Until 2011, chemotherapy was the initial treatment
for metastatic melanoma; however, it only provided a 6-month median
survival time and a 25% 1-year overall survival rate. High-dose
interleukin-2 (IL-2) was the only immunotherapy available, but was
associated with severe toxicities and only benefited a limited
number of patients (4). Currently,
advances in immunotherapy and studies on cell cycle regulatory
molecules have facilitated the creation of immune checkpoint
inhibitors (ICIs), a group of monoclonal antibodies that block
co-inhibitory molecules, such as cytotoxic T-lymphocyte-associated
antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1) and its
ligand, PDL1 (5-7).
Ipilimumab, nivolumab and pembrolizumab were the first class of
medications shown to improve the overall survival of patients with
metastatic melanoma (4).
The present review provides comprehensive evidence
regarding the role of ICIs and their utilization in advanced
melanoma cases. The outcomes of ICIs, such as ipilimumab, nivolumab
and pembrolizumab are highlighted, including the improved survival
rates and response rates associated with their use compared to
traditional chemotherapies, while also focusing on the mechanisms
and demonstrating the potentially adverse effects of these
therapies.
Furthermore, the present review focuses on
combination therapies, including anti-PD1 with anti-CTLA-4,
showcasing their importance compared to monotherapy. The improved
outcomes of combination therapies over traditional therapies are
highlighted, with an emphasis on the need for ongoing research,
optimized treatment approaches and strategies which can be used to
overcome resistance. In addition to discussing the development of
novel biomarkers for assessing ICI therapeutic responses in both
tissue and serum-based prognostic and predictive markers, tumor
metabolic dependencies and targeting the metabolic pathways by
combining ICIs are also discussed. This could provide an improved
efficacy, which, to the best of our knowledge, has not been
described commonly in the available literature focusing on ICIs
used in melanoma.
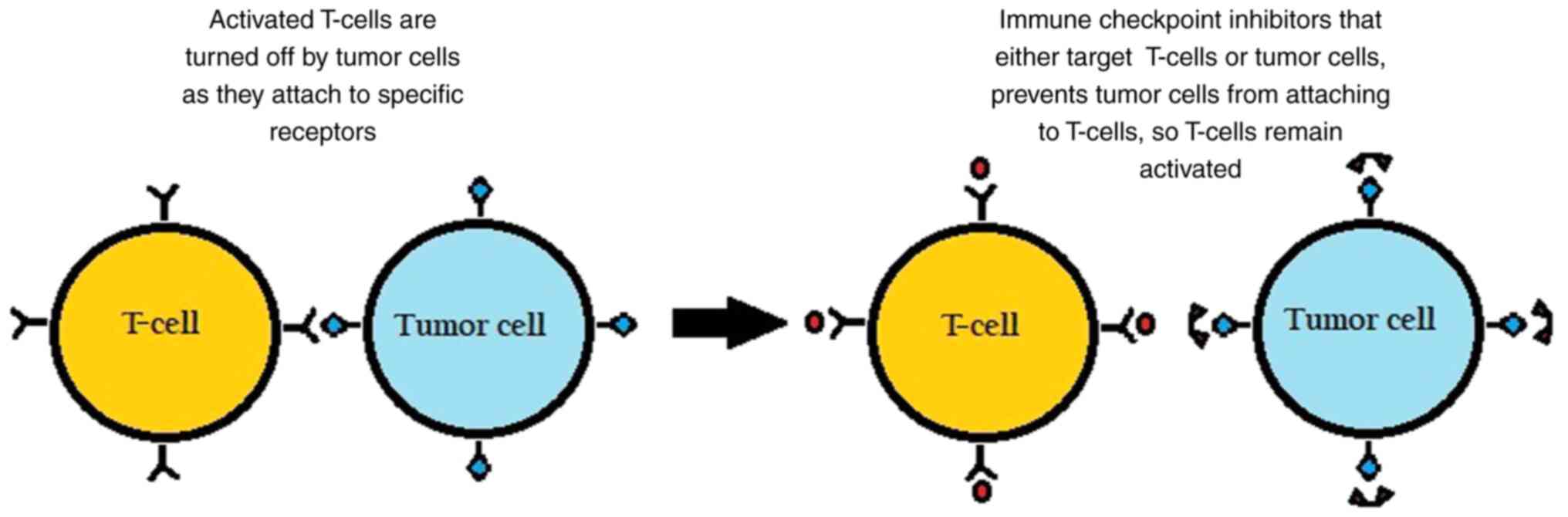
The surface of immune cells, such as T-cells,
assists in the regulation of the immune response through various
receptors. When activated by certain ligands, these receptors
inhibit immune cells from attacking the body's own cells. However,
in cancer, tumor cells can take advantage by binding to these
checkpoint-inhibitory receptors through their own ligands and
suppressing the immune response, as illustrated in Fig. 1.
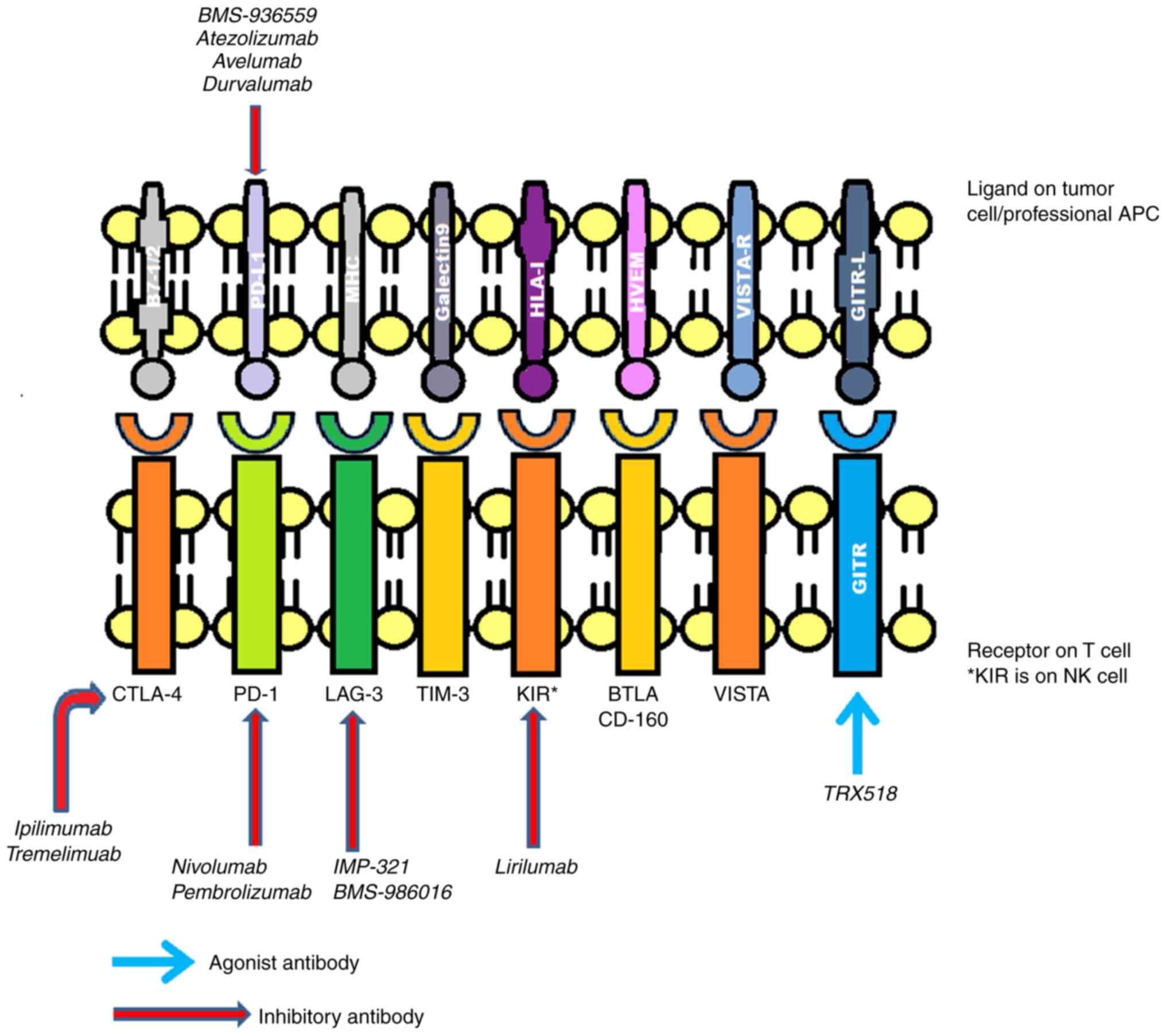
Commonly implicated inhibitory receptors include
(CTLA-4, PD-1, T-cell immunoglobulin domain and mucin domain-3
(TIM-3), killer cell immunoglobulin-like receptor (KIR),
lymphocyte-activation gene 3 (LAG3), glucocorticoid-induced tumor
necrosis factor receptor (GITR), B- and T-lymphocyte attenuator
(BTLA) and V-domain immunoglobulin (Ig)-containing suppressor of
T-cell activation (VISTA) (8-10),
as presented in Fig. 2.
Over time, several drugs have been introduced
targeting these receptors. Ipilimumab was one of the first ICI
drugs to be approved by the Food and Drug Administration (FDA) for
the treatment of metastatic melanoma, which functions by blocking
CTLA-4(11). There are numerous
additional comparable drugs in early phase III, phase II, or
preclinical research. These include pidilizumab, atezolizumab,
durvalumab and tremelimumab (formerly known as ticilimumab)
(12,13). To increase the immune system's
defense against cancer cells, these medications also work against
various immunological checkpoints (12,13).
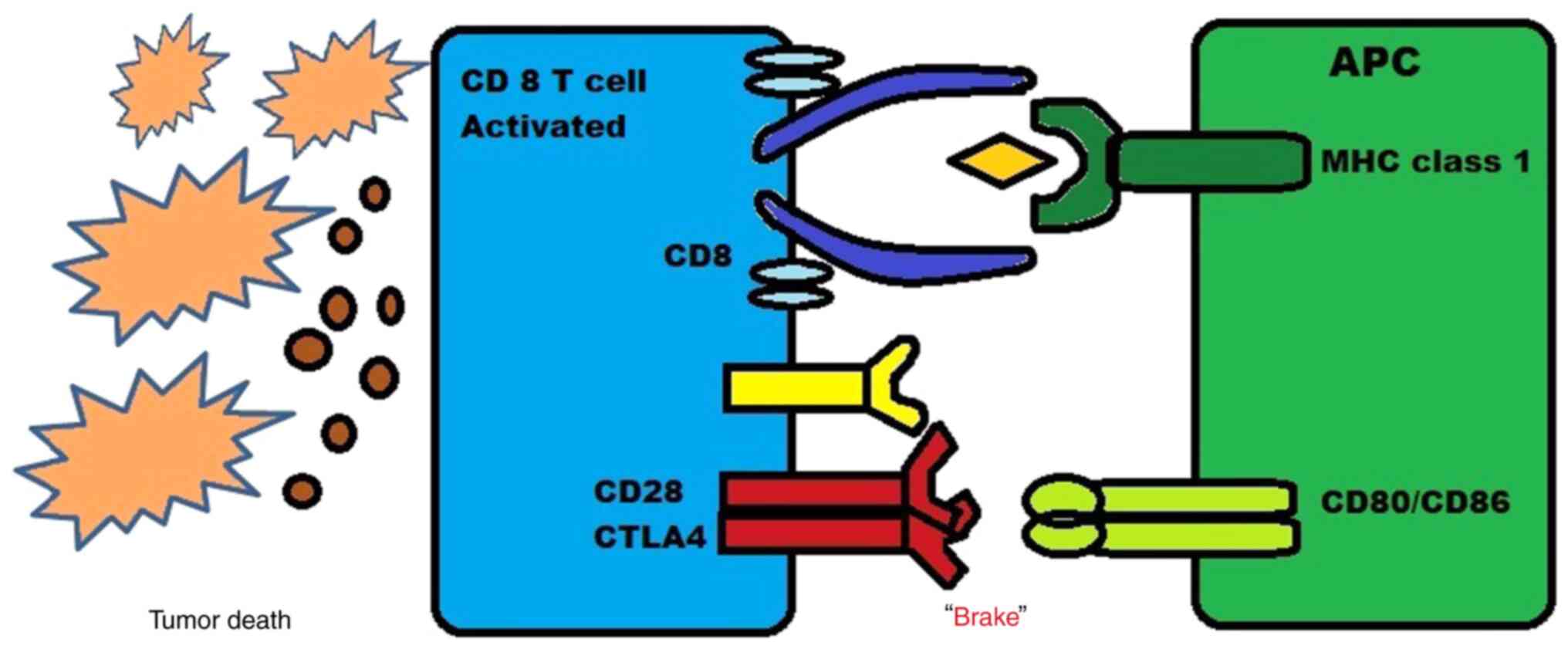
CTLA-4, a B7/CD28 family member, is a coinhibitory
receptor expressed on the surface of T-cells that eventually
inhibits T-cells, and it is expressed by regulatory T-cells (Tregs)
(14). Discovered in 1987, it was
considered to function as a negative regulator of T-cell activation
until the mid-1990s (15-17).
Tregs also play a key role in maintaining immune
homeostasis by inhibiting excessive immune responses. One of the
mechanisms through which Tregs suppress effector T-cell activity is
via CTLA-4 signaling (19). Two
anti-CTLA-4 drugs have been studied in patients with melanoma: i)
Ipilimumab, the first ICI evaluated and approved for the treatment
of melanoma is a fully human immunoglobulin anti-CTLA-4 monoclonal
antibody (20,21); ii) tremelimumab, a fully human
immunoglobulin anti-CTLA-4 monoclonal antibody which is still under
investigation (12).
There are two major mechanisms through which these
drugs act. First, the inhibition of CTLA-4 signaling in cytotoxic
T-cells that specifically target tumors can directly affect these
cells by enabling them to evade a state of anergy and enter an
active proliferative effector phase. Once activated, these effector
T-cells are more likely to penetrate the tumor and exhibit direct
cytotoxic effects on tumor cells, while also releasing cytokines
such as IL-2 and IFN-γ to stimulate an immunogenic tumor
microenvironment. Thus, by blocking the CTLA-4 pathway, T-cells
that were previously inactive can become activated and effectively
target the tumor cells, causing a more powerful immune response
against the cancer. This new approach holds promise as a potential
immunotherapy for the treatment of cancer (22).
The second major mechanism driving these drugs is
the blocking of CTLA-4 signaling in Tregs, which may impair their
ability to halt the activity of effector T-cells. This inhibition
of CTLA-4 signaling can either cause a decrease in the number of
Tregs or reduce their function without affecting their population
size. Therefore, blocking CTLA-4 on Tregs may disrupt this
suppression and lead to increased immune activation against tumor
cells (19,23,24).
Patients with melanoma are treated with the primary
aim of suppressing the molecular interplay between the melanoma
cells and immune effector cells. Ipilimumab, which has mainly been
approved for the treatment of more advanced stages, such as
unresectable or metastatic melanoma, has been shown to be
associated with a marked overall survival rate confirmed from a
phase 3 clinical trial (25). The
interference of ipilimumab on CTLA-4 expressed on the subset of
tumor-specific T-cell proliferation and B7 molecules on
antigen-presenting cells is expected to prevent tumor development
(26).
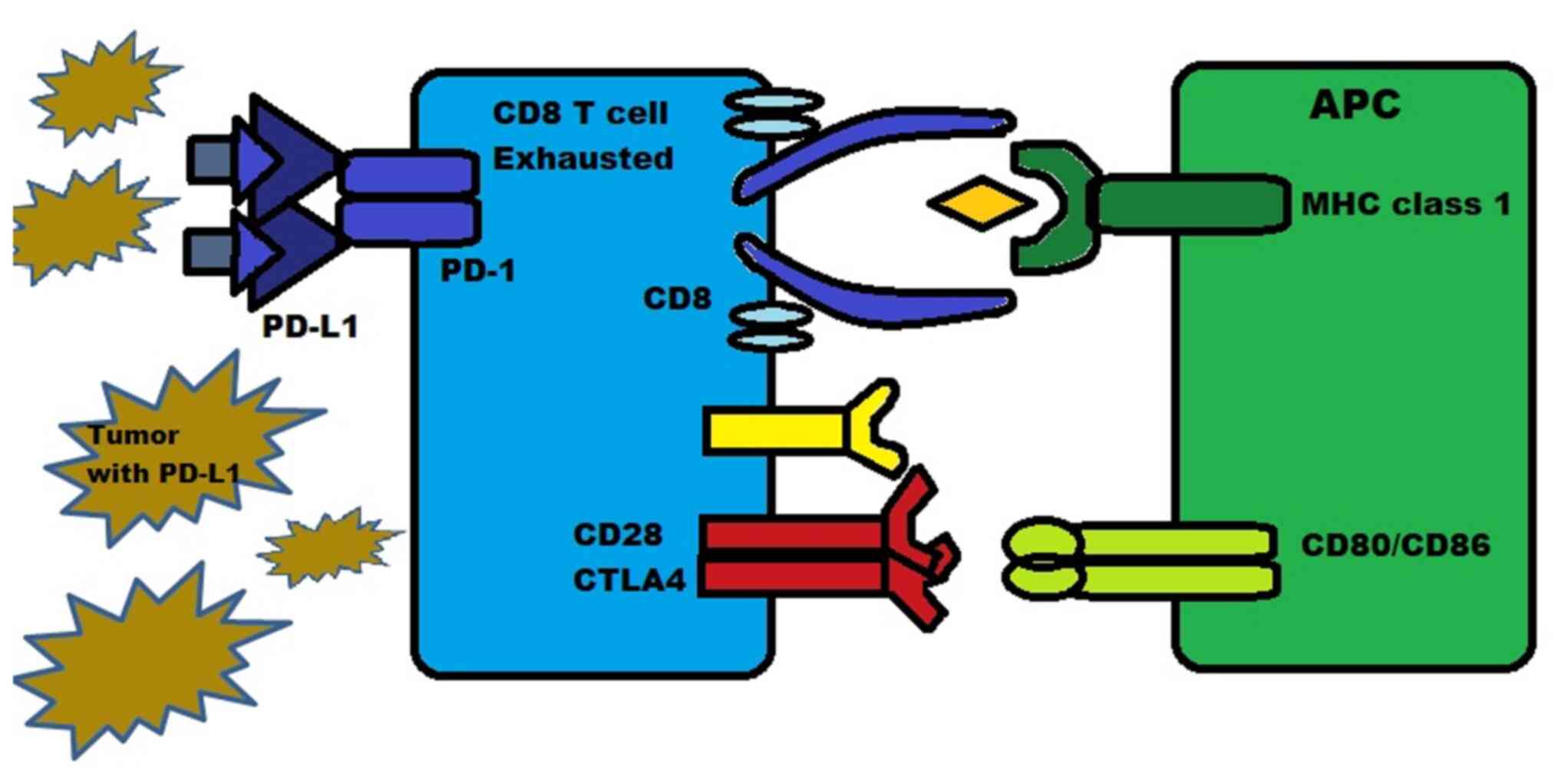
PD-1 is a protein present on the surface of T-cells,
B-cells and natural killer (NK) cells. It functions as an
inhibitory molecule by binding to PD-L1 (or B7-H1) and PD-L2
(B7-H2). PD-L1 is expressed in numerous types of tissue, including
hematopoietic cells and certain tumors such as melanoma, where they
are expressed in 40-50% of cases. PD-L2 is mainly expressed in
hematopoietic cells. The binding of PD-1 to PD-L1/2 inhibits the
death of tumor cells and promotes the conversion of T-effector
cells into Tregs, while also inducing exhaustion in peripheral
T-effector cells, as illustrated in Fig.
4 (27,28).
PD-1 and/or PD-L1 are also expressed on cells, such
as NK cells, monocytes and dendritic cells (27,28). The
PD-1 pathway operates through various mechanisms, such as reducing
the activity of T-cells during an inflammatory response, increasing
the proliferation and suppressive activity of Tregs, and reducing
the lytic activity of B-cells and NK cells (29).
The affinity between PD-1 and PD-L1 is 3-fold
stronger than the affinity between PD-1 and PD-L2. When PD-L1 binds
with PD-1 on T-cells, it results in T-cell exhaustion, dysfunction,
neutralization and the production of IL-10 within the tumor mass.
This process allows tumors that overexpress PD-L1 to protect
themselves from being attacked and killed by CD8+
cytotoxic T-cells (30).
Pro-effector cytokines, namely IL-12 and IFN-γ, can
upregulate the expression of PD-1 and PD-L1/L2, which helps to
prevent excessive T-effector cell activity. It is worth noting that
PD-L1 has also been shown to inhibit CD80, indicating the existence
of complex interactions between CTLA-4, PD-1 and other pathways
(31,32).
The PD-1 and PD-L1 antibody inhibitors were created
with the aim of preventing the PD-1 or PD-L1 side from functioning,
reactivating T-cells and promoting an immune response against
cancer cells (13).
Based on promising results from clinical trials,
antibodies that inhibit PD-1 (such as pembrolizumab, nivolumab-IgG4
fully humanized and dostarlimab), as well as those that inhibit
PD-L1 (such as avelumab, atezolizumab, and durvalumab) are being
evaluated for use in melanoma cases and various other malignancies
(NCT04020809, NCT04274816, NCT03313206, NCT03842943 and
NCT05928962). However, it is not yet known which inhibitor, PD-1 or
PD-L1, is more efficient (13). Of
these, nivolumab and pembrolizumab are the two major FDA-approved
anti-PD-1 monoclonal antibodies available for the treatment of
advanced and metastatic melanomas.
Melanoma cells exhibit increased levels of PD-L1,
which promotes the apoptosis of the likewise increased levels of
T-cells (33). It has also been
found that the circulating melanoma antigen-specific T-cells and
tumor-infiltrating lymphocytes express PD-1 abnormally. It is
considered that melanoma cells are capable of initiating, as well
as sustaining PD-1 signals, T-cell fatigue and dysfunction
(33). Hence, by blocking PD-1 in
patients with melanoma, one could possibly restore abnormal
activation and signaling and eventually recover the immune effect.
Pembrolizumab or lambrolizumab were used in unresectable or
metastatic melanoma in the study by Hamid et al (34), in an aim to elucidate the effects of
PD-1 medications in melanoma.
However, due to the heterogeneous nature of tumors,
the expression of PD-L1 is not uniform throughout. The extent of
PD-L1 expression can differ in various locations within the tumor,
resulting in varying levels of PD-L1 in immunohistochemical
staining. Moreover, the effectiveness of PD-L1/PD-1 inhibitors can
also be influenced by several other factors, such as the type of
cancer, the patient's immune system and the genetic profile of the
tumor. Thus, a more in-depth understanding of these factors is
essential for developing effective treatment strategies that
consider the heterogeneity of tumors and the variability in PD-L1
expression (35).
The combination ICI therapy used in the treatment of
patients with metastatic melanoma primarily involves CTLA-1 and
PD-1 inhibitors. This amplified the inhibitions that can be
simultaneously intervened during different phases of the
interaction among melanoma cells and the immune system. This, for
example, includes anti-CTLA-4 inhibiting the priming stage at the
same time anti-PD-1 inhibits the effector stage (36,37). It
has also been noted that the use of anti-CTLA-4 inhibitors results
in an increased expression of PD-1; hence, using combination
therapy results in a more robust treatment response in patients
with melanoma (38).
An increased understanding of immunological
mechanisms has led to the identification of additional potential
targets for checkpoint inhibition in the treatment of cancer. Some
of these potential targets include BTLA, VISTA, TIM-3, CD47 and
LAG-3. i) The blockade of BTLA has been shown to enhance New York
esophageal squamous cell carcinoma 1) specific CD8+
T-cell function and enhance the efficacy of anti-PD-1 (39-41).
ii) VISTA blockade has been shown to increase T-cell infiltration
and function in tumors, thereby reducing tumor growth (10,42).
iii) TIM-3 blockade causes T-helper-1 cell hyperproliferation and
cytokine release, leading to tumor shrinkage in a mouse model when
combined with anti-CTLA-4 or anti-PD-1 (43-47).
iv) Targeting CD47 with a humanized anti-CD47 monoclonal antibody
in combination with rituximab has shown to lead to objective
responses in half of the heavily pretreated patients with relapsed
or refractory non-Hodgkin's lymphoma, including a complete response
in more than one-third of patients (48). v) An immune pathway known as LAG-3
has been identified as a potential complement to the PD-1/PDL1
pathway in enhancing the immune response against cancer. LAG-3 is
an immune checkpoint receptor that regulates the function of
T-cells. BMS-986016 is a therapy that targets LAG-3 and is
currently under investigation in combination with nivolumab, which
targets PD-1, to enhance the immune response against cancer cells.
The combination of these two therapies has the potential to create
a synergistic effect, leading to improved treatment outcomes for
patients with cancer (49).
The ability of anti-CTLA-4 and anti-PD-1/PD-L1
monoclonal antibodies to target various T-cell activation locations
and phases is the rationale for their combined use. PD-1 is
primarily expressed on antigen-experienced T-cells in peripheral
tissues, while CTLA-4 is expressed by naive T-cells in the lymph
nodes. According to pre-clinical research, combining ICIs is more
effective than treatment with with monotherapy for managing
melanoma (50-53).
In pre-clinical investigations, anti-CTLA-4 and
anti-PD-1/PD-L1 monoclonal antibodies have been shown to induce the
infiltration of CD8+ T-cells and the expansion of an
inducible T-cell co-stimulator (ICOS)+ T helper 1-like
CD4 fraction, which in turn induces the response of CD4+
effector T-cells. Based on this, the sequencing or combination of
nivolumab with ipilimumab in metastatic cutaneous melanoma has been
researched (50-53).
Other combination studies are on nivolumab, relatlimab and
combination therapy with pembrolizumab with low-dose ipilimumab
(54,55). The data from the CheckMate and
RELATIVITIY047 trials on the combination of ICIs are presented in
Table I, which demonstrate a
favorable response for such therapies (54,56,57).
Table II presents data from a
meta-analysis, comparing monotherapy and combination therapy
(47-64).
Along with the major breakthrough in melanoma
treatment with the use of selective BRAF inhibitors, after ~6
months of the median duration, resistance to therapy began to
develop. The BRAF mutation drives the tumor proliferation
exponentially by activating mitogen-activated kinase pathway (MAP),
and the development of resistance to BRAF inhibitors in both MAP
kinase-dependent and MAP kinase-independent pathways (65-67).
Resistance in MAP kinase-dependent pathways includes secondary
mutations in NRAS, the increased expression of COT kinase, CRAF
activation and acquired mutations in MEK1 (65,68-71).
MAP kinase-independent pathways include the upregulation of
platelet-derived growth factor receptor, additional receptor
tyrosine kinases activation including AXL, Erb-B2 receptor tyrosine
kinase 4) and insulin like growth factor 1 receptor, the activation
of PI3K/AKT signaling, and the loss of phosphatase and tensin
homolog (PTEN) (65,69,71-75).
Ipilimumab is a fully human monoclonal antibody
developed to antagonize CTLA-4. A clinical study was conducted on
patients who had unresectable stage III or IV melanoma, where they
were randomly assigned in a 3:1:1 ratio to receive ipilimumab (3
mg/kg) plus the glycoprotein 100 (gp100) vaccine, ipilimumab alone,
or gp100 alone. The patients who received ipilimumab plus gp100 had
a longer median overall survival rate of 10 months compared to 6.4
months for those who received gp100 alone, with a hazard ratio (HR)
for mortality of 0.68 and a statistically significant P-value of
<0.001. The median overall survival rate of patients who
received ipilimumab alone was 10.1 months (HR, 0.66; P=0.003)
compared to those who received gp100 alone (20).
Nivolumab is a monoclonal antibody that inhibits the
interaction of PD-1 with PD-L1. The clinical study performed by
Robert et al (79) compared
the efficacy of nivolumab with the standard therapy of dacarbazine.
In their study, patients who had metastatic melanoma without a BRAF
mutation were randomly divided into two groups (1:1 with nivolumab
at 3 mg/kg once every 2 weeks (n=210) and dacarbazine (n=208). The
survival rate at 1 year was 72.9% in patients treated with
nivolumab compared to 42.1% in patients who were assigned
dacarbazine (HR, 0.42, P<0.001). The objective response rate was
40% with nivolumab compared to 13.9% with dacarbazine (odds ratio,
4.06; P<0.001) (79). Another
randomized controlled trial was carried out between 2012-2014 on
patients with advanced melanoma who progressed after ipilimumab
therapy or a combination of ipilimumab and a BRAF inhibitor if they
were found to be positive for a V600E mutation (80). That study assessed the role of
nivolumab as a second-line treatment in the management of patients
with advanced melanoma. Patients were divided into three groups in
a 2:1 pattern where one group (n=272) received nivolumab at 3 mg/kg
once every 2 weeks and another group (n=133) received the
investigator's choice of chemotherapy (ICC), which was either
dacarbazine or paclitaxel plus carboplatin (80). An interim analysis of that study
found that, in the first 120 patients of the nivolumab group, 38
patients (31.7%) experienced confirmed objective responses, whereas
only 5 out of the 47 patients (10.8%) receiving the ICC treatment
exhibited similar responses. Subsequently, upon further analysis of
that trial, it was revealed that the median overall survival rate
of patients who received nivolumab was 16 months, while for those
who received ICC, it was 14 months (81). The HR was 0.95, indicating that
nivolumab did not improve the survival rate of patients who had
ipilimumab-refractory metastatic melanoma when compared to ICC.
However, nivolumab had a higher overall response rate of 27% vs.
10% for ICC, and the median duration of response was also longer
for nivolumab at 32 months compared to 13 months for ICC (77). Hence, nivolumab exhibiting a higher
overall response rate and a longer duration of response suggests
that it may be a more effective treatment option for some patients
(81). Another study was also
carried out to compare the efficacy of nivolumab compared to
ipilimumab as an adjuvant therapy in patients who had resected
advanced melanoma. In patients with stage III or stage IV melanoma,
adjuvant therapy was administered with either nivolumab (n=453) or
ipilimumab (n=453) and follow-up was performed after 18 months
(82). The 12-month rate of
recurrence-free survival was significantly higher in the nivolumab
group at 70.5%, vs. 60.8% in the ipilimumab group with a HR of 0.65
(P<0.001). It was also noted that treatment-related adverse
events were 14.4% for patients treated with nivolumab and 45.9% for
those treated with ipilimumab. Therefore, patients who received
ipilimumab therapy experienced more severe side-effects than those
who received nivolumab therapy. This suggests that nivolumab may be
a more effective and tolerable treatment option for patients with
stage IIIB, IIIC, or IV melanoma following surgical resection
(82).
Pembrolizumab is a monoclonal antibody which
functions by blocking the PD-1 on T-cells and allowing these
T-cells to identify and kill cancer cells. Similar to nivolumab,
pembrolizumab was also compared with ICC in ipilimumab-refractory
melanoma. A randomized controlled study was conducted on patients
with advanced melanoma and have progressed even after receiving
ipilimumab and/or standard BRAF therapy (83). Patients were divided into three
groups as follows: One group (n=181) received 10 mg/kg
pembrolizumab, one group (n=180) received 2 mg/kg pembrolizumab,
and another group (n=179) received ICC. The 6-month
progression-free survival rate was found to be 38% in patients
treated with pembrolizumab at 10 mg/kg (HR, 0.5 vs. ICC;
P<0.0001), 34% in the 2 mg/kg group (HR, 0.57 vs. ICC;
P<0.0001) and 16% in the ICC group (83). Another study was conducted by Robert
et al (84), this time
comparing pembrolizumab with ipilimumab. Patients with advanced
melanoma were divided at a 1:1:1 ratio to receive pembrolizumab at
10 mg/kg once every 2 weeks or pembrolizumab at 2 mg/kg once every
3 weeks or four doses of ipilimumab at 3 mg/kg for once every 3
weeks. An interim analysis was performed which revealed that the
6-month progression-free survival of the patients treated with
pembrolizumab once every 2 weeks was 47.3% (HR, 0.58 vs.
ipilimumab; P<0.001), 46.4% for those treated with pembrolizumab
once every 3 weeks (HR, 0.58 vs. ipilimumab; P<0.001) and 26.5%
for those treated with ipilimumab (84). A final analysis revealed that the
median overall survival rate was not reached in both pembrolizumab
groups; however, it was noted to be 16 months in the ipilimumab
group (HR, 0.68 for pembrolizumab once every 2 weeks vs.
ipilimumab, P=0.0009; and HR, 0.68 for pembrolizumab once every 3
weeks vs. ipilimumab, P=0.0008) (85). Similarly, in the study by Robert
et al (84) the 24-month
overall survival rate was 55% in the group treated once every 2
weeks, 55% in the group treated once every 3 weeks and 43% in the
ipilimumab group. Not only do nivolumab and pembrolizumab prolong
overall survival, but they also maintain the quality of life of
patients with melanoma (86,87). These findings have led to the FDA
approval of pembrolizumab for ipilimumab and/or BRAF inhibitory
refractory advanced melanoma. A summary of the comparison among
ICIs and their outcomes in patients is presented in Table III.
The prognostic marker for melanoma traditionally
used is the depth of invasion and the associated mitotic count of
the affected cells. With advancements being made, newer prognostic
markers have been found and used. Prognostic and predictive
biomarkers have gained importance, particularly in the treatment of
melanoma.
i) Tumor infiltrating lymphocyte (TIL) patterns are
often divided into grades, such as ‘absent’, which is no presence
of any lymphocytes within the tumor, ‘non-brisk’, which suggest few
foci of lymphocytes within the tumor, or ‘brisk’, which is a large
diffuse infiltration of lymphocytes within the tumor (107). As demonstrated by Clark et
al (107) in 1989, as well as
by others, the presence of brisk TILs in a vertical growth pattern
is often associated with a favorable disease-specific survival and
overall survival rate after non-brisk and absent patterns of TILs
(107,108).
ii) Histotype: The majority of melanoma histotypes
are not considered prognostic when looked at individually from
tumor thickness, and are therefore not included in the American
Joint Committee on Cancer staging system (90,109,110).
However, a nodular melanoma is an independent predictor which can
be used for the measurement of recurrence and its association with
mortality due to melanoma (111).
iii) Digital images trained from AI: New
advancements have allowed for the development of deep
learning-based biomarkers, which can help to stratify the stages of
melanoma into risk groups, and thus associate disease-specific
survival with two independent validating cohorts to accurately
predict the prognosis of patients with early-stage melanoma
(112).
iv) Melanoma cell adhesion molecule (MCAM):
Expressed in 80% of metastatic tumors, MCAM is a cell adhesion
marker (113). Those who are
positive for MCAM have significantly worse 5-year survival rates
than those who are negative for MCAM, and there is an inverse
association between the amount of marker expressed and survival
(114,115).
v) Ki-67: Ki-67 is a unique nuclear antigen that can
function as a marker for cellular proliferation during the active
phase of the cell cycle (116). For
melanomas who have a thickness <1 mm, the risk of metastasis
increases with the expression of Ki-67 and an increased mitotic
rate (117). However, with the
increasing thickness of melanomas, Ki-67 can serve as a more
effective prognostic marker than the mitotic rate, and is often
associated with ulceration within the tumor, necrosis, higher level
Clark's level of invasion, and even vascular invasion (118). In addition, with recurrent
melanomas, higher values of Ki-67 exhibit an independent
association with a decreased overall survival (119).
vi) Lymphatic invasion: In research on primary
melanomas with a thickness >1 mm, D2-40 staining was assessed
for lymphatic invasion, which is an antibody against
sialoglycoprotein that selectively attaches on endothelial cells of
lymphatic vessels and helps detect sentinel lymph node metastasis
(120-122).
vii) Osteopontin: Overexpressed in numerous visceral
malignancies, osteopontin is known as an integrin-binding protein
and used as a biomarker to measure tumor progress and metastasis
(123-125).
It functions as an independent predictor for the prognosis of
melanoma and was found to be associated with increased sentinel
lymph node positivity in a cohort of 345 patients who had primary
melanoma detected using immunohistochemical analysis (126).
viii) Driver mutations: It has been found that BRAF
and NRAS are associated with a significantly lower
melanoma-specific survival in high-risk tumors, such as a stage
>2(127). NF1 mutations has also
been found to be associated with a lower disease-specific survival
and overall survival (128).
However, further research is required to identify patients with
BRAF mutations and uncover the role of BRAF mutations in directing
the treatment strategy.
Therapy with nivolumab affects the frequencies of
innate lymphoid cells (ILCs) in peripheral blood in patients with
melanoma. The frequency, as well as the secretory activity of ILC
subsets, particularly ILC2s, are affected by treatment. Albeit
nivolumab was found to not effectively alter serum cytokine
profiles, pro-inflammatory and angiogenic substances such as IL-1,
IL-6, CCL2, CXCL8 and VEGF had levels outside the normal range in 7
of the 18 cytokines. In addition, the production of IL-5 and IL-13
was affected, which are released during parasite infections and
allergic reactions (152). In
malignant melanoma, type 3 ILC is suspected in tumor suppression
(153). Serum levels of IL-6, CXCL8
and CCL2 in particular, surge during melanoma progression, while
mature NKp44+ ILC3s protect against melanoma (154).
As previously demonstrated, the advancement of
melanoma was comparable with aging, although the treatment outcome
did not differ significantly, and there was no significant change
in the survival outcomes of elderly patients as compared to young
ones. Moreover, it was recommended that both age groups should be
treated in similar manner (155).
Primary and secondary resistance are also a key factor affecting
drug use (84,156). Combination therapy with ipilimumab
and nivolumab, as approved by the FDA, has been proven to be
efficient (157). There is an
increased incidence of melanoma among women of reproductive age. As
opposed to this, postmenopausal women have a relatively low
incidence of the disease, thus raising the possibility that sex
hormones such as estrogen may be involved in the growth of the
disease (158). As a result,
estrogen levels should be considered an important biomarker in
advanced melanoma. Elderly patients aged ≥65 treated with
combination therapy comprising of ipilimumab and nivolumab have not
exhibited a considerable difference in overall mortality. When
prior exposure to ipilimumab is considered, women have a 2.82-fold
increased risk of mortality as compared to prior-exposed males with
ipilimumab (158).
Moderate colitis which does not require the use of
intravenous steroids is consistent with an improved overall
survival of patients with stage IV melanoma when treated with a
single anti-CTLA-4 drug, but not with combination drugs. This holds
true even after the completion of therapy (159). Multiple nonrandomized studies have
shown excellent results in patients who discontinue treatment after
being treated for 1-2 years and disease progression is also
uncommon in the following 2-5 years of treatment termination
(160-162).
This is in contrast to the progression of disease of patients with
non-small cell lung cancer, for whom treatment continuation led to
improved results compared to treatment termination (163).
The use of PD-1 inhibitors, namely nivolumab and
pembrolizumab, and the anti-CTLA-4 drug, ipilimumab, has been shown
to be associated with a steady regression in malignancies,
including metastatic melanoma (164).
PD-1 inhibitors function in the tumor setting, while
CTLA-4 inhibitors act on lymphoid tissue, resulting in a wide and
different set of adverse events (165). Combination therapies with nivolumab
and ipilimumab have been proven to be more effective with a
response rate of 59% as compared to when used alone, with response
rate of 43% for nivolumab and 15-20% for ipilimumab. Moreover, an
increased response rate is associated with an increase in adverse
events, resulting in an overall increase in adverse events with the
combination of nivolumab with ipilimumab, as compared to nivolumab
or ipilimumab monotherapy (36,62).
A CTLA-4 blockade with or without anti-PD-1 antibody
produces adverse events in a dose-dependent manner (166,167).
Considering that older patients are more inclined to develop
rheumatologic events and female patients are also at an increased
risk, the toxicity profile may vary according to age and sex
(168,169).
However, as these molecules are targeted, due to the
resulting immune response, an increase in the incidence of
autoimmune conditions is observed; these adverse events are known
as immune-related adverse events (irAE). If severe irAEs occur with
one of the drugs, then it is a safe practice to re-challenge the
patient with a different class of drug (165). These drugs have the following on
the following systems.
In decreasing order, the first endocrine system that
is most affected by ICIs is the thyroid gland (typically
hypothyroidism observed following a transient thyroiditis-induced
thyrotoxicosis) followed by the rest of the endocrine organs. The
median time frame from the start of the treatment to the
development of thyroid symptoms, most commonly hypothyroidism, is 6
weeks, followed by pituitary (hypophysitis), adrenals (primary
adrenal insufficiency) and β-cells of the pancreas (insulin
deficient diabetes, analogous to type 1 diabetes) (170). These are different from the
side-effects brought on by conventional cytotoxic chemotherapy or
even more recent molecular-targeted medicines, which infrequently
result in endocrine dysfunction (171).
Non-specific adverse events such as maculopapular
rash, pruritus, psoriasiform, eczematous and lichenoid dermatosis
are among the most prevalent (172,173).
Compared to anti-PD-1 monotherapy, the maculopapular rash phenotype
is more prevalent when CTLA-4 inhibition is implemented (21). Bullous pemphigoid, vitiligo-like skin
hypopigmentation/depigmentation and alopecia are other less-common
irCAEs (174,175). Although severe reactions, such as
Stevens-Johnson syndrome, toxic epidermal necrolysis and drug
reaction with eosinophilia and systemic symptoms are uncommon,
cutaneous consequences are typically self-limiting (174-176).
Early diagnosis and the administration of corticosteroids or
antitumor necrosis factor-agents are the foundation of treatment
algorithms for irCAEs (176,177).
However, the use of corticosteroids before or after ICI initiation
may result in a diminished antitumor efficacy. Anti-CTLA-4 and
anti-PD1 therapy have both been associated with reports of vitiligo
(178). The occurrence of skin
hypopigmentation or depigmentation such as vitiligo has been linked
to an extensive anticancer benefit from drug treatment in patients
with melanoma. Vitiligo has been proven as a positive predictive
factor in measuring the tumor response to treatment. In comparison
with the general population, patients with melanoma have a 10-fold
increased incidence of drug-related cutaneous hypopigmentation and
depigmentation (179). Since the
PD-L1:PD1 pathway mostly regulates the peripheral tolerance of
melanosomal proteins (such as tyrosinase and TRP-2), the
interference of PD-1 signaling may result in autoimmune vitiligo
(180). This offers a reasonable
explanation for the onset and durability of depigmentation in
patients receiving immunotherapy.
Case series studies have demonstrated that patients
develop organizing pneumonia, diffuse alveolar damage, acute
respiratory distress syndrome (ARDS) and non-specific interstitial
pneumonia, which is then managed by intravenous and oral steroids
(181-185).
A previous meta-analysis revealed adverse effects
associated with the use of anti-PD-1/PD-L1 monoclonal antibodies
for malignancies with an increased incidence of pancreatitis, and
increased levels of liver enzymes, such as aspartate
aminotransferase and alanine transaminase, elevated creatinine
levels, nephritis and renal failure (164).
A myriad of ongoing clinical trials and practices
have discovered multiple mechanisms leading to resistance to ICIs.
More precisely, these include changes in the tumor microenvironment
prohibiting T-cell interaction, tumor invasion and tumor cell
destruction by effector mechanisms. The key to tumor cell
destruction via effector T-cells is through the processing of tumor
antigens to antigen-presenting cells. The failure of
antigen-presenting components in this pathway is a major cause of
resistance in melanoma (186). β2
microglobulin is an key molecule responsible for the folding and
transportation of major histocompatibility complex-1 to the surface
of cells. Mutations in these molecules have been noted in patients
with melanoma at the time of anti-PD1 treatment failure (187). Other mechanisms responsible for
limiting T-cell trafficking in the tumor microenvironment include
mutations in BRAF, and the inhibition of PTEN. This leads to the
increased expression of immunosuppressive molecules, such as VEGF
(188). It also inhibits the
migration and trafficking of effector T-cells (189). In addition to these tumor-intrinsic
mechanisms, various tumor-extrinsic mechanisms also play a role in
the development of resistance to ICIs. These include the
development of new inhibitory checkpoints, immunosuppressive
cytokines and molecules in the tumor microenvironment suppressing
immune cell function. One such example is the production of
transforming growth factor β (TGF-β) by tumor cells. TGF-β is an
immunosuppressive cytokine that functions by stimulating Tregs and
inhibiting the cytotoxicity of effector T-cells (190).
To summarize, understanding and investigating the
potential mechanisms that lead to resistance to ICIs is crucial in
developing effective strategies to guide therapy. Further studies
are required to identify new mechanisms and develop targeted
therapies to improve the clinical outcome of patients undertaking
immunotherapy.
Tumor cells sustain themselves by utilizing altered
metabolic pathways by using nutrients, such as glucose, tryptophan
and arginine to produce toxic metabolites such as adenosine,
lactate and kynurenine (191,192).
Such toxic metabolites produce an unfavorable environment for the
antitumor cells to function resulting in increased expression of
immune checkpoints and expansion of Tregs (193).
The mechanism that tumor cells use is the mutation
in the myelocytomatosis oncogene (MYC) and PI3K/AKT/mammalian
target of rapamycin (mTOR) signaling pathways. The increased
expression of hypoxia-inducible factor-1-α leads to the
overexpression of the PI3K/AKT/mTOR pathway, as well as glucose
transporters such as glucose transporter 1, leading to increased
glucose consumption and acidification of the tumor microenvironment
(194,195). As hypoxia is generated, glucose
depletion occurs and increased toxic waste is produced within the
tumor microenvironment, resulting in the inhibition of tumor
antigen presentation by APCs (196). Thus, there is an overall decrease
in the antitumor immune response by T-effector, macrophages or NK
cells, while pro-tumor immune cells such as Tregs proliferate to
increase the expression of inhibitory checkpoint ligand PD-1 on
immune cells, inhibiting the antitumor immunity (197). With the advancement of
technologies, newer therapeutic strategies that target the
immunosuppressive tumor microenvironment generated by tumor cells
may be developed to reprogram the behavior of immune cells, leading
to an improved efficacy in terms of the treatment response.
One of the important T-cellular processes is the
activation of the PI3K pathway, which plays a vital role in
proliferation and differentiation. Monotherapy, which inhibits the
PI3K pathway, has not yielded any significant results in the
treatment of cancer; however, combining PI3K inhibitors and the
PD-1-PDL1 blockade has shown some notable results (198). The loss of PTEN, which is a
PI3K-inhibiting tumor suppressor often mutated in tumor cells,
results in the uncontrolled growth of tumor cells and escapes the
immune destruction imposed on it. As previously demonstrated, when
mice with PTEN-null melanoma were treated in vivo with the
PI3Kβ inhibitor, GSK2636771, this resulted in a decreased AKT
phosphorylation and the activation of mTOR targets. Additionally,
when it was combined with an anti-PD1 antibody, it markedly
improved the survival and increased immune response with reduced
tumor cell mass (199). With such
promising results, a number of newer anti-PI3K medicines are being
developed and tested to increase efficacy (NCT01390818). Despite
this, more novel promising approaches are needed to prove the
success of combining anti-PI3K drugs with ICIs in the treatment of
melanoma (200).
The development of ICIs and targeted therapies has
played a crucial role in revolutionizing the management of
melanomas by improving the overall and progression-free survival.
Although both of these therapies have advantages and disadvantages,
combination therapy (ICI + ICI, or ICI + targeted therapies) has
been found to be more effective in improving patient outcomes.
However, there is limited literature available regarding
combination therapies and different types of potential
combinations. There are also insufficient data on patients and
their responses to draw sufficient conclusions. The development of
drug-related adverse effects with the use of combination therapies
is also a debatable question. However, when developing newer ICIs
to achieve a more effective response, a focus should certainly be
placed on the integration of nanotechnology or antibody
engineering. Through these, one can increase drug delivery to a
specific target and thus increase overall response. In addition,
focusing on epigenetic modulation and developing ICIs that target
those changes can enhance the responsiveness of ICIs.
There may be concerns regarding resistance to ICIs
in patients with melanoma. Some patients may have resistance to
certain ICIs from the beginning or may develop them as an acquired
resistance with subsequent treatment after progression of a tumor
with clinical benefit. Further treatment decisions shall be made on
the basis of evaluation of the tumor and factors related to the
patient, focusing on targeted therapeutic drugs, other
immunotherapy drugs, cellular therapies, intralesional therapies,
or chemotherapy. It is important to tailor ICI treatment based on
an individual's genetic makeup and tumor characteristics to
decrease the resistance. The early identification of tumor
biomarkers can predict future responses to particular ICIs and may
help to select a personalized treatment strategy. Furthermore, with
the use of tumor metabolic pathway inhibitors in combination with
ICIs, targeting signaling pathways and immune responses can be
better used to overcome potential resistance to ICIs than when used
alone.
Trials are being conducted on newer inhibitory
immune checkpoint targets, as well as certain inhibitory targets
beyond immune checkpoints. These include LAF-3, TIM-3, B7-H3 and
B7-H4, CD73, etc. which are immune checkpoints, and CEACAM1,
CEACAM5/6, CCL2/CCR2, etc. which are other inhibitory targets
(201). It is essential to maintain
enrollment in clinical trials so that newer ICIs, additional
inhibitory treatments, combination therapy, and mechanisms of
resistance and methods of overcoming the resistance can all be
further investigated.
In conclusion, with the increasing incidence of
melanoma over the past two decades, managing it with different
treatment modalities has become cumbersome. With the limited
effectiveness of the traditional approach using chemotherapy and
immunotherapy, the role of newer treatment modalities should be
given equal emphasis. Novel approaches using ICIs have been a
revolution in the therapeutic approach by unleashing the immune
system's ability to recognize and eliminate cancer cells.
Ipilimumab, nivolumab and pembrolizumab have been shown to lead to
a substantial improvement in the overall survival of patients with
advanced melanoma, particularly in high-risk metastatic melanoma
compared to traditional therapies. However, with ICIs, it is
paramount to monitor any side-effects, and to ensure the optimal
outcome is achieved using personalized treatment approaches.
Not applicable.
Funding: No funding was received.
Not applicable.
VS, VP and AS were involved in the conceptualization
of the study. VS, VP, AS, BV, SB and SA were involved in the
curation of data from the literature for inclusion in the present
review. VS, VP and AS were involved in selecting the relevant
literature. VS, VP and AS were involved in project administration.
VS supervised the study. VS, VP, AS, BV, SB and SA were involved in
the writing of the original draft. VS, VP and AS were involved in
the writing, review and editing of the manuscript. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Finn L, Markovic SN and Joseph RW: Therapy
for metastatic melanoma: The past, present, and future. BMC Med.
10(23)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arnold M, Singh D, Laversanne M, Vignat J,
Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC and Bray
F: Global burden of cutaneous melanoma in 2020 and projections to
2040. JAMA Dermatol. 158:495–503. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosenberg SA, Lotze MT, Yang JC, Topalian
SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan
WM, Seipp CA, et al: Prospective randomized trial of high-dose
interleukin-2 alone or in conjunction with lymphokine-activated
killer cells for the treatment of patients with advanced cancer. J
Natl Cancer Inst. 85:622–632. 1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bronte V and Mocellin S: Suppressive
influences in the immune response to cancer. J Immunother. 32:1–11.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okazaki T, Okazaki IM, Wang J, Sugiura D,
Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, et
al: PD-1 and LAG-3 inhibitory co-receptors act synergistically to
prevent autoimmunity in mice. J Exp Med. 208:395–407.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fourcade J, Sun Z, Pagliano O, Chauvin JM,
Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S,
et al: PD-1 and Tim-3 regulate the expansion of tumor
antigen-specific CD8+ T cells induced by melanoma
vaccines. Cancer Res. 74:1045–1055. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lines JL, Pantazi E, Mak J, Sempere LF,
Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS
and Noelle R: VISTA is an immune checkpoint molecule for human
T-cells. Cancer Res. 74:1924–1932. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hanaizi Z, van Zwieten-Boot B, Calvo G,
Lopez AS, van Dartel M, Camarero J, Abadie E and Pignatti F: The
European medicines agency review of ipilimumab (Yervoy) for the
treatment of advanced (unresectable or metastatic) melanoma in
adults who have received prior therapy: Summary of the scientific
assessment of the committee for medicinal products for human use.
Eur J Cancer. 48:237–242. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tarhini AA: Tremelimumab: A review of
development to date in solid tumors. Immunotherapy. 5:215–229.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang D, Wang T, Liu J, Yu H, Jiao S, Feng
B, Zhou F, Fu Y, Yin Q, Zhang P, et al: Acid-activatable versatile
micelleplexes for PD-L1 blockade-enhanced cancer photodynamic
immunotherapy. Nano Lett. 16:5503–5513. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ottaviano M, De Placido S and Ascierto PA:
Recent success and limitations of immune checkpoint inhibitors for
cancer: A lesson from melanoma. Virchows Arch. 474:421–432.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chambers CA, Sullivan TJ and Allison JP:
Lymphoproliferation in CTLA-4-deficient mice is mediated by
costimulation-dependent activation of CD4+ T-cells.
Immunity. 7:885–895. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tivol EA, Borriello F, Schweitzer AN,
Lynch WP, Bluestone JA and Sharpe AH: Loss of CTLA-4 leads to
massive lymphoproliferation and fatal multiorgan tissue
destruction, revealing a critical negative regulatory role of
CTLA-4. Immunity. 3:541–547. 1995.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Waterhouse P, Penninger JM, Timms E,
Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW:
Lymphoproliferative disorders with early lethality in mice
deficient in Ctla-4. Science. 270:985–988. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Walker LSK and Sansom DM: The emerging
role of CTLA4 as a cell-extrinsic regulator of T cell responses.
Nat Rev Immunol. 11:852–863. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ménard C, Ghiringhelli F, Roux S, Chaput
N, Mateus C, Grohmann U, Caillat-Zucman S, Zitvogel L and Robert C:
Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells
in advanced melanoma: Surrogate marker of efficacy of tremelimumab?
Clin Cancer Res. 14:5242–5249. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Phan GQ, Yang JC, Sherry RM, Hwu P,
Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA,
Freezer LJ, et al: Cancer regression and autoimmunity induced by
cytotoxic T lymphocyte-associated antigen 4 blockade in patients
with metastatic melanoma. Proc Natl Acad Sci USA. 100:8372–8377.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malek TR and Castro I: Interleukin-2
receptor signaling: At the interface between tolerance and
immunity. Immunity. 33:153–165. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reuben JM, Lee BN, Li C, Gomez-Navarro J,
Bozon VA, Parker CA, Hernandez IM, Gutierrez C, Lopez-Berestein G
and Camacho LH: Biologic and immunomodulatory events after CTLA-4
blockade with ticilimumab in patients with advanced malignant
melanoma. Cancer. 106:2437–2444. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ribas A, Comin-Anduix B, Economou JS,
Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB,
Shintaku IP, Glaspy JA, et al: Intratumoral immune cell
infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients
with melanoma undergoing CTLA4 blockade. Clin Cancer Res.
15:390–399. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Amarnath S, Mangus CW, Wang JCM, Wei F, He
A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, et al:
The PDL1-PD1 axis converts human TH1 cells into regulatory T-cells.
Sci Transl Med. 3(111ra120)2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5(200ra116)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun Z, Fourcade J, Pagliano O, Chauvin JM,
Sander C, Kirkwood JM and Zarour HM: IL10 and PD-1 cooperate to
limit the activity of tumor-specific CD8+ T cells.
Cancer Res. 75:1635–1644. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kinter AL, Godbout EJ, McNally JP, Sereti
I, Roby GA, O'Shea MA and Fauci AS: The common gamma-chain
cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of
programmed death-1 and its ligands. J Immunol. 181:6738–6746.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang J, Riella LV, Chock S, Liu T, Zhao X,
Yuan X, Paterson AM, Watanabe T, Vanguri V, Yagita H, et al: The
novel costimulatory programmed death ligand 1/B7.1 pathway is
functional in inhibiting alloimmune responses in vivo. J Immunol.
187:1113–1119. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Krönig H, Julia Falchner K, Odendahl M,
Brackertz B, Conrad H, Muck D, Hein R, Blank C, Peschel C, Haller
B, et al: PD-1 expression on Melan-A-reactive T cells increases
during progression to metastatic disease. Int J Cancer.
130:2327–2336. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Marconcini R, Spagnolo F, Stucci LS,
Ribero S, Marra E, Rosa F, Picasso V, Di Guardo L, Cimminiello C,
Cavalieri S, et al: Current status and perspectives in
immunotherapy for metastatic melanoma. Oncotarget. 9:12452–12470.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Watanabe N, Gavrieli M, Sedy JR, Yang J,
Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et
al: BTLA is a lymphocyte inhibitory receptor with similarities to
CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Murphy KM, Nelson CA and Sedý JR:
Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev
Immunol. 6:671–681. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fourcade J, Sun Z, Pagliano O, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V and
Zarour HM: CD8(+) T cells specific for tumor antigens can be
rendered dysfunctional by the tumor microenvironment through
upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res.
72:887–896. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Le Mercier I, Chen W, Lines JL, Day M, Li
J, Sergent P, Noelle RJ and Wang L: VISTA regulates the development
of protective antitumor immunity. Cancer Res. 74:1933–1944.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein Tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Anderson AC, Anderson DE, Bregoli L,
Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW,
Hirashima M, et al: Promotion of tissue inflammation by the immune
receptor Tim-3 expressed on innate immune cells. Science.
318:1141–1143. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
46
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Ngiow SF, von Scheidt B, Akiba H, Yagita
H, Teng MWL and Smyth MJ: Anti-TIM3 antibody promotes T cell
IFN-γ-mediated antitumor immunity and suppresses established
tumors. Cancer Res. 71:3540–3551. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ascierto PA, Melero I, Bhatia S, Bono P,
Sanborn RE, Lipson EJ, Callahan MK, Gajewski T, Gomez-Roca CA, Hodi
FS, et al: Initial efficacy of anti-lymphocyte activation gene-3
(anti-LAG-3; BMS-986016) in combination with nivolumab (nivo) in
pts with melanoma (MEL) previously treated with anti-PD-1/PD-L1
therapy. J Clin Orthod. 35 (15 Suppl)(S9520)2017.
|
|
50
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie anti-CTLA-4 and
anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rotte A, Jin JY and Lemaire V: Mechanistic
overview of immune checkpoints to support the rational design of
their combinations in cancer immunotherapy. Ann Oncol. 29:71–83.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tarhini A: Immune-mediated adverse events
associated with ipilimumab ctla-4 blockade therapy: The underlying
mechanisms and clinical management. Scientifica (Cairo).
2013(857519)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tawbi HA, Schadendorf D, Lipson EJ,
Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas
HJ, Lao CD, De Menezes JJ, et al: Relatlimab and nivolumab versus
nivolumab in untreated advanced melanoma. N Engl J Med. 386:24–34.
2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Olson DJ, Eroglu Z, Brockstein B,
Poklepovic AS, Bajaj M, Babu S, Hallmeyer S, Velasco M, Lutzky J,
Higgs E, et al: Pembrolizumab plus ipilimumab following
anti-PD-1/L1 failure in melanoma. J Clin Oncol. 39:2647–2655.
2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Weber JS, Gibney G, Sullivan RJ, Sosman
JA, Slingluff CL Jr, Lawrence DP, Logan TF, Schuchter LM, Nair S,
Fecher L, et al: Sequential administration of nivolumab and
ipilimumab with a planned switch in patients with advanced melanoma
(CheckMate 064): An open-label, randomised, phase 2 trial. Lancet
Oncol. 17:943–955. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shoushtari AN, Wagstaff J, Ascierto PA,
Butler MO, Lao CD, Marquez-Rodas I, Chiarion-Sileni V, Dummer R,
Ferrucci PF, Lorigan P, et al: CheckMate 067: Long-term outcomes in
patients with mucosal melanoma. J Clin Orthod. 38 (15
Suppl)(S10019)2020.
|
|
58
|
Pradeep J, Win TT, Aye SN and
Sreeramareddy CT: Efficacy and safety of immune checkpoint
inhibitors for advanced malignant melanoma: A meta-analysis on
monotherapy vs combination therapy. J Cancer. 13:3091–3102.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Amaria RN, Reddy SM, Tawbi HA, Davies MA,
Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, et al:
Neoadjuvant immune checkpoint blockade in high-risk resectable
melanoma. Nat Med. 24:1649–1654. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hodi FS, Chesney J, Pavlick AC, Robert C,
Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK,
Agarwala SS, et al: Combined nivolumab and ipilimumab versus
ipilimumab alone in patients with advanced melanoma: 2-Year overall
survival outcomes in a multicentre, randomised, controlled, phase 2
trial. Lancet Oncol. 17:1558–1568. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hodi FS, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J,
Dummer R, et al: Nivolumab plus ipilimumab or nivolumab alone
versus ipilimumab alone in advanced melanoma (CheckMate 067):
4-Year outcomes of a multicentre, randomised, phase 3 trial. Lancet
Oncol. 19:1480–1492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Postow MA, Chesney J, Pavlick AC, Robert
C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK,
Agarwala SS, et al: Nivolumab and ipilimumab versus ipilimumab in
untreated melanoma. N Engl J Med. 372:2006–2017. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Long GV, Atkinson V, Lo S, Sandhu S,
Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer
RA, et al: Combination nivolumab and ipilimumab or nivolumab alone
in melanoma brain metastases: A multicentre randomised phase 2
study. Lancet Oncol. 19:672–681. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wagle N, Emery C, Berger MF, Davis MJ,
Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE,
Hahn WC, et al: Dissecting therapeutic resistance to RAF inhibition
in melanoma by tumor genomic profiling. J Clin Oncol. 29:3085–3096.
2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gorre ME, Mohammed M, Ellwood K, Hsu N,
Paquette R, Rao PN and Sawyers CL: Clinical resistance to STI-571
cancer therapy caused by BCR-ABL gene mutation or amplification.
Science. 293:876–880. 2001.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ellis LM and Hicklin DJ: Resistance to
targeted therapies: Refining anticancer therapy in the era of
molecular oncology. Clin Cancer Res. 15:7471–7478. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Nazarian R, Shi H, Wang Q, Kong X, Koya
RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al: Melanomas
acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS
upregulation. Nature. 468:973–977. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Johannessen CM, Boehm JS, Kim SY, Thomas
SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP,
Barretina J, et al: COT drives resistance to RAF inhibition through
MAP kinase pathway reactivation. Nature. 468:968–972.
2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Montagut C, Sharma SV, Shioda T, McDermott
U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A,
et al: Elevated CRAF as a potential mechanism of acquired
resistance to BRAF inhibition in melanoma. Cancer Res.
68:4853–4861. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Villanueva J, Vultur A, Lee JT,
Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu
X, Gimotty PA, Kee D, et al: Acquired resistance to BRAF inhibitors
mediated by a RAF kinase switch in melanoma can be overcome by
cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 18:683–695.
2010.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Turke AB, Zejnullahu K, Wu YL, Song Y,
Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L,
et al: Preexistence and clonal selection of MET amplification in
EGFR mutant NSCLC. Cancer Cell. 17:77–88. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043.
2007.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Guix M, Faber AC, Wang SE, Olivares MG,
Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL and Engelman
JA: Acquired resistance to EGFR tyrosine kinase inhibitors in
cancer cells is mediated by loss of IGF-binding proteins. J Clin
Invest. 118:2609–2619. 2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Paraiso KHT, Xiang Y, Rebecca VW, Abel EV,
Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, et
al: PTEN loss confers BRAF inhibitor resistance to melanoma cells
through the suppression of BIM expression. Cancer Res.
71:2750–2760. 2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Maio M, Grob JJ, Aamdal S, Bondarenko I,
Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT,
et al: Five-year survival rates for treatment-naive patients with
advanced melanoma who received ipilimumab plus dacarbazine in a
phase III trial. J Clin Oncol. 33:1191–1196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Eggermont AMM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Prolonged survival in stage III melanoma with
ipilimumab adjuvant therapy. N Engl J Med. 375:1845–1855.
2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ascierto PA, Del Vecchio M, Robert C,
Mackiewicz A, Chiarion-Sileni V, Arance A, Lebbé C, Bastholt L,
Hamid O, Rutkowski P, et al: Ipilimumab 10 mg/kg versus ipilimumab
3 mg/kg in patients with unresectable or metastatic melanoma: A
randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol.
18:611–622. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330.
2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Larkin J, Minor D, D'Angelo S, Neyns B,
Smylie M, Miller WH Jr, Gutzmer R, Linette G, Chmielowski B, Lao
CD, et al: Overall survival in patients with advanced melanoma who
received nivolumab versus investigator's choice chemotherapy in
CheckMate 037: A randomized, controlled, open-label phase III
trial. J Clin Oncol. 36:383–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: Adjuvant nivolumab versus ipilimumab in
resected stage III or IV melanoma. N Engl J Med. 377:1824–1835.
2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ribas A, Puzanov I, Dummer R, Schadendorf
D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD,
et al: Pembrolizumab versus investigator-choice chemotherapy for
ipilimumab-refractory melanoma (KEYNOTE-002): A randomised,
controlled, phase 2 trial. Lancet Oncol. 16:908–918.
2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Robert C, Ribas A, Schachter J, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006):
Post-hoc 5-year results from an open-label, multicentre,
randomised, controlled, phase 3 study. Lancet Oncol. 20:1239–1251.
2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Long GV, Atkinson V, Ascierto PA, Robert
C, Hassel JC, Rutkowski P, Savage KJ, Taylor F, Coon C, Gilloteau
I, et al: Effect of nivolumab on health-related quality of life in
patients with treatment-naïve advanced melanoma: Results from the
phase III CheckMate 066 study. Ann Oncol. 27:1940–1946.
2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Schadendorf D, Dummer R, Hauschild A,
Robert C, Hamid O, Daud A, van den Eertwegh A, Cranmer L, O'Day S,
Puzanov I, et al: Health-related quality of life in the randomised
KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients
with ipilimumab-refractory melanoma. Eur J Cancer. 67:46–54.
2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Nosrati A, Tsai KK, Goldinger SM, Tumeh P,
Grimes B, Loo K, Algazi AP, Nguyen-Kim TDL, Levesque M, Dummer R,
et al: Evaluation of clinicopathological factors in PD-1 response:
derivation and validation of a prediction scale for response to
PD-1 monotherapy. Br J Cancer. 116:1141–1147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhang Y, Liu B, Kotenko S and Li W:
Prognostic value of neutrophil-lymphocyte ratio and lactate
dehydrogenase in melanoma patients treated with immune checkpoint
inhibitors: A systematic review and meta-analysis. Medicine
(Baltimore). 101(e29536)2022.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: Evidence-based changes in the
American joint committee on cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Balch CM, Gershenwald JE, Soong SJ, Soong
SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit
DG, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Hauschild A, Engel G, Brenner W, Gläser R,
Mönig H, Henze E and Christophers E: S100B protein detection in
serum is a significant prognostic factor in metastatic melanoma.
Oncology. 56:338–344. 1999.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jury CS, McAllister EJ and MacKie RM:
Rising levels of serum S100 protein precede other evidence of
disease progression in patients with malignant melanoma. Br J
Dermatol. 143:269–274. 2000.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Mårtenson ED, Hansson LO, Nilsson B, von
Schoultz E, Månsson Brahme E, Ringborg U and Hansson J: Serum
S-100b protein as a prognostic marker in malignant cutaneous
melanoma. J Clin Oncol. 19:824–831. 2001.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Janka EA, Várvölgyi T, Sipos Z, Soós A,
Hegyi P, Kiss S, Dembrovszky F, Csupor D, Kéringer P, Pécsi D, et
al: Predictive performance of serum S100B versus LDH in melanoma
patients: A systematic review and meta-analysis. Front Oncol.
11(772165)2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Friedman RC, Farh KKH, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science.
299(1540)2003.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864.
2001.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Pfeffer SR, Grossmann KF, Cassidy PB, Yang
CH, Fan M, Kopelovich L, Leachman SA and Pfeffer LM: Detection of
exosomal miRNAs in the plasma of melanoma patients. J Clin Med Res.
4:2012–2027. 2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lin N, Zhou Y, Lian X and Tu Y: Expression
of microRNA-106b and its clinical significance in cutaneous
melanoma. Genet Mol Res. 14:16379–16385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Friedman EB, Shang S, de Miera EVS, Fog
JU, Teilum MW, Ma MW, Berman RS, Shapiro RL, Pavlick AC, Hernando
E, et al: Serum microRNAs as biomarkers for recurrence in melanoma.
J Transl Med. 10(155)2012.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wróblewska JP, Lach MS, Ustaszewski A,
Kulcenty K, Ibbs M, Jagiełło I, Suchorska WM and Marszałek A: The
potential role of selected miRNA in uveal melanoma primary tumors
as early biomarkers of disease progression. Genes (Basel).
11(271)2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Tsao SCH, Weiss J, Hudson C, Christophi C,
Cebon J, Behren A and Dobrovic A: Monitoring response to therapy in
melanoma by quantifying circulating tumour DNA with droplet digital
PCR for BRAF and NRAS mutations. Sci Rep. 5(11198)2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Girotti MR, Gremel G, Lee R, Galvani E,
Rothwell D, Viros A, Mandal AK, Lim KH, Saturno G, Furney SJ, et
al: Application of sequencing, liquid biopsies, and patient-derived
xenografts for personalized medicine in melanoma. Cancer Discov.
6:286–299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Clark WH Jr, Elder DE, Guerry D IV,
Braitman LE, Trock BJ, Schultz D, Synnestvedt M and Halpern AC:
Model predicting survival in stage I melanoma based on tumor
progression. J Natl Cancer Inst. 81:1893–1904. 1989.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Clemente CG, Mihm MC Jr, Bufalino R,
Zurrida S, Collini P and Cascinelli N: Prognostic value of tumor
infiltrating lymphocytes in the vertical growth phase of primary
cutaneous melanoma. Cancer. 77:1303–1310. 1996.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Mandalà M and Massi D: Tissue prognostic
biomarkers in primary cutaneous melanoma. Virchows Arch.
464:265–281. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Balch CM, Murad TM, Soong SJ, Ingalls AL,
Halpern NB and Maddox WA: A multifactorial analysis of melanoma:
Prognostic histopathological features comparing Clark's and
Breslow's staging methods. Ann Surg. 188:732–742. 1978.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Lattanzi M, Lee Y, Simpson D, Moran U,
Darvishian F, Kim RH, Hernando E, Polsky D, Hanniford D, Shapiro R,
et al: Primary melanoma histologic subtype: Impact on survival and
response to therapy. J Natl Cancer Inst. 111:180–188.
2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Robinson E, Kulkarni PM, Pradhan JS,
Gartrell RD, Yang C, Acs B, Rohr B, Phelps R, Ferringer T, Horst B,
et al: Prediction of distant melanoma recurrence from primary tumor
digital H&E images using deep learning. J Clin Orthod. 37 (15
Suppl)(S9577)2019.
|
|
113
|
Lehmann JM, Holzmann B, Breitbart EW,
Schmiegelow P, Riethmüller G and Johnson JP: Discrimination between
benign and malignant cells of melanocytic lineage by two novel
antigens, a glycoprotein with a molecular weight of 113,000 and a
protein with a molecular weight of 76,000. Cancer Res. 47:841–845.
1987.PubMed/NCBI
|
|
114
|
Lei X, Guan CW, Song Y and Wang H: The
multifaceted role of CD146/MCAM in the promotion of melanoma
progression. Cancer Cell Int. 15(3)2015.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Pacifico MD, Grover R, Richman PI, Daley
FM, Buffa F and Wilson GD: Development of a tissue array for
primary melanoma with long-term follow-up: Discovering melanoma
cell adhesion molecule as an important prognostic marker. Plast
Reconstr Surg. 115:367–375. 2005.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Weinstein D, Leininger J, Hamby C and
Safai B: Diagnostic and prognostic biomarkers in melanoma. J Clin
Aesthet Dermatol. 7:13–24. 2014.PubMed/NCBI
|
|
117
|
Gimotty PA, Van Belle P, Elder DE, Murry
T, Montone KT, Xu X, Hotz S, Raines S, Ming ME, Wahl P and Guerry
D: Biologic and prognostic significance of dermal Ki67 expression,
mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J
Clin Oncol. 23:8048–8056. 2005.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Ladstein RG, Bachmann IM, Straume O and
Akslen LA: Ki-67 expression is superior to mitotic count and novel
proliferation markers PHH3, MCM4 and mitosin as a prognostic factor
in thick cutaneous melanoma. BMC Cancer. 10(140)2010.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Tu TJ, Ma MW, Monni S, Rose AE, Yee H,
Darvishian F, Polsky D, Berman RS, Shapiro RL, Pavlick AC, et al: A
high proliferative index of recurrent melanoma is associated with
worse survival. Oncology. 80:181–187. 2011.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kahn HJ, Bailey D and Marks A: Monoclonal
antibody D2-40, a new marker of lymphatic endothelium, reacts with
Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol.
15:434–440. 2002.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Niakosari F, Kahn HJ, McCready D,
Ghazarian D, Rotstein LE, Marks A, Kiss A and From L: Lymphatic
invasion identified by monoclonal antibody D2-40, younger age, and
ulceration: Predictors of sentinel lymph node involvement in
primary cutaneous melanoma. Arch Dermatol. 144:462–467.
2008.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881.
2004.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Rudland PS, Platt-Higgins A, El-Tanani M,
Silva Rudland S, Barraclough R, Winstanley JH, Howitt R and West
CR: Prognostic significance of the metastasis-associated protein
osteopontin in human breast cancer. Cancer Res. 62:3417–3427.
2002.PubMed/NCBI
|
|
125
|
Pan HW, Ou YH, Peng SY, Liu SH, Lai PL,
Lee PH, Sheu JC, Chen CL and Hsu HC: Overexpression of osteopontin
is associated with intrahepatic metastasis, early recurrence, and
poorer prognosis of surgically resected hepatocellular carcinoma.
Cancer. 98:119–127. 2003.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Rangel J, Nosrati M, Torabian S, Shaikh L,
Leong SP, Haqq C, Miller JR II, Sagebiel RW and Kashani-Sabet M:
Osteopontin as a molecular prognostic marker for melanoma. Cancer.
112:144–150. 2008.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Thomas NE, Edmiston SN, Alexander A,
Groben PA, Parrish E, Kricker A, Armstrong BK, Anton-Culver H,
Gruber SB, From L, et al: Association between NRAS and BRAF
mutational status and melanoma-specific survival among patients
with higher-risk primary melanoma. JAMA Oncol. 1:359–368.
2015.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Cirenajwis H, Lauss M, Ekedahl H, Törngren
T, Kvist A, Saal LH, Olsson H, Staaf J, Carneiro A, Ingvar C, et
al: NF1-mutated melanoma tumors harbor distinct clinical and
biological characteristics. Mol Oncol. 11:438–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74.
2015.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Van Allen EM, Miao D, Schilling B, Shukla
SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger
SM, et al: Genomic correlates of response to CTLA-4 blockade in
metastatic melanoma. Science. 350:207–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Samstein RM, Lee CH, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Johnson DB, Lovly CM, Flavin M, Panageas
KS, Ayers GD, Zhao Z, Iams WT, Colgan M, DeNoble S, Terry CR, et
al: Impact of NRAS mutations for patients with advanced melanoma
treated with immune therapies. Cancer Immunol Res. 3:288–295.
2015.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Johnson DB, Bordeaux J, Kim JY, Vaupel C,
Rimm DL, Ho TH, Joseph RW, Daud AI, Conry RM, Gaughan EM, et al:
Quantitative spatial profiling of PD-1/PD-L1 interaction and
HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in
metastatic melanoma. Clin Cancer Res. 24:5250–2560. 2018.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Johnson DB, Estrada MV, Salgado R, Sanchez
V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS,
Greenplate AR, et al: Melanoma-specific MHC-II expression
represents a tumour-autonomous phenotype and predicts response to
anti-PD-1/PD-L1 therapy. Nat Commun. 7(10582)2016.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Rodig SJ, Gusenleitner D, Jackson DG,
Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C,
Weber JS, et al: MHC proteins confer differential sensitivity to
CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci
Transl Med. 10(eaar3342)2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Chowell D, Morris LGT, Grigg CM, Weber JK,
Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et
al: Patient HLA class I genotype influences cancer response to
checkpoint blockade immunotherapy. Science. 359:582–587.
2018.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Sanlorenzo M, Vujic I, Floris A, Novelli
M, Gammaitoni L, Giraudo L, Macagno M, Leuci V, Rotolo R, Donini C,
et al: BRAF and MEK inhibitors increase PD-1-positive melanoma
cells leading to a potential lymphocyte-independent synergism with
anti-PD-1 antibody. Clin Cancer Res. 24:3377–3385. 2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Eggermont AMM, Blank CU, Mandala M, Long
GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma. N Engl J Med. 378:1789–1801.
2018.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge
JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A
prospective, multi-institutional, pathologist-based assessment of 4
immunohistochemistry assays for PD-L1 expression in non-small cell
lung cancer. JAMA Oncol. 3:1051–1058. 2017.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Rizk EM, Gartrell RD, Barker LW, Esancy
CL, Finkel GG, Bordbar DD and Saenger YM: Prognostic and predictive
immunohistochemistry-based biomarkers in cancer and immunotherapy.
Hematol Oncol Clin North Am. 33:291–299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Harel M, Ortenberg R, Varanasi SK,
Mangalhara KC, Mardamshina M, Markovits E, Baruch EN, Tripple V,
Arama-Chayoth M, Greenberg E, et al: Proteomics of melanoma
response to immunotherapy reveals mitochondrial dependence. Cell.
179:236–250.e18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Zhou X, Yu S, Zhao DM, Harty JT, Badovinac
VP and Xue HH: Differentiation and persistence of memory CD8(+) T
cells depend on T cell factor 1. Immunity. 33:229–240.
2010.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Kratchmarov R, Magun AM and Reiner SL:
TCF1 expression marks self-renewing human CD8+ T cells.
Blood Adv. 2:1685–1690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative burst after
PD-1 therapy. Nature. 537:417–421. 2016.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Sade-Feldman M, Yizhak K, Bjorgaard SL,
Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT,
Barzily-Rokni M, et al: Defining T cell states associated with
response to checkpoint immunotherapy in melanoma. Cell.
175:998–1013.e20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA,
Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et
al: T-cell-inflamed gene-expression profile, programmed death
ligand 1 expression, and tumor mutational burden predict efficacy
in patients treated with pembrolizumab across 20 cancers:
KEYNOTE-028. J Clin Oncol. 37:318–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Ayers M, Lunceford J, Nebozhyn M, Murphy
E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran
V, et al: IFN-γ-related mRNA profile predicts clinical response to
PD-1 blockade. J Clin Invest. 127:2930–2940. 2017.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Pinato DJ, Howlett S, Ottaviani D, Urus H,
Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al:
Association of prior antibiotic treatment with survival and
response to immune checkpoint inhibitor therapy in patients with
cancer. JAMA Oncol. 5:1774–1778. 2019.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Yang H, Xia L, Chen J, Zhang S, Martin V,
Li Q, Lin S, Chen J, Calmette J, Lu M, et al:
Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced
antitumor immunity. Nat Med. 25:1428–1441. 2019.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Altan-Bonnet G and Mukherjee R:
Cytokine-mediated communication: A quantitative appraisal of immune
complexity. Nat Rev Immunol. 19:205–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Eisenring M, vom Berg J, Kristiansen G,
Saller E and Becher B: IL-12 initiates tumor rejection via lymphoid
tissue-inducer cells bearing the natural cytotoxicity receptor
NKp46. Nat Immunol. 11:1030–1038. 2010.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Cristiani CM, Capone M, Garofalo C,
Madonna G, Mallardo D, Tuffanelli M, Vanella V, Greco M, Foti DP,
Viglietto G, et al: Altered frequencies and functions of innate
lymphoid cells in melanoma patients are modulated by immune
checkpoints inhibitors. Front Immunol. 13(811131)2002.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Joshi K, Atwal D, Ravilla R, Pandey Y,
Yarlagadda N, Kakadia S, Makhoul I, Hutchins L and Mahmoud F:
Immunotherapy outcomes in advanced melanoma in relation to age.
Perm J. 24(19.093)2020.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Seidel JA, Otsuka A and Kabashima K:
Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of
action, efficacy, and limitations. Front Oncol.
8(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Jang SR, Nikita N, Banks J, Keith SW,
Johnson JM, Wilson M and Lu-Yao G: Association between sex and
immune checkpoint inhibitor outcomes for patients with melanoma.
JAMA Netw Open. 4(e2136823)2021.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Anstadt EJ, Chu B, Yegya-Raman N, Han X,
Doucette A, Poirier K, Mohiuddin JJ, Maity A, Facciabene A,
Amaravadi RK, et al: Moderate colitis not requiring intravenous
steroids is associated with improved survival in stage IV melanoma
after anti-CTLA4 monotherapy, but not combination therapy.
Oncologist. 27:799–808. 2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Jansen YJL, Rozeman EA, Mason R, Goldinger
SM, Geukes Foppen MH, Hoejberg L, Schmidt H, van Thienen JV, Haanen
JBAG, Tiainen L, et al: Discontinuation of anti-PD-1 antibody
therapy in the absence of disease progression or treatment limiting
toxicity: Clinical outcomes in advanced melanoma. Ann Oncol.
30:1154–1161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Robert C, Ribas A, Hamid O, Daud A,
Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW,
et al: Durable complete response after discontinuation of
pembrolizumab in patients with metastatic melanoma. J Clin Orthod.
36:1668–1674. 2018.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Betof Warner A, Palmer JS, Shoushtari AN,
Goldman DA, Panageas KS, Hayes SA, Bajwa R, Momtaz P, Callahan MK,
Wolchok JD, et al: Long-term outcomes and responses to retreatment
in patients with melanoma treated with PD-1 blockade. J Clin Oncol.
38:1655–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Waterhouse DM, Garon EB, Chandler J,
McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan
B, et al: Continuous versus 1-year fixed-duration nivolumab in
previously treated advanced non-small-cell lung cancer: CheckMate
153. J Clin Orthod. 38:3863–3873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ,
Li SW, Liu N and Yan CX: Immune-related adverse events associated
with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis.
Front Pharmacol. 8(730)2017.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and efficacy of re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Lebbé C, Meyer N, Mortier L, Marquez-Rodas
I, Robert C, Rutkowski P, Menzies AM, Eigentler T, Ascierto PA,
Smylie M, et al: Evaluation of two dosing regimens for nivolumab in
combination with ipilimumab in patients with advanced melanoma:
Results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol.
37:867–875. 2019.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Tarhini AA, Lee SJ, Hodi FS, Rao UNM,
Cohen GI, Hamid O, Hutchins LF, Sosman JA, Kluger HM, Eroglu Z, et
al: Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus
high-dose interferon alfa-2b for resected high-risk melanoma: North
American intergroup E1609. J Clin Oncol. 38:567–575.
2020.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Betof AS, Nipp RD, Giobbie-Hurder A,
Johnpulle RAN, Rubin K, Rubinstein SM, Flaherty KT, Lawrence DP,
Johnson DB and Sullivan RJ: Impact of age on outcomes with
immunotherapy for patients with melanoma. Oncologist. 22:963–971.
2017.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Zamami Y, Niimura T, Okada N, Koyama T,
Fukushima K, Izawa-Ishizawa Y and Ishizawa K: Factors associated
with immune checkpoint inhibitor-related myocarditis. JAMA Oncol.
5:1635–1637. 2019.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Tan MH, Iyengar R, Mizokami-Stout K, Yentz
S, MacEachern MP, Shen LY, Redman B and Gianchandani R: Spectrum of
immune checkpoint inhibitors-induced endocrinopathies in cancer
patients: A scoping review of case reports. Clin Diabetes
Endocrinol. 5(1)2019.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Minkis K, Garden BC, Wu S, Pulitzer MP and
Lacouture ME: The risk of rash associated with ipilimumab in
patients with cancer: A systematic review of the literature and
meta-analysis. J Am Acad Dermatol. 69:e121–e128. 2013.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Coleman E, Ko C, Dai F, Tomayko MM, Kluger
H and Leventhal JS: Inflammatory eruptions associated with immune
checkpoint inhibitor therapy: A single-institution retrospective
analysis with stratification of reactions by toxicity and
implications for management. J Am Acad Dermatol. 80:990–997.
2019.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Sibaud V, Meyer N, Lamant L, Vigarios E,
Mazieres J and Delord JP: Dermatologic complications of
anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol.
28:254–263. 2016.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Sibaud V: Dermatologic reactions to immune
checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin
Dermatol. 19:345–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Inno A, Metro G, Bironzo P, Grimaldi AM,
Grego E, Di Nunno V, Picasso V, Massari F and Gori S: Pathogenesis,
clinical manifestations and management of immune checkpoint
inhibitors toxicity. Tumori. 103:405–421. 2017.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Kumar V, Chaudhary N, Garg M, Floudas CS,
Soni P and Chandra AB: Current diagnosis and management of immune
related adverse events (irAEs) Induced by immune checkpoint
inhibitor therapy. Front Pharmacol. 8(49)2017.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Bryce J and Boers-Doets CB: Non-rash
dermatologic adverse events related to targeted therapies. Semin
Oncol Nurs. 30:155–168. 2014.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Geisler AN, Phillips GS, Barrios DM, Wu J,
Leung DYM, Moy AP, Kern JA and Lacouture ME: Immune checkpoint
inhibitor-related dermatologic adverse events. J Am Acad Dermatol.
83:1255–1268. 2020.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ,
Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, et al:
Lymphatic endothelial cells induce tolerance via PD-L1 and lack of
costimulation leading to high-level PD-1 expression on CD8 T cells.
Blood. 120:4772–4782. 2012.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Sano T, Uhara H, Mikoshiba Y, Kobayashi A,
Uchiyama R, Tateishi K, Yamamoto H and Okuyama R: Nivolumab-induced
organizing pneumonia in a melanoma patient. Jpn J Clin Oncol.
46:270–272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Nakashima K, Naito T, Omori S, Yoshikawa
S, Endo M, Kiyohara Y and Takahashi T: Organizing pneumonia induced
by nivolumab in a patient with metastatic melanoma. J Thorac Oncol.
11:432–433. 2016.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Koelzer VH, Rothschild SI, Zihler D, Wicki
A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A and Mertz
KD: Systemic inflammation in a melanoma patient treated with immune
checkpoint inhibitors-an autopsy study. J Immunother Cancer.
4(13)2016.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Watanabe S, Kimura H, Takato H, Waseda Y,
Hara J, Sone T, Abo M, Maeda S, Matsushita T and Kasahara K: Severe
pneumonitis after nivolumab treatment in a patient with melanoma.
Allergol Int. 65:487–489. 2016.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Nishino M, Sholl LM, Hatabu H, Ramaiya NH
and Hodi FS: Anti-PD-1-related pneumonitis during cancer
immunotherapy. N Engl J Med. 373:288–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Mir MA: T-cell costimulation and its
applications in diseases. Dev Costimulatory Mol Immunother Dis.
29:255–292. 2015.
|
|
187
|
Tarazona R, Duran E and Solana R: Natural
killer cell recognition of melanoma: New clues for a more effective
immunotherapy. Front Immunol. 6(649)2015.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Rieth J and Subramanian S: Mechanisms of
intrinsic tumor resistance to immunotherapy. Int J Mol Sci.
19(1340)2018.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
190
|
O'Donnell JS, Long GV, Scolyer RA, Teng
MWL and Smyth MJ: Resistance to PD1/PDL1 checkpoint inhibition.
Cancer Treat Rev. 52:71–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI View Article : Google Scholar
|
|
192
|
Ahn CS and Metallo CM: Mitochondria as
biosynthetic factories for cancer proliferation. Cancer Metab.
3(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
193
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
194
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv.
2(e1600200)2016.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Robey IF, Lien AD, Welsh SJ, Baggett BK
and Gillies RJ: Hypoxia-inducible factor-1alpha and the glycolytic
phenotype in tumors. Neoplasia. 7:324–330. 2005.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Franco F, Jaccard A, Romero P, Yu YR and
Ho PC: Metabolic and epigenetic regulation of T-cell exhaustion.
Nat Metab. 2:1001–1012. 2020.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Jiang Y, Li Y and Zhu B: T-cell exhaustion
in the tumor microenvironment. Cell Death Dis.
6(e1792)2015.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Sirico M, D'Angelo A, Gianni C, Casadei C,
Merloni F and De Giorgi U: Current state and future challenges for
PI3K inhibitors in cancer therapy. Cancers (Basel).
15(703)2023.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Li X, Wenes M, Romero P, Huang SCC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441.
2019.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Marin-Acevedo JA, Kimbrough EO and Lou Y:
Next generation of immune checkpoint inhibitors and beyond. J
Hematol Oncol. 14(45)2021.PubMed/NCBI View Article : Google Scholar
|