Introduction
Rheumatic illnesses are a general umbrella term for
almost 200 different disorders affecting the musculoskeletal system
(1). The causes and therapies for
the almost 100 different types of arthritis have been
well-documented. In humans, rheumatoid arthritis (RA) and
osteoarthritis are the most well-known types of arthritis. RA, also
referred to as an autoimmune disease or a progressive disease, is
an example of a chronic systemic condition (2). It is characterized by destructive joint
disease and prolonged inflammation, although its etiology is
uncertain (3). There are two
subtypes of this disease, namely ‘seropositive’ and ‘seronegative’.
Elevated blood levels of rheumatoid factor (RF) autoantibodies and,
more recently, antibodies to citrullinated protein/peptide antigens
(ACPAs) can be used to determine seropositivity (4). RF autoantibodies are a type of antibody
that are produced by the immune system.
RA is far more common in certain populations than in
others. France (0.19%) and Italy (0.41%) have been found to have a
lower reported prevalence of RA compared to other countries of the
world, where it varies from 0.19 to 1.1% (5,6). The
prevalence of RA in Germany and Sweden has been reported to be
~0.65% (7,8). RA has been reported to affect between
0.5 and 1% of North Americans (8-10).
RA is estimated to afflict 0.75% of individuals in India (10,11).
Furthermore, coronary artery calcium (CAC) as it is
commonly known, is a characteristic of coronary atherosclerosis
(12). Consistent, dependable and
convincing evidence of a substantial association between CAC and
significant cardiovascular events has also been found in a number
of asymptomatic individuals (13,14).
Initially, CAC was evaluated using chest X-ray, fluoroscopy, or
digital subtraction of fluoroscopy; following the introduction of
electron beam computed tomography and multidetector computed
tomography, these devices were found to be more useful. The most
useful information on cardiovascular risk, or the likelihood of
suffering a heart attack or stroke, may be obtained from coronary
calcium scores for females aged 35 to 70 years and for males aged
40 to 60 years (15).
The disease activity score (DAS) was developed by a
group of Dutch rheumatologists with the goal of offering a
standardized method to compare and contrast results from clinical
trials of novel medications for the treatment of RA. Clinical
practices often use disease activity measurements related to RA.
The DAS28, which evaluates a total of 28 joints, is one such
instrument.
The present study aimed to examine the association
between cardiovascular risk (using CAC) and CIMT in patients with
RA and healthy controls, using the DAS28 as a proxy.
Patients and methods
Study population
The present case-control study was carried out at a
tertiary care center over the course of 2 academic years
(2020-2022). Each individual who met the American College of
Rheumatology (ACR) criteria (Table
SI) for a diagnosis of RA was included in the analysis as the
cases, while those who were healthy, and age- and sex-matched
individuals served as the controls. All patients were subjected to
routine blood and radiological investigations. Patients with
Inflammatory joint disease other than RA, a history of any
cardiovascular/cerebrovascular events, known diabetes and obesity
[body mass index (BMI) >30 kg/m2] and patients who
were critically ill were excluded from the study. The Institutional
Ethical Committee of Era's Lucknow Medical College and Hospital,
Lucknow, India gave its approval for the research to go on. All
patients were asked to provide their written and informed
permission.
All controls and patients with RA who were
identified using the aforementioned criteria were referred to the
Radiology Department of the same hospital for evaluation of CIMT by
carotid intima-media thickness ultrasonography and coronary artery
calcium(CAC) burden by dual-energy computed tomography under the
supervision of competent radiologists. These criteria categorize
newly presented patients with confirmed clinical synovitis in at
least one joint when no other medical condition seems to fit into
the category. A score of ≥6 fulfils the requirements for definite
RA. The classification criteria used in the present study for the
patients with RA are listed in Table
SI (16).
DAS
Dutch rheumatologists first created the DAS in order
to standardize and compare outcomes in RA medication clinical
trials. Over time, the DAS28 has also found its way into everyday
clinical settings. DAS28 is a metric for assessing the efficacy of
RA therapy and management. Treatments may reduce the inflammation
caused by RA, which in turn attenuates the damage inflicted to
joints that causes disability and suffering. As a result, DAS28
(Table SII) (17) plays a crucial role in determining the
optimal strategy for the disease management of each individual
affected.
Estimation of CAC score
The estimation of the CAC score was achieved using a
384-slice dual source machine, SOMATOM Force (Siemens
Healthineers), for coronary calcium scoring at the Department of
Radiology, Era's Lucknow Medical College and Hospital. The arteries
which were focused on were the following: Left main, left
circumflex, right circumflex and left anterior descending. The CAC
score calculated using the Agatston score.
Method of calculation
The calculation is based on the weighted density
score given to the highest attenuation value (HU) multiplied by
area of the calcification speck. The density factor assigned to
each HU corresponds as 130-199 HU=1; 200-299 HU=2; 300-399 HU=3 and
400+ HU= 4. For example, if a calcified speck has maximum
attenuation value of 400 HU and occupies 8 sq mm area, then its
calcium score is 32. The severity is then graded as demonstrated in
Table SIII (18).
Statistical analysis
Statistical analysis was performed using SPSS
version 21.0 statistical analysis software (IBM Corp.). The values
are presented as number and percentage, or as the mean ± standard
deviation (SD). Categorical variables were analyzed using the
Chi-squared test or Fisher's exact test and continuous variables
were analyzed using the unpaired student's t-test. Bivariate
Pearson's (r-value) correlation analysis was used to determine the
correlations among variables. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
The present study examined 45 patients with RA who
fulfilled the aforementioned inclusion criteria. These patients
were then classified as the cases and another 45 healthy
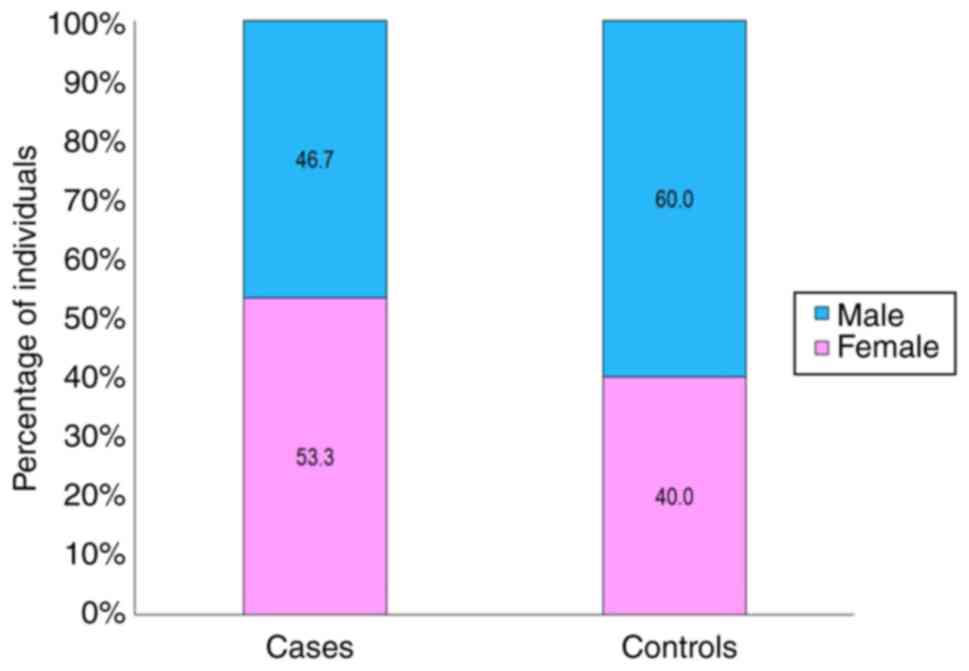
individuals were included as the controls. As demonstrated in
Fig. 1, although the sex ratios
between the patients with (53.3% females) and the controls (60%
males) were comparable, the RA patient group had a higher number of
females. The demographic profiles of both groups are presented in
Table I.
 | Table IDemographic details of the
participants in the present study. |
Table I
Demographic details of the
participants in the present study.
| Demographic
characteristics | Total (n=90) | Cases (n=45) | Controls (n=45) | t-test (P-value) |
|---|
| Mean age ± SD (range)
in years | 50.66±12.35 | 49.07±12.38 | 52.24±12.26 | 1.22 (0.224) |
| Mean BMI ± SD (range)
in kg/m2 | 24.75±1.73 | 24.60±1.89 | 24.90±1.66 | 0.782 (0.436) |
Only 2 (4.4%) of the 45 patients with RA had a DAS
≤2.6, which is indicative of remission. A total of 40 patients of
45 cases (88.9%), had disease activity ratings that ranged from
moderate to high (range, >3.2), whereas 3 patients, which is
equivalent to 6.7% of the sample, had low disease activity scores
(range, 2.6-3.2) (Table II).
 | Table IIThe comorbidities and therapy of the
patients with RA. |
Table II
The comorbidities and therapy of the
patients with RA.
| Parameter | No. of cases | Percentage |
|---|
| Comorbidities | | |
|
Asthma | 5 | 11.1 |
|
AKI | 2 | 4.4 |
|
COPD | 5 | 11.1 |
|
CKD | 2 | 4.4 |
|
Hypothyroidism | 7 | 15.6 |
|
Interstitial
lung disease | 4 | 8.9 |
|
No
comorbidity | 28 | 62.2 |
| Therapy | | |
|
Not on
therapy | 2 | 4.4 |
|
On DMARDS
treatment for <6 months | 6 | 13.3 |
|
On DMARDS
treatment for >6 months | 37 | 82.2 |
| DAS | | |
|
DAS <2.6
(remission) | 2 | 4.4 |
|
DAS 2.6-3.2
(low disease activity) | 3 | 6.7 |
|
DAS 3.3-5.2
(moderate disease activity) | 16 | 35.6 |
|
DAS >5.2
(high disease activity) | 24 | 53.3 |
Compared with the controls, the cases had a
significantly higher total leucocyte count (TLC; 9311±2877 vs.
8049±1780 per microliter), erythrocyte sedimentation rate (ESR) in
the first hour (19.18±6.34 vs. 16.62±3.56 mm/h), C-reactive protein
(CRP; 11.36±5.84 vs. 2.44±1.69 mg/l), serum urea (24.76±7.96 vs.
21.99±3.53 mg/dl), serum potassium (4.18±0.69 vs. 3.95±0.23
mmol/l), serum magnesium (1.95±0.18 vs. 2.02±0.14 mg/dl), serum
glutamic pyruvic transaminase (SGPT; 44.82±18.60 vs. 37.69±9.58
units/l), serum alkaline phosphatase (ALP; 96.02±36.12 vs.
81.96±21.19 IU/l), serum cholesterol (160.88±30.11 vs. 145.35±17.40
mg/dl), serum high-density lipoprotein (HDL; 7.20±15.83 vs.
43.91±8.17 mg/dl), serum low-density lipoprotein (LDL; 107.74±23.75
vs. 98.11±16.66 mg/dl) and the LDL-HDL ratio (2.85±1.58 vs.
1.57±3.27 mg/dl) (Table III). The
results of the analysis of the correlation between the DAS, and
BMI, CRP, serum cholesterol, serum triglycerides, serum HDL, serum
LDL and serum very low-density lipoprotein (VLDL) are presented in
Table IV. An inverse correlation
was observed between the DAS and HDL levels. In addition, a strong
correlation was found between the DAS and the CRP level. However, a
moderate or weak correlation was observed between DAS and the
remaining variables, although some correlations were
significant.
 | Table IIIComparison of the parameters between
the cases and controls. |
Table III
Comparison of the parameters between
the cases and controls.
| | Cases (n=45) | Controls
(n=45) | Statistical
significance |
|---|
| Parameter | Average values | Mean | SD | Mean | SD | t value | P-value |
|---|
| Hemoglobin
(mg/dl) | Males, 14-18 mg/dl;
females,12-16 mg/dl | 11.86 | 1.86 | 12.44 | 1.98 | -1.438 | 0.154 |
| TLC
(cells/cumm) | 4,000-11,000
cells/cumm | 9311 | 2877 | 8049 | 1780 | 2.502 | 0.014 |
| Platelets
(lakh) | 1.5-2.5 lakh | 2.90 | 1.06 | 2.60 | 0.74 | 1.559 | 0.123 |
| HbA1c (%) | Below 5.7% | 5.42 | 0.40 | 5.30 | 0.53 | 1.215 | 0.227 |
| RBS (mg/dl) | 70-140 mg/dl | 141.84 | 30.21 | 135.20 | 22.70 | 1.180 | 0.241 |
| ESR in first-hour
(mm/h) | 0 to 15 mm/h in
males; 0 to 20 mm/h in females | 19.18 | 6.34 | 16.62 | 3.56 | 2.357 | 0.021 |
| CRP (mg/dl) | <5 mg/l | 11.36 | 5.84 | 2.44 | 1.69 | 9.840 |
<0.001 |
| Serum urea
(mg/dl) | 5 to 20 mg/dl | 24.76 | 7.96 | 21.99 | 3.53 | 2.138 | 0.035 |
| Serum creatinine
(mg/dl) | 0.7 to 1.3
mg/dl | 0.96 | 0.55 | 0.91 | 0.54 | 0.447 | 0.656 |
| Serum sodium
(mEq/l) | 135 to 145
mEq/l | 140.47 | 3.63 | 140.04 | 2.75 | 0.622 | 0.536 |
| Serum potassium
(mEq/l) | 3.5 to 5.5
mEq/l | 4.18 | 0.69 | 3.95 | 0.23 | 2.160 | 0.033 |
| Serum magnesium
(mg/dl) | 1.6-2.5 mg/dl | 1.95 | 0.18 | 2.02 | 0.14 | -2.235 | 0.028 |
| SGPT (U/l) | 7 to 56 U/l | 44.82 | 18.60 | 37.69 | 9.58 | 2.287 | 0.025 |
| SGOT (U/l) | 8 to 45 U/l | 38.49 | 11.25 | 34.33 | 9.92 | 1.859 | 0.066 |
| Serum total
bilirubin (mg/dl) | 0.1 to 1.2
mg/dl | 0.93 | 0.30 | 0.91 | 0.30 | 0.248 | 0.805 |
| Serum ALP
(IU/l) | 44 to 147 IU/l | 96.02 | 36.12 | 81.96 | 21.19 | 2.253 | 0.027 |
| Serum cholesterol
(mg/dl) | <200 mg/dl | 160.88 | 30.11 | 145.35 | 17.40 | 2.996 | 0.004 |
| Serum triglycerides
(mg/dl) | <150 mg/dl | 179.69 | 34.94 | 176.64 | 16.34 | 0.530 | 0.598 |
| Serum HDL
(mg/dl) | >40 mg/dl | 37.20 | 15.83 | 43.91 | 8.17 | -2.527 | 0.013 |
| Serum LDL
(mg/dl) | <100 mg/dl | 107.74 | 23.75 | 98.11 | 16.66 | 2.227 | 0.029 |
| Serum VLDL (mg/dl)
LD | 2 to 30 mg/dl | 35.94 | 6.99 | 35.33 | 3.27 | 0.530 | 0.598 |
| LDL-HDL ratio | below 5:1 | 2.85 | 1.58 | 1.57 | 0.50 | 5.219 |
<0.001 |
 | Table IVCorrelation of DAS score with
cardiovascular risk factors. |
Table IV
Correlation of DAS score with
cardiovascular risk factors.
| Cardiovascular risk
factors | r value | Level of
correlation | P-value |
|---|
| BMI | -0.055 | Weak | 0.606 |
| CRP | 0.730 | Strong |
<0.001 |
| Serum
cholesterol | 0.319 | Mild | 0.002 |
| Serum TGL | 0.127 | Weak | 0.232 |
| Serum HDL | -0.219 | Weak | 0.038 |
| Serum LDL | 0.462 | Mild | 0.033 |
| Serum VLDL | 0.127 | Weak | 0.232 |
None of the controls had moderate risk (score
101-400) or severe risk (score ≥401), and the majority of the
controls exhibited no evidence of CAD (CAC score 0; 88.9%). The
remaining controls had a minimum risk (score 1-10) or mild risk
(score 11-100). By contrast, according to the CAC scores, only
22.2% of the patients with RA exhibited no signs of CAD, another
22.2% had a low risk, and the remaining 55.5% had a mild to severe
risk. This difference was found to be statistically significant.
The proportion of cases was higher as compared to controls in all
the risk categories: Minimal (22.2 vs. 6.7%), mild (13.3 vs. 4.4%),
moderate (24.4 vs. 0.0%) and severe risk (17.8 vs. 0.0%) (Table V).
 | Table VComparison of CAC score between the
groups. |
Table V
Comparison of CAC score between the
groups.
| | Cases (n=45) | Controls
(n=45) | |
|---|
| CAC score | Total (n=90) | No. of
participants | % | No. of
participants | % | P-value (Fisher's
exact test) |
|---|
| 0 (No evidence of
CAD) | 50 (55.6) | 10 | 22.2 | 40 | 88.9 |
<0.001 |
| 1-10 (Minimal) | 13 (14.4) | 10 | 22.2 | 3 | 6.7 |
<0.001 |
| 11-100 (Mild) | 8 (8.9) | 6 | 13.3 | 2 | 4.4 |
<0.001 |
| 101-400
(Moderate) | 11 (12.2) | 11 | 24.4 | 0 | 0.0 |
<0.001 |
| ≥401 (Severe) | 8 (8.9) | 8 | 17.8 | 0 | 0.0 |
<0.001 |
| Mean CAC score ± SD
(range) | 123.70±263.49
(0-1,350) | 246.80±330.81
(0-1,350) | 0.600±0.251
(0-13) | N/A |
The range of CIMT in the patients with RA was
0.712-1.310 mm, while that in the controls was 0.412-0.518 mm. In
the patients with RA, the CIMT was determined to be 1.050±0.218 mm,
which was substantially larger than the value of the control group
(0.479±0.040 mm) (Table VI).
 | Table VIComparison of the CIMT between the
groups. |
Table VI
Comparison of the CIMT between the
groups.
| Group | No. of
subjects | Min. | Max. | Mean | S.D. | t-test
(P-value) |
|---|
| Cases | 45 | 0.712 | 1.310 | 1.050 | 0.218 | 17.277
(0.001)a |
| Controls | 45 | 0.412 | 0.518 | 0.479 | 0.040 | |
| Total | 90 | 0.412 | 1.310 | 0.764 | 0.327 | |
The CIMT exhibited a significant correlation with
CRP, serum cholesterol, triglycerides, serum HDL (inverse), LDL and
VLDL. A strong correlation was found between CIMT and CRP was
strong; however, a moderate or weak correlation was found between
CIMT and the remaining variables, although some correlations were
significant. The CAC score also exhibited a significant correlation
with CRP, serum cholesterol and serum triglycerides, serum VLDL and
serum LDL. A moderate correlation was found between the CAC score
and CRP, serum triglycerides and VLDL. The CAC scores and CIMT
levels of both sexes were comparable. Age was not found to
significantly correlate with the aforementioned two markers (CAC
and CIMT) (Table VII).
 | Table VIICorrelation of CIMT and CAC scores
with cardiovascular risk factors. |
Table VII
Correlation of CIMT and CAC scores
with cardiovascular risk factors.
| | CIMT | CAC score |
|---|
| Cardiovascular risk
factors | r value | Level of
correlation | P-value | r value | Level of
correlation | P-value |
|---|
| BMI | -0.068 | Weak | 0.522 | -0.046 | Weak | 0.664 |
| CRP | 0.880 | Strong |
<0.001 | 0.659 | Moderate |
<0.001 |
| Serum
cholesterol | 0.319 | Mild | 0.002 | 0.306 | Mild | 0.003 |
| Serum
triglycerides | 0.356 | Mild | 0.001 | 0.567 | Moderate | <0.001 |
| Serum HDL | -0.240 | Weak | 0.023 | -0.45 | Weak | 0.671 |
| Serum LDL | 0.462 | Mild |
<0.001 | 0.336 | Mild |
<0.001 |
| Serum VLDL | 0.356 | Mild | 0.001 | 0.567 | Moderate |
<0.001 |
| Age, years | 0.145 | Weak | 0.174 | 0.058 | Weak | 0.590 |
Discussion
As a chronic inflammatory joint condition, RA
restricts everyday activities due to the pain, stiffness, and
exhaustion that patients experience regularly. The link between
inflammation and the development of cardiovascular disease was
recognized only ~10 years ago. Researchers found that patients with
RA had a 1.5-2.0-fold greater risk of developing coronary artery
disease (CAD) than the general population (18). Another study demonstrated that
patients with RA had a prevalence of myocardial infarction (MI)
that was >3-fold higher than that of patients without RA
(19). Thus, CAD is already more
likely to occur before RA is even diagnosed clinically. The signs
and symptoms of CAD vary with the disease process throughout time.
In certain cases, there may be no obvious symptoms of damage.
The present study aimed to evaluate CIMT and
computed tomography-CAC, two relatively novel cardiovascular risk
indicators, in patients with RA. For this purpose, the present
study recruited 45 patients with RA and 45 individuals who served
as the healthy controls. The enrolled patients with RA had an
average age of 49.07±12.38 years (ranging from 24 to 80 years),
with 53.3% being female. Specifically, the controls in the present
study were matched for both sex and age. Although RA may develop at
any age, the majority of epidemiological research has shown that
the condition is most common in individuals who are in their 50 and
60s. Other studies have suggested a later onset of the illness
(19-23).
The mean age of patients with RA included in the
clinical studies by Schott et al (24) (53.3 years) and Targońska-Stepniak
et al (25) (42.6±8.0 years;
range, 27-59) was also very close to that in the present study. In
the study by Udachkina et al (26), the median age of the patients was 56
years.
In the present study, two-thirds (53.3%) of the
patients had a DAS >5.2 (high disease activity), and16 patients
had a DAS of 3.3-5.2 (35.6%) (moderate disease activity). Out of
the 45 patients with RA, 2 (4.4%) were not on therapy, 6 (13.3%)
were on disease-modifying antirheumatic drugs (DMARDs) treatment
for <6 months, and the remaining 37 (82.22%) were on DMARDS
treatment for >6 months. The DAS exhibited a substantial
correlation with serum cholesterol, LDL and CRP levels among the
CAD risk factors. Sengul et al (27) examined Turkish individuals with RA
and their DAS28-ESR and DAS28-CRP criteria. While the DAS28-CRP was
based on DAS28 without the ESR, the DAS28-ESR was based on the DAS
with 28 joints (27). According to
that study, the DAS28-CRP and DAS28-ESR exhibited a substantial
association, whereas the individual components only exhibited a
modest correlation (27).
In the present study, significant differences in the
laboratory parameters (TLC, first-hour ESR, CRP, serum urea, serum
potassium, serum magnesium, SGPT and serum ALP, and lipid levels)
of the RA cases and healthy controls were observed. The TLC, ESR
and CRP levels are inflammatory markers and have been found to be
increased in ~40% of patients with RA (28). It has been shown that
anti-inflammatory medications increase total, HDL and LDL
cholesterol levels in patients with RA (29,30).
Oxidative alterations caused by chronic inflammation modify the
structure of HDL and decrease apolipoprotein-A1 in patients with
active RA (31).
In the present study, the CAC score of the patients
with RA was significantly higher compared with that of the controls
(246.80±330.81 vs. 0.600±0.251). Of note, ~55.6% of patients with
RA had been found to have a mild to severe risk of developing CAD
(score, 11->400). A significant correlation between CAC and CRP,
and lipid levels (apart from HDL) was found. These findings are
supported by the findings in the study by Wahlin et al
(32), who studied the computed
tomography-CAC of 22 patients with RA (mean age, 65 years). They
also found that 55.5% of the patients had a CAC score
>10(32).
The average age of the 60 female patients with RA in
the study by Bernardes et al (33) was 53.6±10.4 years, and their average
DAS28 score was 4. Age, BMI, hyperglycemia and cholesterol levels
were shown to have a substantial correlation with the mean coronary
calcium score, which was 35.192±117.786. In the present study, the
CIMT varied significantly (1.050±0.218 vs. 0.479±0.040 mm) between
the experimental and control groups. CIMT was substantially linked
with levels of lipids and CRP.
Targońska-Stepniak et al (25) found similar results when they
evaluated CIMT values in 74 patients with RA without cardiovascular
risk, with an average age of 46.4±0.6 years. A total of 31
age-matched control participants had notably higher CIMT values
than the controls (25).
As a chronic inflammatory disorder, RA affects more
than only the joints and muscles. Multiple risk variables that are
also linked to cardiovascular risk govern it. Moreover, medication
for RA also has a cardiovascular impact. Aging also contributes as
a common risk factor between RA and cardiovascular disorders. Thus,
the screening of all patients with RA for cardiovascular disease
and associated risks is recommend at regular intervals.
Furthermore, according to the data indicating a much larger
percentage of these surrogate indicators in patients with RA, it is
imperative that more stringent measures be implemented to decrease
the cardiovascular risk in this population.
In conclusion, the present study demonstrated that
the CIMT and CAC differed substantially between healthy individuals
and patients with RA. This difference was shown to be statistically
significant. The diagnosis and planning of risk reduction measures
for patients with RA may be aided by these surrogate indicators of
early atherosclerosis. Consistent results between CIMT and CAC, and
established CV risk variables, such as dyslipidemia, suggest that
these two methods could assist prevention and treatment efforts.
Chronic inflammation significantly increases the risk of developing
atherosclerosis, as suggested by the link between the disease
activity score, the CIMT and CAC.
Supplementary Material
Classification criteria for rheumatoid
arthritis.
Disease activity score.
Severity grading of CAC score.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was conceptualized and planned by JF. All
the data were collected, analyzed and entered into tables by MZS.
JF and MZS analyzed the data and modified the final study. VS and
ZS provided scientific input and helped editing protocols,
contributed data or analysis tools and performed the analyses. SM
helped pool information and edited the final manuscript, collected
the data, and contributed data or analysis tools. Performed the
analysis and Edited the paper. AAJ and ZU helped collect patient
profiles and raw data. All authors have reviewed and approved the
final manuscript. JF and MZS confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The Institutional Ethical Committee of Era's Lucknow
Medical College and Hospital, Lucknow, India gave its approval for
the research to go on. All patients were asked to provide their
informed permission.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohsin Z, Asghar AA, Faiq A, Khalid I,
Ul-Haque I, Rehman S, Ahmed SI, Basalat ST, Aimen A, Shafique S, et
al: Prevalence of rheumatic diseases in a tertiary care hospital of
Karachi. Cureus. 10(e2858)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shi Y, Wu Y, Ren Y, Jiang Y and Chen Y:
Infection risks of rituximab versus non-rituximab treatment for
rheumatoid arthritis: A systematic review and meta-analysis. Int J
Rheum Dis. 22:1361–1370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Okada Y, Eyre S, Suzuki A, Kochi Y and
Yamamoto K: Genetics of rheumatoid arthritis: 2018 status. Ann
Rheum Dis. 78:446–453. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smolen JS, Aletaha D, Koeller M, Weisman
MH and Emery P: New therapies for treatment of rheumatoid
arthritis. Lancet. 370:1861–1874. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rossini M, Rossi E, Bernardi D, Viapiana
O, Gatti D, Idolazzi L, Caimmi C, Derosa M and Adami S: Prevalence
and incidence of rheumatoid arthritis in Italy. Rheumatol Int.
34:659–664. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Biver E, Beague V, Verloop D, Mollet D,
Lajugie D, Baudens G, Neirinck P and Flipo RM: Low and stable
prevalence of rheumatoid arthritis in northern France. Joint Bone
Spine. 76:497–500. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Englund M, Jöud A, Geborek P, Felson DT,
Jacobsson LT and Petersson IF: Prevalence and incidence of
rheumatoid arthritis in southern Sweden 2008 and their relation to
prescribed biologics. Rheumatology (Oxford). 49:1563–1569.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hense S, Ramos AL, Callhoff J, Albrecht K,
Zink A and Hoffmann F: Prevalence of rheumatoid arthritis in
Germany based on health insurance data: Regional differences and
first results of the PROCLAIR study. Z Rheumatol. 75:819–827.
2016.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
9
|
Hunter TM, Boytsov NN, Zhang X, Schroeder
K, Michaud K and Araujo AB: Prevalence of rheumatoid arthritis in
the United States adult population in healthcare claims databases,
2004-2014. Rheumatol Int. 37:1551–1557. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jean S, Hudson M, Gamache P, Bessette L,
Fortin PR, Boire G and Bernatsky S: Temporal trends in prevalence,
incidence, and mortality for rheumatoid arthritis in Quebec,
Canada: A population-based study. Clin Rheumatol. 36:2667–2671.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bernatsky S, Dekis A, Hudson M, Pineau CA,
Boire G, Fortin PR, Bessette L, Jean S, Chetaille AL, Belisle P, et
al: Rheumatoid arthritis prevalence in Quebec. BMC Res Notes.
7(937)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Demer LL and Tintut Y: Vascular
calcification: Pathobiology of a multifaceted disease. Circulation.
117:2938–2948. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oei HH, Vliegenthart R, Hak AE, del Sol A,
Hofman A, Oudkerk M and Witteman JC: The association between
coronary calcification assessed by electron beam computed
tomography and measures of extracoronary atherosclerosis: The
rotterdam coronary calcification study. J Am Coll Cardiol.
39:1745–1751. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hoffmann U, Massaro JM, Fox CS, Manders E
and O'Donnell CJ: Defining normal distributions of coronary artery
calcium in women and men (from the Framingham Heart Study). Am J
Cardiol. 102:1136–1141. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yadav A, Mala M, Yadav GAM and Kumar LN:
Effect of cigarette smoking on blood levels of lipid and
atherogenic lipid ratios. Natl J Lab Med. 9:1–3. 2020.
|
|
16
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American college of rheumatology/European league against
rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar BS, Suneetha P, Mohan A, Kumar DP
and Sarma KVS: Comparison of disease activity score in 28 joints
with ESR (DAS28), clinical disease activity index (CDAI), health
assessment questionnaire disability index (HAQ-DI) & routine
assessment of patient index data with 3 measures (RAPID3) for
assessing disease activity in patients with rheumatoid arthritis at
initial presentation. Indian J Med Res. 146:S57–S62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Solomon DH, Goodson NJ, Katz JN, Weinblatt
ME, Avorn J, Setoguchi S, Canning C and Schneeweiss S: Patterns of
cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis.
65:1608–1612. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maradit-Kremers H, Crowson CS, Nicola PJ,
Ballman KV, Roger VL, Jacobsen SJ and Gabriel SE: Increased
unrecognized coronary heart disease and sudden deaths in rheumatoid
arthritis: A population-based cohort study. Arthritis Rheum.
52:402–411. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Doran MF, Pond GR, Crowson CS, O'Fallon WM
and Gabriel SE: Trends in incidence and mortality in rheumatoid
arthritis in Rochester, Minnesota, over a forty-year period.
Arthritis Rheum. 46:625–631. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Riise T, Jacobsen BK and Gran JT:
Incidence and prevalence of rheumatoid arthritis in the county of
Troms, Northern Norway. J Rheumatol. 27:1386–1389. 2000.PubMed/NCBI
|
|
22
|
Linos A, Worthington JW, O'Fallon WM and
Kurland LT: The epidemiology of rheumatoid arthritis in Rochester,
Minnesota: A study of incidence, prevalence, and mortality. Am J
Epidemiol. 111:87–98. 1980.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gabriel SE, Crowson CS and O'Fallon WM:
The epidemiology of rheumatoid arthritis in Rochester, Minnesota,
1955-1985. Arthritis Rheum. 42:415–420. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schott LL, Kao AH, Cunningham A, Wildman
RP, Kuller LH, Sutton-Tyrrell K and Wasko MC: Do carotid artery
diameters manifest early evidence of atherosclerosis in women with
rheumatoid arthritis? J Womens Health (Larchmt). 18:21–29.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Targońska-Stepniak B, Drelich-Zbroja A and
Majdan M: The relationship between carotid intima-media thickness
and the activity of rheumatoid arthritis. J Clin Rheumatol.
17:249–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Udachkina EV, Novikova DS, Popkova TV,
Kirillova IG, Markelova EI, Gorbunova YN, Karateev DE, Luchikhina
EL, Demidova NV, Borisova MA, et al: Progression of Carotid Artery
Atherosclerosis during treatment to target in patients with early
rheumatoid arthritis. Rheumatol Sci Pract. 56:449–455. 2018.
|
|
27
|
Sengul I, Akcay-Yalbuzdag S, Ince B,
Goksel-Karatepe A and Kaya T: Comparison of the DAS28-CRP and
DAS28-ESR in patients with rheumatoid arthritis. Int J Rheum Dis.
18:640–645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Emery P: The dunlop-dottridge lecture:
Prognosis in inflammatory arthritis: The value of HLA genotyping
and the oncological analogy. J Rheumatol. 24:1436–1442.
1997.PubMed/NCBI
|
|
29
|
van Sijl AM, Peters MJ, Knol DL, de Vet
RH, Sattar N, Dijkmans BA, Smulders YM and Nurmohamed MT: The
effect of TNF-alpha blocking therapy on lipid levels in rheumatoid
arthritis: A meta-analysis. Semin Arthritis Rheum. 41:393–400.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Robertson J, Peters MJ, McInnes IB and
Sattar N: Changes in lipid levels with inflammation and therapy in
RA: A maturing paradigm. Nat Rev Rheumatol. 9:513–523.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Charles-Schoeman C, Watanabe J, Lee YY,
Furst DE, Amjadi S, Elashoff D, Park G, McMahon M, Paulus HE,
Fogelman AM and Reddy ST: Abnormal function of high-density
lipoprotein is associated with poor disease control and an altered
protein cargo in rheumatoid arthritis. Arthritis Rheum.
60:2870–2879. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wahlin B, Meedt T, Jonsson F, Henein MY
and Wållberg-Jonsson S: Coronary artery calcification is related to
inflammation in rheumatoid arthritis: A long-term follow-up study.
Biomed Res Int. 2016(1261582)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bernardes M, Madureira A, Oliveira A,
Martins MJ, Lucas R, Costa L, Pereira JG, Ventura F, Ramos I and
Martins E: Coronary artery calcium score in female rheumatoid
arthritis patients: Associations with apolipoproteins and disease
biomarkers. Int J Rheum Dis. 22:1841–1856. 2019.PubMed/NCBI View Article : Google Scholar
|