburg JDH, van
Wezel T, Oosting J, Corver WE, Kapiteijn E and Morreau H: Targeting
EML4-ALK gene fusion variant 3 in thyroid cancer. Endocr Relat
Cancer. 28:377–389. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kawamoto H, Kaneko Y, Ryu K and Kuwano K:
Thyroid metastasis from lung adenocarcinoma with EML4-ALK
rearrangement. BMJ Case Rep. 2016(bcr2016217541)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McCusker MG, Russo A, Scilla KA, Mehra R
and Rolfo C: How I treat ALK-positive non-small cell lung cancer.
ESMO Open. 4 (Suppl 2)(e000524)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jassem J: Alectinib in
crizotinib-resistant, ALK-positive NSCLC. Lancet Oncol. 17:134–135.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Song Z, Wang M and Zhang A: Alectinib: A
novel second generation anaplastic lymphoma kinase (ALK) inhibitor
for overcoming clinically-acquired resistance. Acta Pharm Sin B.
5:34–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Can AS and Köksal G: Thyroid metastasis
from small cell lung carcinoma: A case report and review of the
literature. J Med Case Rep. 9(231)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang Q and Wang Z: Metastases to the
thyroid gland: What can we do? Cancers (Basel).
14(3017)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
LaPar DJ, Bhamidipati CM, Lau CL, Jones DR
and Kozower BD: The Society of Thoracic Surgeons General Thoracic
Surgery Database: Establishing generalizability to national lung
cancer resection outcomes. Ann Thorac Surg. 94:216–221; discussion
221. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hegerova L, Griebeler ML, Reynolds JP,
Henry MR and Gharib H: Metastasis to the thyroid gland: Report of a
large series from the Mayo Clinic. Am J Clin Oncol. 38:338–342.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonoff MB and D'Cunha J: Non-small cell
lung cancer: The era of targeted therapy. Lung Cancer (Auckl).
3:31–41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dong J, Li B, Lin D, Zhou Q and Huang D:
Advances in targeted therapy and immunotherapy for non-small cell
lung cancer based on accurate molecular typing. Front Pharmacol.
10(230)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chia PL, Mitchell P, Dobrovic A and John
T: Prevalence and natural history of ALK positive non-small-cell
lung cancer and the clinical impact of targeted therapy with ALK
inhibitors. Clin Epidemiol. 6:423–432. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Camidge DR, Kono SA, Flacco A, Tan AC,
Doebele RC, Zhou Q, Crino L, Franklin WA and Varella-Garcia M:
Optimizing the detection of lung cancer patients harboring
anaplastic lymphoma kinase (ALK) gene rearrangements potentially
suitable for ALK inhibitor treatment. Clin Cancer Res.
16:5581–5590. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sgambato A, Casaluce F, Maione P and
Gridelli C: Targeted therapies in non-small cell lung cancer: A
focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer
Ther. 18:71–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Desai A and Lovly CM: Strategies to
overcome resistance to ALK inhibitors in non-small cell lung
cancer: A narrative review. Transl Lung Cancer Res. 12:615–628.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hizal M, Bilgin B, Paksoy N, Atcı MM,
Kahraman S, Kılıçkap S, Güven DC, Keskinkılıç M, Ayhan M, Eren Ö,
et al: The percentage of ALK-positive cells and the efficacy of
first-line alectinib in advanced non-small cell lung cancer: Is it
a novel factor for stratification? (Turkish Oncology Group Study).
J Cancer Res Clin Oncol. 149:4141–4148. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Esagian SM, Grigoriadou GI, Nikas IP,
Boikou V, Sadow PM, Won JK and Economopoulos KP: Comparison of
liquid-based to tissue-based biopsy analysis by targeted next
generation sequencing in advanced non-small cell lung cancer: A
comprehensive systematic review. J Cancer Res Clin Oncol.
146:2051–2066. 2020.PubMed/NCBI View Article : Google Scholar
|