Introduction
The coronavirus disease 2019 (COVID-19) pandemic,
caused by severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), is considered one of the greatest global public
health crises. Initial respiratory infections can rapidly progress
to severe pneumonia in predisposed individuals. Of note, ~33% of
patients hospitalized due to COVID-19-associated pneumonia develop
acute respiratory distress syndrome (ARDS), and 16-26% of patients
require intensive care unit (ICU) admission and treatment with
invasive mechanical ventilation (IMV) (1). Other complications, such as sepsis,
acute coronary syndrome (ACS), acute renal failure (ARF) and
thromboembolic events, occur in patients undergoing treatment in
the ICU. The mortality rate among critically ill patients with
COVID-19 is as high as 78% (2),
depending on the geographic and social characteristics of the
population.
SARS-CoV-2 infection reduces the expression of
angiotensin-converting enzyme II (ACE2) receptors on the surface of
type II pneumocytes, and cardiac, renal, brain, intestinal and
endothelial cells, leading to an imbalance in the renin-angiotensin
system (RAS) (3). An overactivated
angiotensin II (Ang II)-Ang II type I receptor (AT1R) axis promotes
inflammatory signaling, cytokine storms, massive endothelial cell
injury and coagulation disorders (4).
Previous studies have found that hypertension is one
of the most common comorbidities among patients with COVID-19
(5,6). The proposed link between hypertension
and COVID-19 is the interruption of the classical RAS pathway due
to the downregulation of the ACE2 receptors, and hyperactivation of
the Ang II-AT1R axis. Hypertension itself induces endothelial
inflammation, and weakens the immune response, causing target organ
damage that may increase susceptibility to serious complications of
COVID-19(3). The association between
hypertension and the severe form of COVID-19 remains unclear, as
certain studies have reported that hypertension increases the
severity or mortality from COVID-19 (2,5,7), while others have not found such
evidence (8,9).
In May 2023, the World Health Organization declared
the end of the COVID-19 pandemic. Epidemiologists have predicted
that COVID-19 will continue with annual fluctuations in infection
and may persist beyond 2025(10).
Considering that an estimated 1.28 billion adults worldwide have
hypertension (11), and no specific
drug has yet been approved for the treatment of COVID-19, the aim
of the present study was to investigate whether hypertension
contributes to the development of complications in patients
undergoing treatment in the ICU for COVID-19.
The present study aimed to assess the incidence of
complications in critically ill patients with COVID-19-associated
pneumonia and a history of hypertension, as compared with patients
without hypertension. The predictive significance of hypertension
in the incidence of complications in critically ill patients with
COVID-19 pneumonia was determined.
Patients and methods
Study design
The present retrospective single-center study was
conducted between January 1 and December 31, 2021, at the
Department of Anesthesiology, Resuscitation, and Intensive Care of
the Cantonal Hospital in Zenica, Bosnia and Herzegovina. The study
report followed the Strengthening the Reporting of Observational
Studies in Epidemiology (STROBE) guidelines (12).
Ethics approval
The Research Hospital Ethics Committee of the Zenica
Cantonal Hospital approved the study protocol (approval no.
00-03-35-337-23/23). The study followed the Declaration of Helsinki
(13), and written informed consent
was obtained from all subjects or their next-of-kin.
Patient selection
The present study included data from 372 critically
ill adults diagnosed with COVID-19. COVID-19 infection was
confirmed by the reverse transcription PCR of nasopharyngeal swab
samples. The criteria for admission to the ICU were as follows:
Respiratory distress with dyspnea and tachypnea (>30/min),
oxygen saturation in room air <90%, a ratio of partial pressure
of oxygen in arterial blood to fraction of inspired oxygen
(PaO2/FiO2) <300 mmHg, and the progression
of pulmonary infiltrates by >50% within 48 h on a chest
radiography (14). Patients with
incomplete medical records, those aged <18 years, those
resuscitated immediately upon admission to the ICU, those
hospitalized <24 h in the ICU, or those with COVID-19 infection,
but admitted to the ICU for reasons other than pneumonia (e.g.,
pregnancy, trauma, tumor surgery or chronic organ dysfunction) were
excluded from the study. Patients who met the study criteria were
divided into the hypertension group (HTA group; study group), which
included 245 patients with a history of hypertension, and a non-HTA
group (control group), which included 127 patients without
hypertension. Hypertension was defined as an office systolic blood
pressure (SBP) ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg
according to the European Society of Cardiology (ESC) and the
European Society of Hypertension (ESH) (15). All patients received the same
therapeutic protocol, including respiratory support, ventilation
strategy, corticosteroids (methylprednisolone), anticoagulants (low
molecular weight heparin), antibiotics (second generation
cephalosporins), antivirals (remdesivir) and immunomodulatory
therapy (tocilizumab). Patients in the HTA group continued with
previously prescribed antihypertensive medications.
Data collection
Data were retrieved from the electronic medical
records by two researchers. Demographic and clinical data included
age, sex, comorbidities, time from onset of symptoms to
hospitalization (T1), time from hospitalization to ICU admission
(T2) and the length of stay in the ICU (T3). Hemodynamic parameters
recorded at ICU admission, such as SBP, DBP, mean BP (MBP) and
heart rate (HR) were also collected. Initial BP was classified into
grades according to the ESC and ESH in both groups. The type and
number of anti-hypertensive drugs were documented in the HTA
group.
Study outcomes
The primary outcomes were intra-group differences in
the incidence of the following complications: IMV, sepsis, ACS,
ARF, hemodialysis, cerebrovascular insults (CVIs), thromboembolic
complications, pleural abnormalities, surgical procedures and total
complications per patient. The secondary aim was to determine the
predictive value of hypertension in the incidence of complications
in critically ill patients with COVID-19.
Definitions of complications
IMV was defined as a therapeutic option for ARDS
refractory to non-invasive respiratory support, altered
consciousness and the progression of gas analysis abnormalities (pH
≤7.25, PaO2 ≤45 mmHg, SpO2 <85% with
FiO2 0.5). Sepsis was defined according to The Third
International Consensus Definitions for Sepsis and Septic Shock.
ACS was defined according to The Fourth Universal Definition of
Myocardial Infarction. ARF and hemodialysis were defined according
to the Kidney Disease Improving Global Outcomes Clinical Practice
Guidelines for Acute Kidney Injury. CVI was defined as an acute,
focal or global neurological disorder caused by a cerebral
infarction or hemorrhage. Thromboembolic complications (acute limb
ischemia, pulmonary embolism and intestinal infarction) were
defined as thrombotic occlusions of circulation in the extremities,
lungs and mesenteric/bowel blood vessels. Pleural abnormalities
(pneumothorax and pneumomediastinum) were defined as the presence
of air in the pleural space or mediastinum. Surgical procedures
(arterial thrombectomy, amputation of ischemic limbs and
laparotomy) were defined as therapeutic treatments for
thromboembolic and ischemic events or gastrointestinal bleeding,
and were performed under general, spinal or regional
anesthesia.
Statistical analysis
Statistical analysis was performed using SPSS v.
25.0 (IBM Corp.). The normality of the data distribution was
confirmed using the Kolmogorov-Smirnov test. Categorical variables
are presented as frequencies (percentages) and compared using the
Pearson's χ2 test and Fisher's exact test. Continuous
variables are presented as the mean ± standard deviation or as a
median (interquartile range) and tested using an unpaired Student's
t-test or Mann-Whitney U test. A multivariate logistic regression
analysis (adjusted for potential confounders) was used to determine
the predictive accuracy of hypertension for the incidence of
complications. The predictive discriminative power of hypertension
was determined using receiver operating characteristic (ROC) curve
analysis. The area under the curve (AUC), specificity, sensitivity,
positive predictive value (PPV) and negative predictive value were
calculated. A two-sided P<0.05, 95% confidence interval and an
expected study power of 80% were considered to indicate a
statistically significant difference.
Results
Baseline demographic and clinical
characteristics of the patients in the two groups
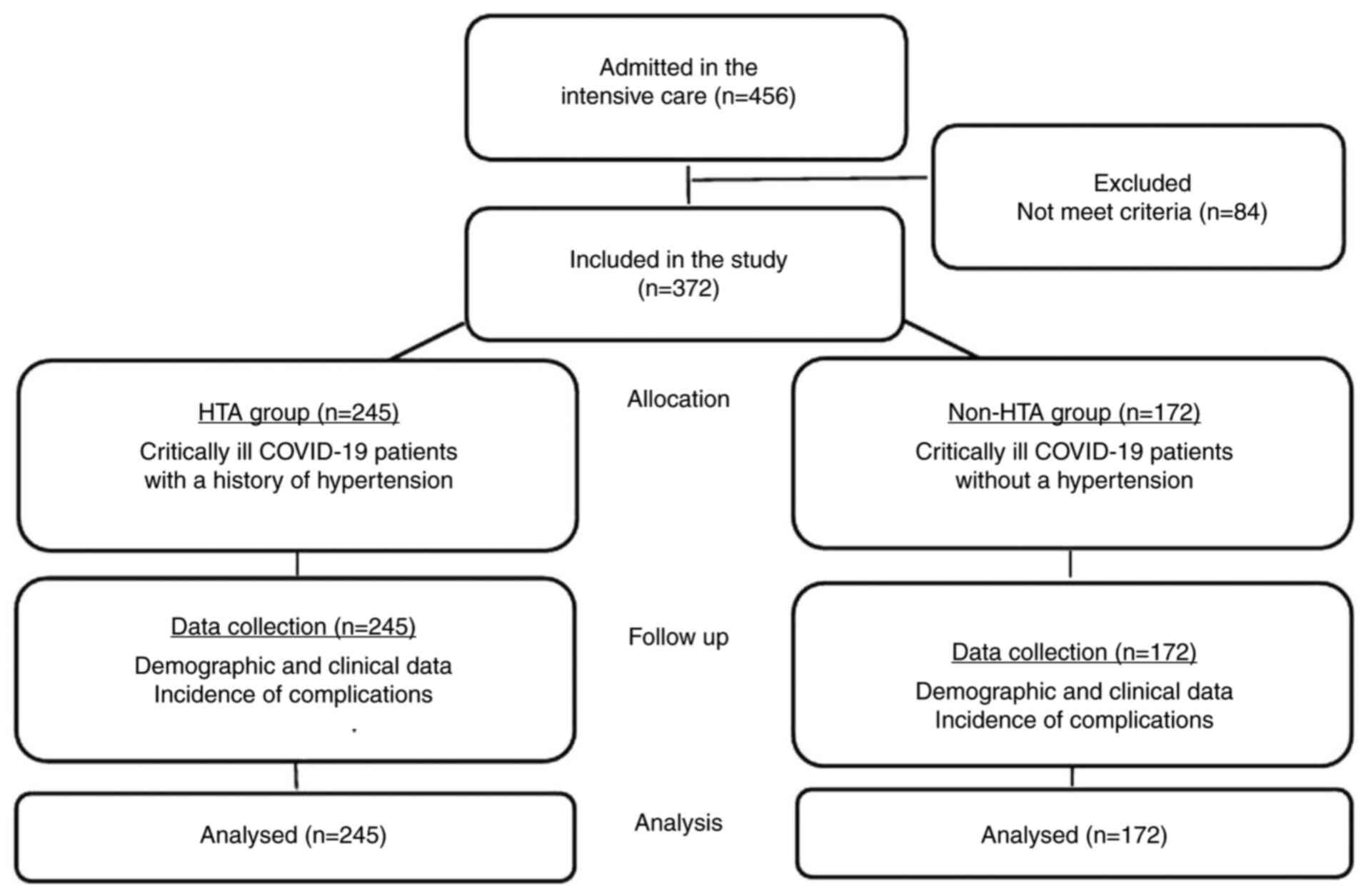
In 2021, 456 adult patients with COVID-19 were
admitted to the ICU. A total of 372 patients met the eligibility
criteria, and were included in the present study and analyzed.
There were 245 (65.8%) patients with hypertension and 127 (34.2%)
without a history of hypertension (Fig.
1).
The median age was higher in the HTA group [67
(61.5-72.3) years] than in the control group [62 (53.1-68.5) years;
P<0.001]. There were more male than female patients in both
groups, although no significant difference was found. Coronary
heart disease (36.3%) was the most common comorbidity in the HTA
group, whereas chronic obstructive pulmonary disease (14.2%) was
the most common comorbidity in the control group. The average
number of comorbidities per patient was higher in the HTA group
(2.14±0.97 vs. 0.94±0.34; P<0.001). Significantly higher SBP,
DBP, MBP and HR values at ICU admission were observed in the HTA
group (P<0.001). In the HTA group, initial BP was most
frequently classified as grade 1 hypertension [80 patients
(32.7%)]. In the control group, initial BP was most often
classified as high-normal [42 patients (33.2%)]. ACE inhibitors
were the most common anti-hypertensive therapy administered to 183
patients (74.6%) in the HTA group (Table
I).
 | Table IBaseline demographic and clinical
characteristics of the study participants. |
Table I
Baseline demographic and clinical
characteristics of the study participants.
| Parameters | HTA group, n=245
(65.8%) | non-HTA group, n=127
(34.2%) | P-value |
|---|
| Age, years (median,
IQR) | 67 (61.5-72.3) | 62 (53.1-68.5) |
0.001a |
| Sex, n (%) | | | 0.067b |
|
Male | 141 (57.6) | 86 (67.7) | |
|
Female | 104 (42.4) | 41 (32.3) | |
| Comorbidities,
n (%) | | | |
|
Coronary
heart disease | | |
0.001b |
|
Yes | 89 (36.3) | 12 (9.4) | |
|
No | 156 (63.7) | 115 (90.6) | |
|
Diabetes
mellitus | | |
0.001b |
|
Yes | 81 (33.1) | 17 (13.4) | |
|
No | 164 (66.9) | 110 (86.6) | |
|
COPD | | | 0.162b |
|
Yes | 23 (9.4) | 18 (14.2) | |
|
No | 222 (90.6) | 109 (85.8) | |
|
Hypothyroidism | | | 0.096b |
|
Yes | 21 (8.6) | 5 (3.9) | |
|
No | 224 (91.4) | 122 (96.1) | |
|
Chronic
kidney disease | | | 0.174c |
|
Yes | 9 (3.7) | 1 (0.8) | |
|
No | 236 (96.3) | 126 (99.2) | |
|
Cerebrovascular
disease | | | 0.171b |
|
Yes | 8 (3.6) | 8 (6.3) | |
|
No | 237 (96.4) | 199 (93.6) | |
| Average of
comorbidities | | | |
|
per patient
(mean ± SD) | 2.14±0.97 | 0.94±0.34 |
0.001d |
| T1, days (median,
IQR) | 7.0 (5.0-10.0) | 7.0 (5.0-9.0) | 0.121a |
| T2, days (median,
IQR) | 3.0 (1.0-5.0) | 3.0 (2.0-5.0) | 0.070a |
| T3, days (median,
IQR) | 6.0 (4.0-8.5) | 7.0 (4.0-9.0) | 0.346a |
| SBP, mmHg (mean ±
SD) | 145.04±17.15 | 132.28±7.86 |
0.001d |
| DBP, mmHg (mean ±
SD) | 106.97±15.48 | 96.19±10.46 |
0.001d |
| MBP, mmHg (mean ±
SD) | 88.04±12.85 | 79.48±9.81 |
0.001d |
| Heart rate,
beat/min (mean ± SD) | 101.88±21.19 | 91.96±15.88 |
0.001d |
| Hypertension grade,
n (%) | | |
0.001b |
|
Optimal BP,
<120 mmHg | 26 (10.6) | 25 (19.6) | |
|
Normal BP,
120-130/80-84 mmHg | 29 (11.8) | 36 (28.3) | |
|
High-normal
BP, 130-139/85-89 mmHg | 43 (17.6) | 42 (33.2) | |
|
Grade 1 HTA,
140-159/90-99 mmHg | 80 (32.7) | 24 (18.9) | |
|
Grade 2 HTA,
160-179/100-109 mmHg | 43 (17.6) | / | / |
|
Grade 3 HTA,
>180/110 mmHg | 24 (9.8) | / | / |
| Type of
anti-hypertensive drugs, n (%) | | | |
|
ACE
inhibitors | 183 (74.6) | / | / |
|
Angiotensin
receptor blockers | 31 (12.7) | / | / |
|
Calcium
channel blockers | 67 (27.3) | / | / |
|
β-blockers | 112 (45.7) | / | / |
|
Diuretics | 100 (40.8) | / | / |
| Average of
antihypertensive drugs | | | |
|
per patient
(mean ± SD) | 2.70±1.34 | / | / |
Comparison of the incidence of
complications between groups
A significantly higher incidence of IMV
(P<0.001), sepsis (P<0.001), ACS (P<0.043), ARF
(P<0.007), hemodialysis (P<0.018), CVIs (P<0.037),
surgical procedures (P<0.09) and total complications per patient
(P<0.001) was noted in the HTA group. There were 27 (11%)
patients without complications in the HTA group compared with 43
(33.9%) in the control group (P<0.001; Table II).
 | Table IIIncidence and distribution of
complications according to the groups. |
Table II
Incidence and distribution of
complications according to the groups.
| Complications | HTA group, n=245
(65.8%) | non-HTA group,
n=127 (34.2%) | 95 % CI | P-value |
|---|
| IMV, n (%) | | | 0.00-0.00 |
0.001a |
|
Yes | 215 (87.8) | 85 (66.9) | | |
|
No | 30 (12.2) | 42 (33.1) | | |
| Sepsis, n (%) | | | 0.00-0.00 |
0.001a |
|
Yes | 141 (57.6) | 35 (27.6) | | |
|
No | 34 (27.6) | 92 (72.4) | | |
| Acute coronary
syndrome, n (%) | | | 0.05-0.06 |
0.043a |
|
Yes | 42 (17.1) | 12 (9.4) | | |
|
No | 203 (82.9) | 115 (90.6) | | |
| Acute renal
failure, n (%) | | | 0.00-0.01 | 0.007a |
|
Yes | 50 (20.4) | 12 (9.4) | | |
|
No | 195 (79.6) | 115 (90.6) | | |
| Hemodialysis, n
(%) | | | 0.02-0.03 |
0.018b |
|
Yes | 10 (4.1) | 0 (0) | | |
|
No | 235 (95.9) | 127(100) | | |
| Cerebrovascular
insults, n (%) | | | 0.04-0.05 |
0.037b |
|
Yes | 8 (3.3) | 0 (0) | | |
|
No | 237 (96.7) | 127(100) | | |
| Thromboembolic
events, n (%) | | | 0.07-0.08 | 0.055a |
|
Yes | 26 (10.6) | 6 (4.7) | | |
|
No | 219 (89.4) | 121 (55.3) | | |
| Pleural
abnormalities, n (%) | | | 0.12-0.13 | 0.131b |
|
Yes | 15 (6.1) | 3 (2.4) | | |
|
No | 230 (93.9) | 124 (97.6) | | |
| Surgical
procedures, n (%) | | | 0.01-0.01 |
0.009b |
|
Yes | 14 (4.6) | 1 (0.8) | | |
|
No | 231 (95.4) | 126 (99.2) | | |
| Average of
complications | | | | |
|
per patient,
(mean ± SD) | 2.11±1.1 | 1.2±0.7 | 0.00-0.00 |
0.001c |
| Distribution of
complications | | | |
0.010b |
|
No
complications, n (%) | 27 (11.0) | 43 (33.9) | 0.00-0.00 | |
|
1
complication, n (%) | 43 (17.6) | 34 (2.8) | 0.04-0.04 | |
|
2
complications, n (%) | 76.(31.1) | 31 (24.4) | 0.18-0.19 | |
|
3
complications, n (%) | 75 (30.6) | 15 (11.8) | 0.00-0.00 | |
|
4
complications, n (%) | 19 (7.8) | 3 (2.4) | 0.03-0.04 | |
|
5
complications, n (%) | 5 (2.0) | 0 (0.0) | 0.16-0.17 | |
Predictive significance of
hypertension for the incidence of complications
Univariate regression analysis revealed that
hypertension had a potential predictive significance for the
incidence of IMV (P<0.001), sepsis (P<0.001), ACS
(P<0.040), surgical interventions (P<0.036) and total
complications per patient (P<0.001; Table III).
 | Table IIIUnivariate regression analysis. |
Table III
Univariate regression analysis.
| | Univariate
regression analysis | |
|---|
| Complications | OR | 95% CI | P-value |
|---|
| IMV | 3.541 | 2.08-6.02 | 0.001 |
| Sepsis | 3.564 | 2.24-5.67 | 0.001 |
| Acute coronary
syndrome | 1.983 | 1.00-3.91 | 0.040 |
| Acute renal
failure | 2.457 | 1.25-4.80 | 0.009 |
| Hemodialysis | 8.730 | 0.00-0.00 | 0.999 |
| Cerebrovascular
insults | 8.656 | 0.00-0.00 | 0.999 |
| Thromboembolic
events | 2.394 | 0.95-5.97 | 0.061 |
| Pleural
abnormalities | 2.696 | 0.76-9.49 | 0.123 |
| Surgical
procedures | 8.803 | 1.15-6.71 | 0.036 |
| Total complications
per patients | 1.987 | 1.61-2.44 | 0.001 |
After adjusting for age, a history of coronary heart
disease and diabetes mellitus, multivariate regression analysis
confirmed the independent predictive significance of hypertension
on the incidence of IMV (OR=1.696; P<0.02), sepsis (OR=1.807;
P<0.01) and total complications per patient (OR=3.101;
P<0.001) in critically ill patients with COVID-19 (Table IV).
 | Table IVMultivariate regression analysis. |
Table IV
Multivariate regression analysis.
| | Multivariate
analysis |
|---|
| | Unadjusted
model | Adjusted
modela |
|---|
| Complications | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| IMV | 2.076 | 0.64-4.17 | 0.001 | 1.696 | 0.92-2.91 | 0.02 |
| Sepsis | 2.416 | 0.59-4.36 | 0.001 | 1.807 | 0.97-3.58 | 0.01 |
| Acute coronary
syndrome | 0.537 | 0.20-1.41 | 0.208 | / | / | / |
| Acute renal
failure | 0.443 | 0.16-1.18 | 0.104 | / | / | / |
| Surgical
procedures | 0.512 | 0.19-1.37 | 0.193 | / | / | / |
| Total complications
per patients | 4.025 | 0.91-7.99 | 0.001 | 3.101 | 1.57-6.10 | 0.001 |
ROC curve analysis demonstrated the predictive
discriminative power of hypertension for the incidence of IMV (AUC,
0.67; PPV, 71.7%; P<0.05), sepsis (AUC, 0.69; PPV, 77.5%;
P<0.026) and total complications per patient (AUC, 0.71; PPV,
81.4%; P<0.000; Table V).
 | Table VPredictive discriminatory power of
hypertension on the incidence of complications. |
Table V
Predictive discriminatory power of
hypertension on the incidence of complications.
| | IMV | Sepsis | Total complications
per patient |
|---|
| AUC | 0.67 | 0.69 | 0.71 |
| Sensitivity % | 67.1 | 69.6 | 73.4 |
| Specificity % | 60.9 | 64.0 | 63.2 |
| PPV % | 71.7 | 77.5 | 81.4 |
| NPV % | 34.5 | 37.7 | 50.1 |
| 95% CI | 0.48-0.70 | 0.54-0.79 | 0.65-0.81 |
| P-value | 0.05 | 0.026 | 0.001 |
Discussion
The present retrospective study investigated the
predictive significance of pre-existing hypertension on the
incidence of complications in critically ill patients with
COVID-19-associated pneumonia. The results revealed that patients
with a history of hypertension had a higher incidence of
complications during treatment in the ICU. Hypertension was
identified as an independent positive predictor of IMV, sepsis and
total complications per patient. Hypertension increased the OR for
IMV by 1.696-fold, sepsis by 1.807-fold and total complications per
patient by 3.101-fold.
The COVID-19 pandemic has officially ended; however,
the virus remains widespread worldwide. The emergence of more
transmissible and virulent variants of SARS-CoV-2 will continue to
pose a threat to global health (16). Patients with chronic diseases, such
as hypertension, are still at risk of developing severe forms of
COVID-19 and related complications. The prevalence of hypertension
in patients with COVID-19 is dependent on the rate of hypertension
in the general population and the form of COVID-19. In the present
study, hypertension was the most prevalent comorbidity, present in
65.8% of the critically ill patients. Cummings et al
(17) reported a 63% prevalence of
hypertension in patients in the ICU.
In the present study, the median age and rates of
coronary heart disease and diabetes mellitus were higher in the HTA
group than those in the control group. To avoid confounding factors
within baseline characteristics, the multivariate regression
analysis was adjusted for age, a history of coronary heart disease
and diabetes mellitus. Both groups were predominantly male, which
was consistent with previous findings (7,9,18).
In the present study, in 18.9% of the patients in
the control group, the mean value of BP at ICU admission was
classified as grade 1 hypertension, although they had no history of
hypertension. This could have been associated with
COVID-19-specific mechanisms, such as cardiovascular consequences
of unopposed Ang II-AT1R axis (vasoconstriction, renal salt and
water retention, increased sympathetic tone), hypoxia and
inflammation (3), but also
non-specific mechanisms, such as corticosteroid therapy, fluid and
hemodynamic management, sleep deprivation and psychosocial
stress.
Despite a large body of research, the difference in
complication rates between patients critically ill with COVID-19
with and without hypertension has been limited. The present study
found a higher incidence of all the assessed complications in the
HTA group. The most common complications were IMV, sepsis, ARF and
ACS. Chen et al (18)
reported a similar distribution of complications in hypertensive
patients with COVID-19 hospitalized inside and outside the ICU,
including ARDS, heart failure, sepsis and ARF.
During the COVID-19 pandemic, IMV was the main
supportive treatment for hypoxemic ARDS caused by viral alveolar
damage and immune-cell infiltration. The IMV rate was 29.1-94%,
depending on differences in intubation criteria, ICU resources and
target study populations (19,20). In
the present study cohort, the incidence of IMV was 80.6%. IMV was
required in 215 (87.8%) patients in the HTA group and in 88 (66.9%)
patients in the control group.
COVID-19-related sepsis is of viral origin and is
based on a hyperinflammatory response to viral infection and
consequent immunosuppression, which induces cell apoptosis,
hemodynamic instability, metabolic failure and multi-organ damage
(21). Herein, sepsis rates of 57.6
and 27.6% were recorded in the HTA and control groups,
respectively. Hypertension delays viral clearance, which could
contribute to a higher sepsis rate in the HTA group (3).
The mechanisms involved in COVID-19-related ARF
include direct viral injury to the kidneys, systemic effects of
infection and secondary effects (hemodynamic failure and
therapeutic consequences) (22). In
the present study, ARF occurred in 50 (20.4%) patients in the HTA
group, of whom 10 (20%) required hemodialysis. In the control
group, ARF occurred in 12 (9.4%) patients and there was no need for
hemodialysis. These findings suggested an adverse effect of
pre-existing hypertension on the compensatory capacity of target
organs.
The reported rate of COVID-19-related ACS is
16.1-23.8% (23). Cardiotropic viral
properties and hyperinflammation cause endotheliitis, a
supply/demand imbalance, and a hypercoagulable state, leading to
coronary thrombosis. In the present study, the ACS rates were 17.1
and 9.4% in the HTA and control groups, respectively. Hypertension
may worsen the rate of ACS with chronically increased sympathetic
activity, arterial stiffness and impaired myocardial relaxation
(3).
Despite routine thromboprophylaxis, thromboembolic
events were recorded in 10.6 and 4.7% of the patients in the HTA
and control groups, respectively. Klok et al (24) reported a 31% incidence of thrombotic
complications in patients with COVID-19in the ICU. The discrepancy
in the results between the studies could be explained by the
different classifications of complications.
In the literature, the incidence of CVI (ischemic
and hemorrhagic) in patients critically ill with COVID-19 was found
to be up to 6% (25). In the present
study, the rate of CVI in the HTA group was 3.3% and there were no
CVIs in the control group. Such a low rate of CVIs was probably
underestimated, due to the difficulty of neurological assessment in
intubated and sedated patients.
A higher rate of pleural abnormalities was noted in
the HTA group due to a greater need for IMV; however, there was no
statistically significant difference between the groups.
As was expected, surgical procedures were performed
more often in the HTA group due to thromboembolic complications,
and acute ischemic and hemorrhagic events were more common in this
group.
Previous studies have evaluated the impact of a
history of hypertension on the severity of COVID-19, although with
very inconsistent results, due to large heterogeneity across the
studies, including patient populations (7,9,18), disease course (7,9,18), the definition of hypertension
(8,18,26),
statistical analysis (2,9,26,27,28)
and study outcomes (29,30). In order to minimize bias, the present
study focused exclusively on complications in critically ill
patients with COVID-19 and pre-existing hypertension, and included
multivariate regression analysis adjusted for potential
confounders. These results provided evidence that a history of
hypertension increased the incidence of complications in critically
ill patients with COVID-19, and revealed hypertension as a
predictor of IMV, sepsis and total complications per patient. There
lies the strength of the present study. The study limitations
include its retrospective, single-center design and the absence of
external validation. The present study also did not evaluate ICU
mortality rates or follow-up of patients following ICU and hospital
discharge.
In conclusion, pre-existing hypertension predicts a
higher incidence of complications in critically ill patients with
COVID-19. Early identification and effective treatment of COVID-19
patients with a history of hypertension are paramount to avoid
disease progression and complications.
Acknowledgements
The authors would like to thank M.Sc. Senada
Čaušević (Cantonal Hospital Zenica, University of Zenica, Faculty
of Medicine Zenica, Bosnia and Herzegovina), for her assistance in
proofreading and language editing.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK and NR contributed equally to the conception and
design of the study, as well as to the acquisition, analysis and
interpretation of the data; in the writing the first draft of the
manuscript; and in revising the manuscript. AK and RN confirm the
authenticity of all the raw data. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of the Cantonal
Hospital Zenica approved the study protocol (approval no.
00-03-35-337-23/23). The study followed the Declaration of
Helsinki, and written informed consent was obtained from all
subjects or their next-of-kin.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tzotzos SJ, Fischer B, Fischer H and
Zeitlinger M: Incidence of ARDS and outcomes in hospitalized
patients with COVID-19: A global literature survey. Crit Care.
24(516)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tavares CAM, Bailey MA and Girardi ACC:
Biological context linking hypertension and higher risk for
COVID-19 severity. Front Physiol. 19(599729)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peng M, He J, Xue Y, Yang X, Liu S and
Gong Z: Role of hypertension on the severity of COVID-19: A review.
J Cardiovasc Pharmacol. 78:648–655. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fathi M, Vakili K, Sayehmiri F,
Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M and Eilami O: The
prognostic value of comorbidity for the severity of COVID-19: A
systematic review and meta-analysis study. PLoS One.
16(e0246190)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ayed M, Borahmah AA, Yazdani A, Sultan A,
Mossad A and Rawdhan H: Assessment of clinical characteristics and
mortality-associated factors in COVID-19 critical cases in Kuwait.
Med Princ Pract. 30:185–192. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shalaeva EV, Shadmanov AК, Azizova FL,
Mirakhmedova KT, Messerli FH, Franco OH and Saner H: Is lone
hypertension a risk factor for more severe COVID-19 outcomes? Glob
Heart. 17(17)2022.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Xavier LG, Neto RS, Morais MHO,
Cronemberger PJ, Martins MCC and Rosal M: Clinical aspects of
hypertensive patients with COVID-19 hospitalized in a campaign
hospital in Northeast Brazil. Int J Cardiovasc Sci.
36(e2022003)2023.
|
|
10
|
Scudellari M: How the pandemic might play
out in 2021 and beyond. Nature. 584:22–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
World Health Organisation. Hypertension.
2023; March 16. https://www.who.int/news-room/fact-sheets/detail/hypertension.
Accessed May 15, 2024.
|
|
12
|
Vandenbroucke JP, von Elm E, Altman DG,
Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ and
Egger M: STROBE Initiative. Strengthening the reporting of
observational studies in epidemiology (STROBE): Explanation and
elaboration. Epidemiology. 18:805–835. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
World Medical Association. World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chawla R, Dixit SB, Zirpe KG, Chaudhry D,
Khilnani GC, Mehta Y, Khatib KI, Jagiasi BG, Chanchalani G, Mishra
RC, et al: ISCCM guidelines for the use of non-invasive ventilation
in acute respiratory failure in adult ICUs. Indian J Crit Care Med.
24:61–81. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Williams B, Mancia G, Spiering W, Rosei
EA, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak
A, et al: 2018 ESC/ESH guidelines for the management of arterial
hypertension: The task force for the management of arterial
hypertension of the European society of cardiology and the European
society of hypertension. J Hypertens. 36:1953–2041. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sarker R, Roknuzzaman ASM, Hossain MJ,
Bhuiyan MA and Islam MR: The WHO declares COVID-19 is no longer a
public health emergency of international concern: Benefits,
challenges, and necessary precautions to come back to normal life.
Int J Surg. 109:2851–2852. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cummings MJ, Baldwin MR, Abrams D,
Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani
LE, Hastie J, et al: Epidemiology, clinical course, and outcomes of
critically ill adults with COVID-19 in New York city: A prospective
cohort study. Lancet. 395:1763–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen J, Liu Y, Qin J, Ruan C, Zeng X, Xu
A, Yang R, Li J, Cai H and Zhang Z: Hypertension as an independent
risk factor for severity and mortality in patients with COVID-19: A
retrospective study. Postgrad Med J. 98:515–522. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang Y, Lu X, Li Y, Chen H, Chen T, Su N,
Huang F, Zhou J, Zhang B, Yan F and Wang J: Clinical course and
outcomes of 344 intensive care patients with COVID-19. Am J Respir
Crit Care Med. 201:1430–1434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aleva FE, van Mourik L, Broeders MEAC,
Paling AJ and de Jager CPC: COVID-19 in critically ill patients in
North Brabant, the Netherlands: Patient characteristics and
outcomes. J Crit Care. 60:111–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin HY: The severe COVID-19: A sepsis
induced by viral infection? And its immunomodulatory therapy. Chin
J Traumatol. 23:190–195. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gabarre P, Dumas G, Dupont T, Darmon M,
Azoulay E and Zafrani L: Acute kidney injury in critically ill
patients with COVID-19. Intensive Care Med. 46:1339–1348.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prasitlumkum N, Chokesuwattanaskul R,
Thongprayoon C, Bathini T, Vallabhajosyula S and Cheungpasitporn W:
Incidence of myocardial injury in COVID-19-infected patients: A
systematic review and meta-analysis. Diseases. 8(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Klok FA, Kruip MJHA, van der Meer NJM,
Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J,
Stals MAM, Huisman MV and Endeman H: Incidence of thrombotic
complications in critically ill ICU patients with COVID-19. Thromb
Res. 191:145–147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vogrig A, Gigli GL, Bnà C and Morassi M:
Stroke in patients with COVID-19: Clinical and neuroimaging
characteristics. Neurosci Lett. 743(135564)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang S, Zhang Q, Wang P, Ye H, Jing X,
Zhang Z, Zhu S, Luo T and Zheng Z: Clinical features of
hypertensive patients with COVID-19 compared with a normotensive
group: Single-center experience in China. Open Med (Wars).
16:367–374. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lippi G, Wong J and Henry BM: Hypertension
in patients with coronavirus disease 2019 (COVID-19): A pooled
analysis. Pol Arch Intern Med. 130:304–309. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sisnieguez CE, Espeche WG and Salazar MR:
Arterial hypertension and the risk of severity and mortality of
COVID-19. Eur Respir J. 55(2001148)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sakurai K, Chubachi S, Asakura T, Namkoong
H, Tanaka H, Azekawa S, Shimada T, Otake S, Nakagawara K, Fukushima
T, et al: Prognostic significance of hypertension history and blood
pressure on admission in Japanese patients with coronavirus disease
2019: Integrative analysis from the Japan COVID-19 task force.
Hypertens Res. 47:639–648. 2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang V, Fisher M, Hou W, Zhang L and
Duong TQ: Incidence of new-onset hypertension post-COVID-19:
Comparison with influenza. Hypertension. 80:2135–2148.
2023.PubMed/NCBI View Article : Google Scholar
|