Introduction
The effects of exercise on the diameter of blood
vessels and blood flow within skeletal muscles are profound
(1-3);
however, it has only recently garnered sufficient attention in the
medical community (4). Wearing
myopia glasses is a common method to correct myopia. Myopia glasses
are concave lenses, which can cause peripheral hyperopic defocus on
the retina (5,6). Peripheral hyperopic defocus accelerates
the progression of myopia (7,8).
Therefore, various myopia glasses have been introduced clinically
to correct peripheral hyperopic defocus and prevent the progression
of myopia (9,10). However, their effectiveness is
limited (11,12).
It would be of interest to determine the reasons for
this limitation. Apart from inducing peripheral hyperopic defocus
on the retina, it is worthy to examine whether myopia glasses have
other effects on the eyes. From an optical theory perspective, it
would be prudent to determine whether wearing myopia glasses can
also affect eyeball movement, and to determine such an effect in an
actual situation. In the case that eyeball movement is affected,
the effect this has on the eyeballs should be examined. Any
measures that need to be taken in response to this should also
perhaps be determined. For this purpose, the present study was
designed in an aim to shed some light on the aforementioned
questions.
Subjects and methods
General information of the study
subjects
In a self-control study, 30 subjects from a
150-person hospital team were recruited to participate in an
experiment to test eye movement. A total of 7 male and 23 female
subjects, aged 18-55 years, were each instructed to wear both 0.00
D and -10.00 D glasses during the experiment. Under both conditions
and with the same fixation distance, the amount of movement of the
eyes when shifting gaze from a central point to a point light
source on the left or right was measured, and the difference
between the measurements obtained with the two glasses was
compared. The present study followed the principles of the
Declaration of Helsinki, in which all subjects signed a consent
form when informed of the objectives, risks and benefits of the
study. Ethical approval was obtained from the Institutional Review
Boards at the Ethics Committee of Chongqing Aier Eye Hospital
(Chongqing, China; identifier, no. 202215).
The inclusion criteria were as follows: The
corrected visual acuity of both eyes was normal, the dioptre of
both eyes was 0-3.00 D, and the age was 18-55 years. The exclusion
criteria were as follows: Patients with strabismus or a history of
eye surgery.
Inspection equipment included the Spark Mi Up pupil
distance measuring instrument (Shamir Optical Industry Ltd.)
(Fig. 1); a slit lamp holder and a
point light source; frames with different pupil distances; two
-10.00 D concave lenses; and two 0.00 D glasses.
Examination principle
The Spark Mi Up pupillary distance measuring
instrument captures the central reflection points on the cornea and
measures their distance from the nasal midline to obtain the
monocular pupillary distance for each eye.
The Spark Mi Up pupillary distance measuring
instrument obtains images of the reflected light points of the
cornea to calculate the pupillary distance. When the eyeball
rotates to the left and right, the movement range of the eyeball
can be calculated by measuring the monocular pupillary distance
change. The calculation formula is as follows: The rotation amount
of the eyeball (mm)=the monocular pupillary distance except for the
central gaze point-the monocular pupillary distance at the central
gaze point.
Experimental procedures
The ophthalmic slit lamp holder was fixed 50 cm in
front of the Spark Mi Up pupil distance measuring instrument (magic
mirror). The subjects were instructed to sit directly in front of
the display. Their lower jaw was placed on the lower jaw support
with the frontal part leaning against the frontal support. The
height of the support was adjusted to ensure that the subjects were
seated upright, the head position did not deviate, and the height
of the eyes was the same as that of the central point of the pupil
distance metre. The point light sources were fixed at the centres
of two sides of the screen of the measuring instrument. Ensuring
that the heads of the participants could not move during the test,
the participants were allowed to first gaze at the central point of
the display screen and then at the point light sources on either of
the two sides of the display screen. The changes in the amount of
eyeball movement from gazing at the central point to gazing at the
left or right point light were recorded for the 0.00 D glass and
-10.00 D glasses conditions (Fig.
2).
Statistical analysis
SPSS 19.0 statistical software (SPSS Inc.) was used
for statistical analysis. The measurement data were subjected to a
Shapiro-Wilk normality test' and confirmed to conform to the normal
distribution, and are expressed as the mean ± SD. The differences
were tested by paired sample t-tests. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
After wearing the concave lens, the range of eye
movement was markedly reduced, and this difference was significant
(Table I). All 30 subjects were
first requested to gaze at the right point light source from the
central fixation point. The difference between the rotation
distance of the right eye when wearing the 0.00 D glass and that
wearing the -10.0 D concave lens was 0.73±0.45 mm (t=8.93,
P<0.01). The difference between the rotations of the left eye
with the two glasses was 0.73±0.43 mm (t=9.34, P<0.01). Both
differences were statistically significant.
 | Table IComparison of eye movement between
wearing 0.00 D glasses and wearing -10.0 D lenses. |
Table I
Comparison of eye movement between
wearing 0.00 D glasses and wearing -10.0 D lenses.
| | Shift from central to
right | Shift from central to
left |
|---|
| | OD | OS | OD | OS |
|---|
| | 0.0 D | -10.0 D | Difference | 0.0 D | -10.0D | Difference | 0·00 D | -10.0 D | Difference | 0.0 D | -10.0 D | Difference |
|---|
| Mean (mm) | 1.32±0.43 | 0.58±0.32 | 0.73±0.45 | 1.40±0.38 | 0.67±0.33 | 0.73±0.43 | 1.58±0.35 | 0.78±0.36 | 0.80±0.45 | 1.70±0.34 | 0.74±0.36 | 0.96±0.52 |
| t-value | 8.93 | 9.34 | 9.80 | 10.07 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 |
When the subjects were requested to view the left
point light source from the central fixation point, the difference
between the rotation distance of the left eye when wearing the 0.00
D glasses and that when wearing the -10.0 D concave lenses was
0.96±0.52 mm (t=10.07, P<0.01). The difference between the
rotations of the right eye with the two glasses was 0.80±0.45 mm
(t=9.80, P<0.01).
Discussion
Wearing myopic glasses limits eyeball
movement
In the present study, the subjects were human. The
results revealed that apart from hyperopic defocus, the amount of
eyeball movement was significantly reduced after wearing the
concave lens. When the subjects wore the -10.0 D concave lenses,
compared with wearing the 0.00 D glasses, the eyeball movement
amount from the point of viewing at the front central gaze to the
target of the peripheral visual field was significantly reduced
when looking left or right, and the difference was statistically
significant. Thus, the hypothesis that hyperopic defocus causes
myopia cannot exclude the factor of decreased eyeball movement.
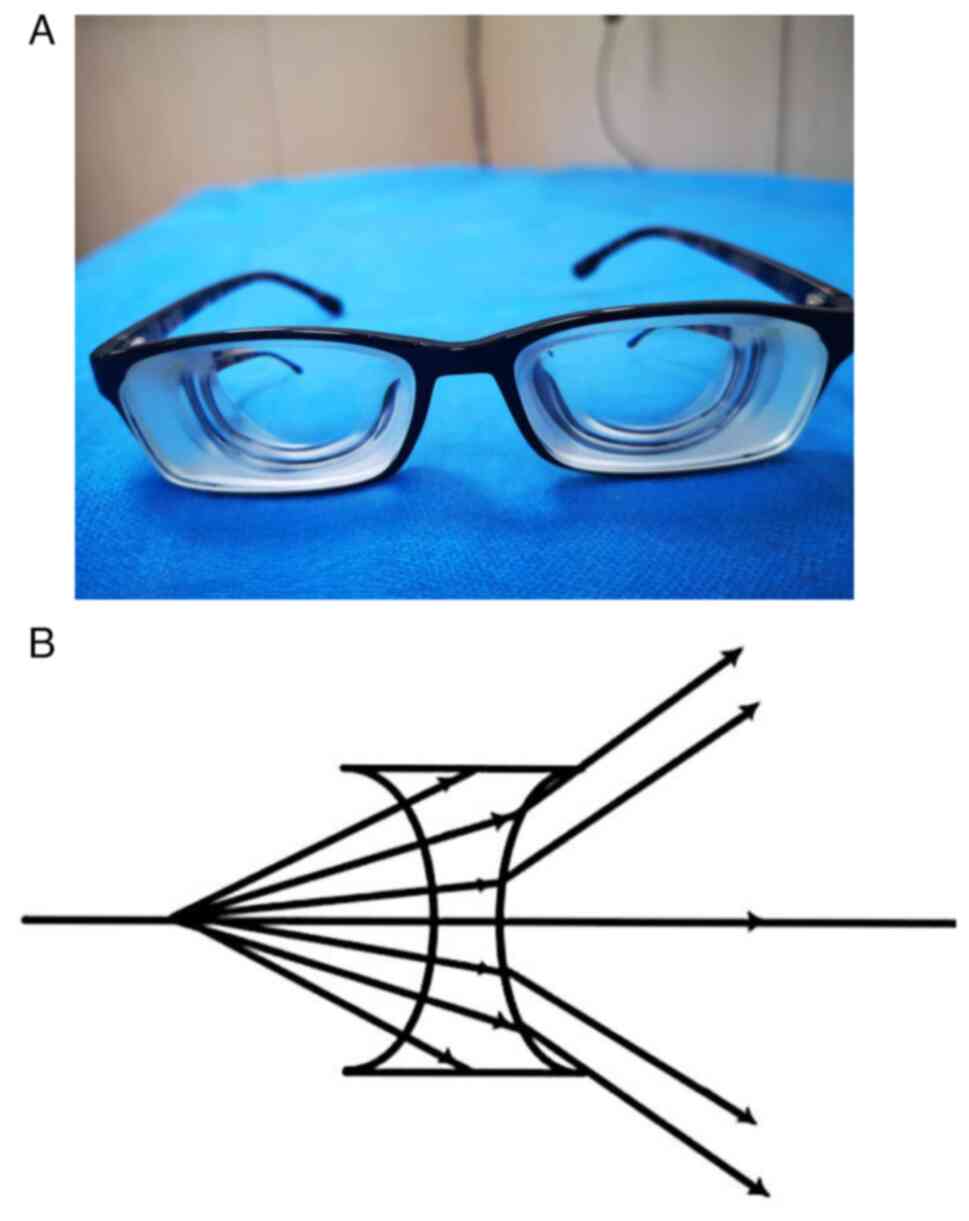
The difference can be explained by the optical
principle: When wearing high-dioptre myopia glasses (e.g., -10.0 D)
(Fig. 3A), as the concave lens
spreads out the light from the macular centre when it passes
through the lens (Fig. 3B), the
eyeball can see the peripheral visual field without large movement;
that is, after wearing the -10.0 D concave lens, the movement
amount of the eyeball is decreased when looking at the same
external visual field. When a larger visual field is required, as
the light reaches the edge of the concave lens and the lens frame,
the eyeball movement has to be replaced by the deflection of the
head, which further reduces the amount of movement of the
eyeball.
When wearing myopia glasses, the frequency of
eyeball movement per day remains unaltered; however, the amplitude
of each eye movement and the intensity of extraocular muscle
movement are significantly reduced. This phenomenon has not
previously attracted notable attention, at least to the best of our
knowledge. It would thus be of interest to determine its effect on
the eyes.
Restricted eye movement can reduce the
blood supply to the sclera and anterior chamber
Exercise has profound effects on the human vascular
system (1-3).
McIntosh et al (4) reported
that the effect of exercise on the diameter and blood flow of
arteries within skeletal muscles has been significantly
underestimated in the past, only receiving attention recently.
There is evidence to suggest that even single sessions of
moderate-intensity exercise can increase blood flow velocity within
arteries and affect their diameter. Consistent exercise over a
period of weeks to months can improve basal blood flow and arterial
diameter within skeletal muscles. Similar to other skeletal
muscles, the restriction of eye movement inevitably alters the
blood flow, luminal shear stress, arterial pressure and tangential
wall stress within the extraocular muscles, leading to a reduction
in arterial diameter and changes in vascular dilation function,
ultimately reducing blood flow speed (13-16).
Eye movements in humans are controlled by six extraocular muscles:
The superior rectus, inferior rectus, medial rectus, lateral
rectus, inferior oblique and superior oblique muscles. The
prolonged restriction of eye movement inevitably leads to the
narrowing of ocular muscular arteries and a decrease in blood flow.
The terminals of the muscular artery are the episcleral arteries
and the anterior ciliary arteries. The episcleral arteries are
formed by the branches of multiple muscular arteries and the short
posterior ciliary arteries (Fig.
4A), with the exception that the external rectus muscle has
only one muscular artery, and the other three rectus muscles have
two muscular arteries. The effects of changes in the diameter and
blood flow of muscular arteries, due to the abundance of these
arteries, on the blood supply to the sclera cannot be overlooked.
The anterior ciliary arteries are the continuation of the muscular
arteries of the rectus muscles. The episcleral arteries are
responsible for the blood supply of the sclera. The anterior
ciliary arteries participate in the blood supply of the ciliary
body and iris (Fig. 4B).
Therefore, limited eye movement not only affects the
blood supply to the sclera, but also affects the blood supply to
the ciliary body and iris. The occurrence and development of myopia
are closely related to scleral ischemia, and the remodeling of the
sclera and elongation of the eye axis due to scleral ischemia and
hypoxia are recognized pathological processes in the development of
myopia (17-21).
However, the exact cause of ischemia remains unclear. Nevertheless,
this suggests that any factors exacerbating scleral ischemia should
be avoided, and any factors improving scleral blood supply should
be emphasized. Additionally, the occurrence and development of
myopia are associated with accommodative lag, as extensively
evidenced in the literature (22-25).
Ischemia of the ciliary body and iris will undoubtedly affect the
normal functioning of eye regulation. Therefore, wearing myopic
glasses, reducing eye movement, will decrease scleral blood supply
and affect eye regulation, which is a high-risk factor for
accelerating the development of myopia.
For adolescents, the most common behaviors that
restrict eye movement, aside from wearing myopia glasses, are
likely to include reading at close distances, doing homework and
staring at a blackboard or screen, while the behaviors that
increase eye movement are outdoor exercise. The occurrence and
development of juvenile myopia have a clear association with the
time spent participating in outdoor activities. Long-term close
reading can lead to the development of myopia, and increasing the
time spent participating in outdoor activities can reduce the
incidence of myopia (26-30).
This is a recognized phenomenon. The reason has always been
unclear; however, it cannot be ruled out that it is related to
changes in eye movement. The eye movement amplitude is guided by
the target seen. The target seen in outdoor activities is not
fixed. The wider the field of view, the more rapid the target
transformation, and the greater the eye movement amplitude and
frequency. In the classroom, during long-term close reading, such
as reading a book, looking at a computer, or looking the teaching
screen, one can see a tiny field of view, and the gaze target is
relatively fixed, which is bound to limit the amplitude and amount
of eye movement.
In conclusion, the present study experimentally
confirms that wearing myopia glasses not only restricts eye
movement, but also allows for the quantitative assessment of the
degree of restricted eye movement based on different diopter
values. While higher diopter myopia glasses impose more severe
movement restrictions and require greater attention, it is crucial
to note that any degree of myopia glasses leads to persistent,
long-term, cumulative effects on eye movement. The limitations
imposed by near-distance reading on eye movement amplitude are
notable, with the severity of eye movement restriction directly
related to the duration of reading. Therefore, it is recommended
that patients wearing myopia glasses and adolescents who
unavoidably engage in prolonged near-distance reading, homework, or
focus on educational videos should enhance active eye movement or
engage in outdoor activities to compensate for restricted eye
movement, increase scleral blood supply, and thereby delay or
prevent the onset and progression of myopia.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Nanan District
Health Committee and Science and Technology Bureau of Chongqing
Joint Medical Research Project (grant no. CQNAKWNH2020-10).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and LT were responsible for the entire project
and have equal contributions, including the literature search,
creating figures, study design, data collection, data analysis,
data interpretation, manuscript writing, funding acquisition, and
project administration. HY, CC, YP, YT, JW and LA were involved in
data collection, data management and data validation. All authors
have read and approved the final manuscript. JT and LT confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Aier Eye Hospital, Chongqing, China.
Informed consent form was obtained from the participants
(volunteers). The analysis used anonymous clinical data that were
obtained after each participant agreed to participate by written
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Green DJ and Smith KJ: Effects of exercise
on vascular function, structure, and health in humans. Cold Spring
Harb Perspect Med. 8(a029819)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Spence AL, Carter HH, Naylor LH and Green
DJ: A prospective randomized longitudinal study involving 6 months
of endurance or resistance exercise. Conduit artery adaptation in
humans. J Physiol. 591:1265–1275. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Laughlin MH and Roseguini B: Mechanisms
for exercise training-induced increases in skeletal muscle blood
flow capacity: Differences with interval sprint training versus
aerobic endurance training. J Physiol Pharmacol. 59 (Suppl
7):S71–S88. 2008.PubMed/NCBI
|
|
4
|
McIntosh MC, Anglin DA, Robinson AT, Beck
DT and Roberts MD: Making the case for resistance training in
improving vascular function and skeletal muscle capillarization.
Front Physiol. 15(1338507)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shang L, Liu W, Song Y and Jiang J:
Methodology research on peripheral refractive measurement under
spectacle correction. Chin J Optom Ophthalmol Vis Sci. 12:204–208.
2010.
|
|
6
|
Dai YS, Lin DD, Lu P, Chen H and Jiang J:
Study of peripheral refraction with single-vision spectacle lenses
in myopic children. International Eye Science. 13:339–342.
2013.
|
|
7
|
Erdinest N, London N, Lavy I, Berkow D,
Landau D, Levinger N and Morad Y: Peripheral defocus as it relates
to myopia progression: A mini-review. Taiwan J Ophthalmol.
13:285–292. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Benavente-Pérez A, Nour A and Troilo D:
Axial eye growth and refractive error development can be modified
by exposing the peripheral retina to relative myopic or hyperopic
defocus. Invest Ophthalmol Vis Sci. 55:6765–6773. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lupon M, Nolla C and Cardona G: New
designs of spectacle lenses for the control of myopia progression:
A scoping review. J Clin Med. 13(1157)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo H, Li X, Zhang X, Wang H and Li J:
Comparing the effects of highly aspherical lenslets versus defocus
incorporated multiple segment spectacle lenses on myopia control.
Sci Rep. 13(3048)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walline JJ, Lindsley KB, Vedula SS, Cotter
SA, Mutti DO, Ng SM and Twelker JD: Interventions to slow
progression of myopia in children. Cochrane Database Syst Rev.
1(CD004916)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lawrenson JG, Shah R, Huntjens B, Downie
LE, Virgili G, Dhakal R, Verkicharla PK, Li D, Mavi S, Kernohan A,
et al: Interventions for myopia control in children: A living
systematic review and network meta-analysis. Cochrane Database Syst
Rev. 2(CD014758)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Green DJ, Hopman MT, Padilla J, Laughlin
MH and Thijssen DH: Vascular adaptation to exercise in humans: Role
of hemodynamic stimuli. Physiol Rev. 97:495–528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Naylor LH, O'Driscoll G, Fitzsimons M,
Arnolda LF and Green DJ: Effects of training resumption on conduit
arterial diameter in elite rowers. Med Sci Sports Exerc. 38:86–92.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dinenno FA, Tanaka H, Monahan KD,
Clevenger CM, Eskurza I, DeSouza CA and Seals DR: Regular endurance
exercise induces expansive arterial remodelling in the trained
limbs of healthy men. J Physiol. 534:287–295. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maiorana A, O'Driscoll G, Taylor R and
Green D: Exercise and the nitric oxide vasodilator system. Sports
Med. 33:1013–1035. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu H, Chen W, Zhao F, Zhou Q, Reinach PS,
Deng L, Ma L, Luo S, Srinivasalu N, Pan M, et al: Scleral hypoxia
is a target for myopia control. Proc Natl Acad Sci USA.
115:E7091–E7100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao F, Zhang D, Zhou Q, Zhao F, He M,
Yang Z, Su Y, Zhai Y, Yan J, Zhang G, et al: Scleral HIF-1α is a
prominent regulatory candidate for genetic and environmental
interactions in human myopia pathogenesis. EBioMedicine.
57(102878)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tang X, Liu L and Yang Q: Effects of
hypoxia on the activation of endoplasmic reticulum stress response
and on the scleral remodeling in human fetal scleral fibroblasts.
Recent Adv Ophthalmol. 42:529–533. 2022.
|
|
20
|
Yu Q and Zhou JB: Scleral remodeling in
myopia development. Int J Ophthalmol. 15:510–514. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Metlapally R and Wildsoet CF: Scleral
mechanisms underlying ocular growth and myopia. Prog Mol Biol
Transl Sci. 134:241–248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prousali E, Haidich AB, Tzamalis A, Ziakas
N and Mataftsi A: ‘The role of accommodative function in myopic
development: A review’. Semin Ophthalmol. 37:455–461.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schor S: The influence of interactions
between accommodation and convergence on the lag of accommodation.
Ophthalmic Physiol Opt. 19:134–150. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaphle D, Varnas SR, Schmid KL, Suheimat
M, Leube A and Atchison DA: Accommodation lags are higher in myopia
than in emmetropia: Measurement methods and metrics matter.
Ophthalmic Physiol Opt. 42:1103–1114. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Norazman FNN, Mohd-Ali B, Syed Mohd Dardin
SF, Mohamad Shahimin M, Mohamad Fadzil N, Mohd Saman MN and Mohidin
N: Baseline accommodation and binocular vision measures in Malay
schoolchildren enrolled in the myopia control study using spectacle
lenses in Kuala Lumpur. Clin Optom (Auckl). 16:45–52.
2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu PC, Tsai CL, Wu HL, Yang YH and Kuo HK:
Outdoor activity during class recess reduces myopia onset and
progression in school children. Ophthalmology. 120:1080–1085.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jin JX, Hua WJ, Jiang X, Wu XY, Yang JW,
Gao GP, Fang Y, Pei CL, Wang S, Zhang JZ, et al: Effect of outdoor
activity on myopia onset and progression in school-aged children in
northeast China: The Sujiatun Eye Care Study. BMC Ophthalmol.
15(73)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Martínez-Albert N, Bueno-Gimeno I and
Gené-Sampedro A: Risk Factors for Myopia: A Review. J Clin Med.
12(6062)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang J and Deng G: Protective effects of
increased outdoor time against myopia: A review. J Int Med Res.
48(0300060519893866)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karthikeyan SK, Ashwini DL, Priyanka M,
Nayak A and Biswas S: Physical activity, time spent outdoors, and
near work in relation to myopia prevalence, incidence, and
progression: An overview of systematic reviews and meta-analyses.
Indian J Ophthalmol. 70:728–739. 2022.PubMed/NCBI View Article : Google Scholar
|