Introduction
Prostatic cancer is one of the most highly prevalent
malignancies affecting males globally (1). The spectrum of prostatic neoplasms
includes a variety of rare histological variants. Among these,
prostatic pleomorphic giant cell carcinoma (PGCC) is an extremely
rare and poorly understood subtype, recently listed in the World
Health Organization (WHO) Classification of Tumors of the Urinary
System as a variant of acinar adenocarcinoma (2,3). Based
on current data, PGCC is an aggressive form of acinar
adenocarcinoma associated with a poor prognosis, despite treatment
(4). PGCC typically demonstrates a
small portion of more differentiated, yet high-grade, typical
prostatic acinar adenocarcinoma (5).
It is characterized by giant, bizarre cells with pleomorphic nuclei
upon a conventional histological examination (2,3,6).
Some PGCC cases may also contain components of other
tumor types, such as squamous cell carcinoma, neuroendocrine
carcinoma and prostatic ductal adenocarcinoma (2). When compared to conventional prostate
carcinoma, PGCC frequently exhibits high Gleason grade
characteristics and currently falls into the International Society
of Urological Pathology (ISUP) grade group 5 (3,6,7), based on the recent 5th edition WHO
classification of male genital and urinary tumors (2). Moreover, previous chemotherapy,
hormonal therapy and radiation therapy are commonly related to
occurrences of PGCC, particularly androgen deprivation therapy
(3,8). Given its rarity, prostatic PGCC often
presents significant diagnostic and treatment challenges,
exacerbated by its aggressive nature and poor prognosis. The
scarcity of prostatic PGCC case reports in the medical literature
underscores the need for more comprehensive data on this rare
pathologic entity. The present study reports a case of a
65-year-old male patient with prostatic PGCC and also provides a
brief review of the literature with the aim of shattering further
light on this rare entity.
Case report
Patient information
A 65-year-old male patient visited Smart Health
Tower (Sulaimani, Iraq) and complained of severe dysuria, nocturia,
and frequent, urgent, and difficult urination for a period of 3
months. He had previously visited numerous urologists, and they
diagnosed his condition as benign prostatic hypertrophy with
prostatitis. He developed acute urinary retention while on medical
treatment for prostatitis. An 18 French Foley catheter was inserted
for him for 1 week; however, following its removal, the patient was
unable to urinate again. Consequently, the Foley catheter was
inserted again and kept for 2 months, being changed once every 2
weeks. Despite receiving multiple different types of antibiotics
and alpha-blockers, his condition remained the same. He then
complained of a generalized body ache, weakness, anorexia, weight
loss, constipation, back pain and insomnia with severe lower
urinary tract symptoms (LUTS).
Clinical examination
Upon an examination, the patient was found to be
pale, have a low body weight, and have a soft abdomen with
tenderness over the lower abdomen. His urine bag contained turbid
urine. A digital rectal examination revealed a significantly
enlarged and painful prostate bulging into the rectum, with a
smooth surface and no nodularity. The upper portion could not be
reached due to its large size.
Diagnostic assessment
Laboratory investigations, including a complete
blood count, renal function tests and prostate-specific antigen
(PSA), were performed and all yielded normal results. The patient
was sent for pelvic magnetic resonance imaging (MRI) for more
detailed information about the prostate and pelvic organs. The
report of the pelvic MRI revealed a large prostatic mass measuring
9x9x11 cm with a well-defined outline and multiple areas of cystic
degeneration. Some of the cysts exhibited fluid-fluid levels
(indicating variably-aged internal hemorrhage). The mass originated
from the prostate with a marked pressure effect on the rest of the
prostate, urinary bladder and rectum. No definite invasion to the
surrounding organs and no associated pelvic lymphadenopathy were
observed. There was a focal bone lesion involving the left pubic
bone, suggestive of bone metastasis. A well-defined enhancing mass
was located in the left gluteal region under the gluteus maximus
muscle measuring 16x11 mm. The urinary bladder wall was thin and
there was no ascites. The case was presented to a multidisciplinary
team, which included urologists, general surgeons, radiologists,
pathologists and uro-oncologists; the decision was to perform a
prostate biopsy for tissue diagnosis. Following preparation, the
patient underwent a transrectal, 12-core prostate biopsy. Upon a
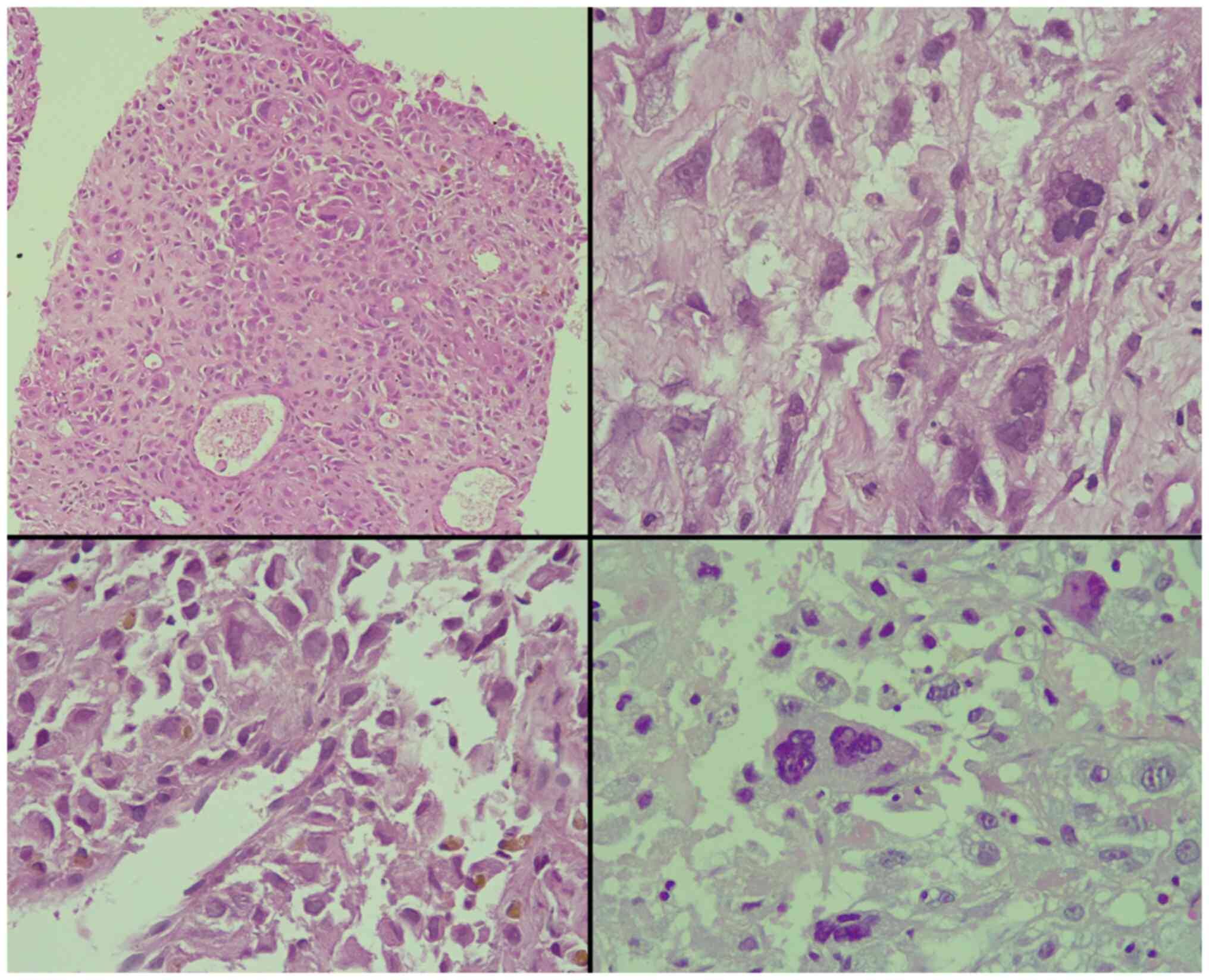
histopathological examination, it was found that 80% of the tissue
involved by the tumor had a Gleason score of 10 (5+5) for
conventional prostate carcinoma, composed of dyscohesive, large,
and pleomorphic cells with abundant eosinophilic cytoplasm and
bizarre, hyperchromatic nuclei with irregular nuclear outlines,
intranuclear inclusions, large macronucleoli and multinucleation
(Fig. 1). Perineural invasion was
observed; however, intraductal carcinoma, extraprostatic extension
and lymphovascular invasion were not observed. An
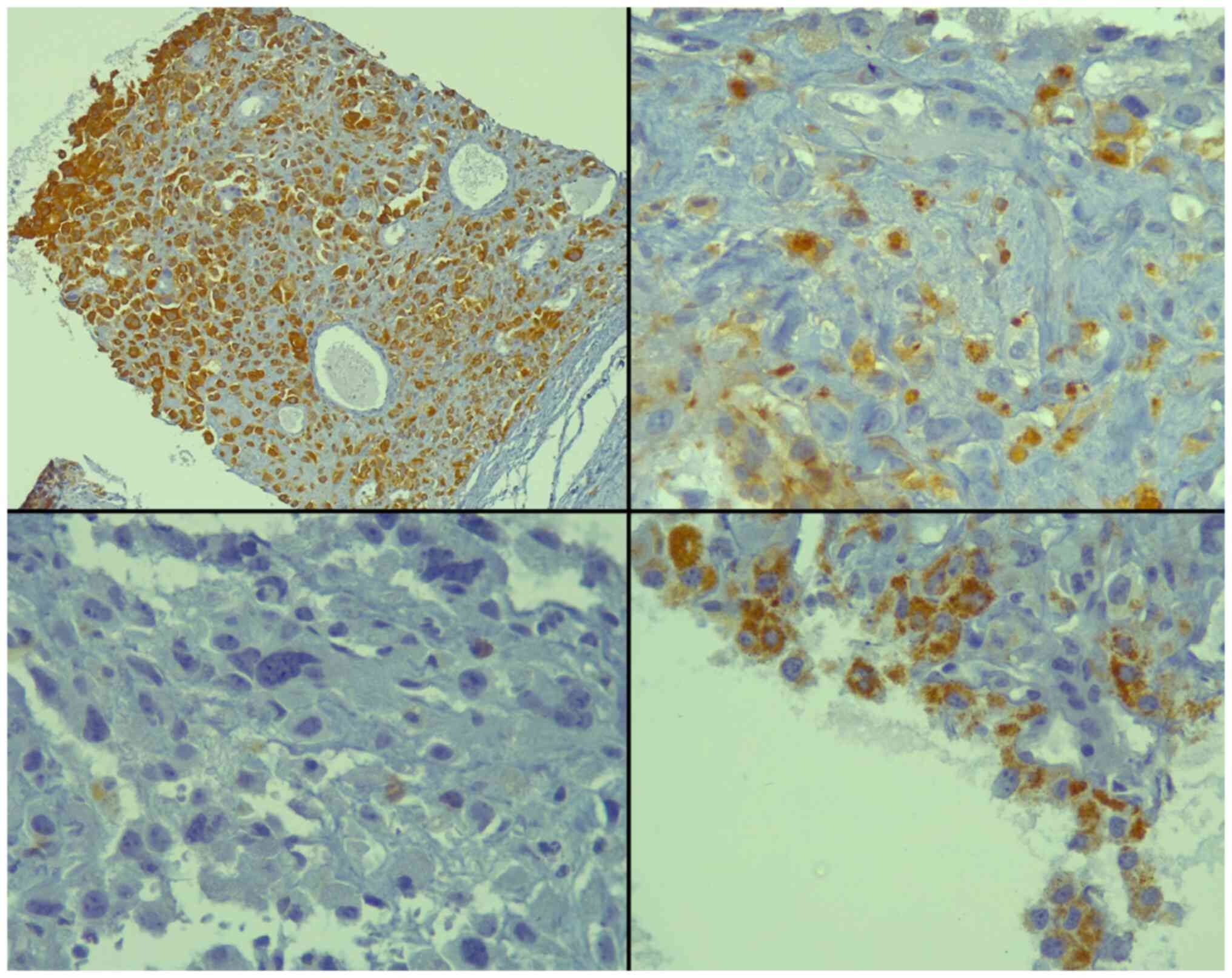
immunohistochemical analysis revealed positivity of the tumor cells
for the pan-epithelial markers AE1/AE3 and alpha-methylacyl-CoA
racemase (AMACR), and focal positivity for sphingolipid activator
protein-2 (SAP) (Fig. 2). Of note,
all staining protocols and immunohistochemistry were conducted in
an external facility and not at the authors' institute. All the
other markers studied were negative, including PSA, melanin A,
desmin, CD34, CK7, CK20, caudal type homeobox 2 (CDX2), GATA
binding protein 3 (GATA3) and thyroid transcription factor-1
(TTF-1) (data not shown). Hence, the diagnosis of PGCC of the
prostate was made.
Therapeutic intervention and
follow-up
While waiting for the results of the
histopathological examination of the prostate biopsy, the patient
developed acute abdominal distension and pain with repeated
vomiting, for which he was admitted to the emergency room
and was diagnosed with intestinal obstruction. Following 2 days of
conservative treatment (as at that time, the family refused any
surgical intervention, the gastrointestinal tract surgeon was
obliged to manage the patient by inserting a nasogastric tube,
antibiotics and IV fluid), the gastrointestinal tract surgeon
decided to do a laparotomy and end colostomy for him. After 4 days,
his condition deteriorated, and he developed severe sepsis with
wound dehiscence. He was admitted to the intensive care unit, and
after 2 weeks of follow-up, the patient passed away from multiorgan
failure.
Discussion
PGCC, a relatively rare tumor variant, has been
identified in several organs, including the hepatobiliary system,
pancreas, thyroid, urinary bladder, endometrium and kidney
(3,5,9). In the
context of the prostate, the first PGCC case was documented by Mai
et al in 1996(10). This
variant of carcinoma is interesting due to its unique histological
presentation and diagnostic challenges, particularly in
differentiating it from other forms of cancer.
Standard prostate cancer generally exhibits cells
that possess relatively uniform nuclei, even in high-grade cases
(7). This lack of pleomorphism
serves as a key characteristic that sets poorly differentiated
prostate cancer apart from urothelial carcinoma and allows for
differentiation from sarcomatoid carcinoma due to the absence of
spindle cells (3,5). However, in uncommon cases, the
histological features of pleomorphic giant cell adenocarcinoma of
the prostate can overlap with those of urothelial carcinoma, which
poses a diagnostic challenge, given that the treatments for these
two diseases are markedly different.
Clinically, determining whether a sizable tumor at
the bladder neck is of bladder or prostatic origin can be difficult
on imaging and even during cystoscopy. Experienced urologists have
submitted numerous cases of ‘bladder tumors’ that were initially
misdiagnosed by pathologists as urothelial carcinomas. Upon further
review and the implementation of immunohistochemistry, these tumors
were identified as high-grade prostatic adenocarcinomas (7). Previous reports of prostatic PGCC cases
(7,11) have detailed similarities to
conventional prostate cancer, including an aggressive clinical
course, an increased incidence in older patients, an association
with a high Gleason grade for standard prostate carcinoma
components, and typically focal, negative, or weak staining for
standard prostatic immunohistochemical markers (3).
Pleomorphic giant cell adenocarcinoma has very
particular histological features. The tumor cells display marked
pleomorphism and varying levels of cohesiveness. Even in the
presence of extreme atypia, more conventional features can still be
observed. If PGCC is associated with a more traditional acinar
adenocarcinoma, the latter component generally exhibits a high
Gleason grade, thereby categorizing this unique form of prostate
cancer under ISUP grade group 5, even in the absence of more
classical features (2,6,12). In
terms of morphology, PGCCs consist of unusually large cells that
typically comprise a small fraction of the overall tumor. These
cells are distinctively epithelial and form a cohesive structure,
which aids in distinguishing them from the diverse, pleomorphic
individual cells seen in sarcomatoid prostate adenocarcinoma
(7). Atypical mitoses are frequently
observed, and necrosis has been documented in the literature, with
the case reported by Larnaudie et al (13) illustrating hemorrhagic and necrotic
features. Another common observation is the clearing of the
cytoplasm, while spindle cell aspects have been described, albeit
being rare, as reported by Larnaudie et al (13). PGCC has been shown to be related to
other histological variants of prostatic carcinoma, including
squamous cell carcinoma, intraductal carcinoma and neuroendocrine
carcinoma (13). In the six cases
documented by Parwani et al (5), each one exhibited an additional
component of either small cell, squamous cell, or prostatic ductal
carcinoma. The tumor in the patient depicted herein displayed
clusters of loosely cohesive cells characterized by abundant
eosinophilic cytoplasm and large, hyperchromatic nuclei with
intranuclear inclusions, sizable macronucleoli and
multinucleation.
Diagnostic difficulties may arise when only the
pleomorphic component is present. The ability to recognize the
prostatic origin of the tumor is crucial, particularly in cases of
isolated metastasis, as the treatment modalities diverge
substantially, with one leaning towards hormonotherapy and
chemotherapy (13). To confirm the
origin of PGCC, the use of prostatic marker immunohistochemistry,
such as prostate-specific membrane antigen (PSMA), PSA, NK3
homeobox 1 (NKX3.1) and androgen receptor, is recommended, as
previously suggested by Alharbi et al (7) and corroborated by Bilé-Silva et
al (4).
El-Zaatari et al (3) emphasized the importance of a
comprehensive panel of immunohistochemical markers for correct PGCC
diagnosis. Their review of 51 PGCC cases revealed that numerous
cases exhibited weak or no staining for any marker of prostatic
differentiation. Intriguingly, despite being commonly performed,
PSA staining was positive in only 2 out of 22 cases, whereas 10
cases exhibited weak and focal staining and the others were
entirely negative (3). The study by
Bilé-Silva et al (4) also
revealed a lower PSA positivity, ranging from 5 to 20%. However,
this low expression should not be construed as a negative PSA
expression, as it may result from various therapies (4). NKX3.1 emerged as a superior
prostate-specific marker for distinguishing urothelial carcinoma
and poorly differentiated prostatic adenocarcinoma, exhibiting a
high sensitivity and specificity for the latter (14). Yet, Alharbi et al.'s study
showed that its staining was not consistent, with some cases
showing only focal positivity or even negativity in the pleomorphic
component (7). Another promising
marker is homeobox B13 (HOXB13), exhibiting a high sensitivity and
specificity for prostatic tissue. A 2017 study suggested its
utility in confirming the prostatic origin of metastatic lesions
(13). However, the study by Alharbi
et al (7) revealed that
HOXB13 staining varied significantly among PGCC cases, with some
cases even showing complete negativity. The cases reported in the
study by Parwani et al (5)
were all positive for cytokeratin AE1/AE3. Lastly, the majority of
the PGCC cases in the study by Alharbi et al (7) exhibited negativity for GATA3, p63, and
thrombomodulin, which is useful for ruling out urothelial
carcinoma. All these findings emphasize the necessity for a
comprehensive panel of markers to ensure an accurate diagnosis of
PGCC. The case in the present study was positive for PSAP, AMACR,
and AE1/AE3, while being negative for PSA, CK7, CK20, GATA3,
melan-A, TTF-1, desmin, CD34 and CDX2.
Prior radiation, hormonal therapy, and chemotherapy
appear to be frequently associated with the PGCC phenotype
(4,8,15). There
is substantial evidence to indicate that PGCC develops as a result
of the dedifferentiation of high-grade, traditional prostate
carcinoma. The conventional prostatic adenocarcinoma component has
coexisted with all known PGCC cases. Notably, 11 out of 12 cases
with earlier traditional prostatic cancer, including the cases
reported in the study by El-Zaatari et al (3,5,6), had undergone radiation, chemo-, or
hormonal therapy prior to presenting with PGCC. This suggests that
prior treatment modalities may contribute to PGCC development
(3,5,7). This is
supported by observations of the frequent loss/weak staining of
prostatic differentiation markers in these tumors, pointing towards
a loss of prostatic differentiation (3). Further confirming this theory, the
study by Bilé-Silva et al (4)
demonstrated that all the patients with PGCC had previously been
treated with hormonal therapy.
The most recently published study by Bilé-Silva
et al (4), which included the
largest series of patients with extensive PGCC, provides a
comparative survival analysis between cases with PGCC and cases
with conventional high-grade prostate adenocarcinoma. The findings
indicate a significant difference in cancer-specific survival,
suggesting the aggressive nature of PGCC (4). This is also supported by the findings
of the cases reported by Alharbi et al (7).
Further research is required in order to confirm
these findings. Improving the understanding of this variant of
prostate cancer could allow for an earlier and more accurate
diagnosis, leading to more effective therapeutic approaches that
could potentially enhance patient outcomes.
In conclusion, PGCC of the prostate is an aggressive
variant with a dismal prognosis. It frequently occurs in patients
who have received prior prostatic cancer-directed therapy.
Prostatic marker immunohistochemistry, such as PSMA, PSA, NKX3.1
and androgen receptor can be used to confirm whether the PGCC is of
prostatic origin. The early recognition of this entity may
contribute to more effective therapy, as physicians could opt for
more aggressive treatments.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SSF was a major contributor to the conception of the
study, as well as in the literature search for related studies. DMH
and FHK were involved in the literature review, in the writing of
the manuscript, and in the examination and interpretation of the
patient's data. FMF, SHM, BAA and HMR were involved in the
literature review, in the design of the study, in the critical
revision of the manuscript and in the processing of the figures.
RMA and AMA were the pathologists who performed the
histopathological diagnosis of the patient. BAA and FHK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for his participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahmood ZH, Mohemed FM, Fatih BN, Qadir AA
and Abdalla SH: Cancer publications in one year (2022); a
cross-sectional study. Barw Med J. 1(Issue 2)2023.
|
|
2
|
Moch H, Amin MB, Berney DM, Compérat EM,
Gill AJ, Hartmann A, Menon S, Raspollini MR, Rubin MA, Srigley JR,
et al: The 2022 World Health Organization classification of tumours
of the urinary system and male genital organs-part A: Renal,
penile, and testicular tumours. Eur Urol. 82:458–468.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
El-Zaatari ZM, Thomas JS, Divatia MK, Shen
SS, Ayala AG, Monroig-Bosque P, Shehabeldin A and Ro JY:
Pleomorphic giant cell carcinoma of prostate: Rare tumor with
unique clinicopathological, immunohistochemical, and molecular
features. Ann Diagn Pathol. 52(151719)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bilé-Silva A, Lopez-Beltran A, Rasteiro H,
Vau N, Blanca A, Gomez E, Gaspar F and Cheng L: Pleomorphic giant
cell carcinoma of the prostate: Clinicopathologic analysis and
oncological outcomes. Virchows Archiv. 482:493–505. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parwani AV, Herawi M and Epstein JI:
Pleomorphic giant cell adenocarcinoma of the prostate: Report of 6
cases. Am J Surg Pathol. 30:1254–1259. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lopez-Beltran A, Eble JN and Bostwick DG:
Pleomorphic giant cell carcinoma of the prostate. Arch Pathol Lab
Med. 129:683–685. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alharbi AM, De Marzo AM, Hicks JL, Lotan
TL and Epstein JI: Prostatic adenocarcinoma with focal pleomorphic
giant cell features: A series of 30 cases. Am J Surg Pathol.
42:1286–1296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santoni M, Conti A, Burattini L, Berardi
R, Scarpelli M, Cheng L, Lopez-Beltran A, Cascinu S and Montironi
R: Neuroendocrine differentiation in prostate cancer: Novel
morphological insights and future therapeutic perspectives. Biochim
Biophys Acta. 1846:630–637. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Samaratunga H, Delahunt B, Egevad L,
Adamson M, Hussey D, Malone G, Hoyle K, Nathan T, Kerle D, Ferguson
P and Nacey JN: Pleomorphic giant cell carcinoma of the urinary
bladder: An extreme form of tumour de-differentiation.
Histopathology. 68:533–540. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mai K, Burns B and Morash D: Giant-cell
carcinoma of the prostate. J Urol Pathol. 5:167–174. 1996.
|
|
11
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA: Grading Committee. The 2014
International Society of Urological Pathology (ISUP) consensus
conference on Gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alhamar M, Tudor Vladislav I, Smith SC,
Gao Y, Cheng L, Favazza LA, Alani AM, Ittmann MM, Riddle ND,
Whiteley LJ, et al: Gene fusion characterisation of rare aggressive
prostate cancer variants-adenosquamous carcinoma, pleomorphic
giant-cell carcinoma, and sarcomatoid carcinoma: An analysis of 19
cases. Histopathology. 77:890–899. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Larnaudie L, Compérat E, Conort P and
Varinot J: HOXB13 a useful marker in pleomorphic giant cell
adenocarcinoma of the prostate: A case report and review of the
literature. Virchows Archiv. 471:133–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Epstein JI, Egevad L, Humphrey PA and
Montironi R: ISUP Immunohistochemistry in Diagnostic Urologic
Pathology Group. Best practices recommendations in the application
of immunohistochemistry in the prostate: Report from the
International Society of Urologic Pathology consensus conference.
Am J Surg Pathol. 38:e6–e19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aparicio AM, Shen L, Tapia EL, Lu JF, Chen
HC, Zhang J, Wu G, Wang X, Troncoso P, Corn P, et al: Combined
tumor suppressor defects characterize clinically defined aggressive
variant prostate cancers. Clin Cancer Res. 22:1520–1530.
2016.PubMed/NCBI View Article : Google Scholar
|