Introduction
The outcome of surgically managed chronic subdural
hematoma (CSDH) is usually improved, with a median surgical
intervention-to-resolution time achieved until 160 days
(interquartile range, 85-365 days) (1-3).
However, the reappearance of hematoma occurs in up to 33% of
patients, which is related to higher morbidity and mortality rates
(4-10).
The primary pathogenetic mechanisms of the condition remain
uncertain; however, there is evidence of an interaction connecting
inflammatory, fibrinolytic and angiogenic pathways (2,3).
Numerous studies have recognized possible clinical, radiological
and surgical risk factors for the recurrence of hematomas,
including an advanced age, the male sex and other characteristics
inherent to the hematoma (4-6,10-12).
In extremely old patients suffering from multiple major
comorbidities, there are extensive repercussions, suggesting that
those at an older age are at a higher risk of developing recurrent
hematomas (4). A variety of
neurological symptoms frequently arise, ranging from mild focal
symptoms related to the long tracts to coma and mortality (2,3). The
diagnosis is typically complete with a computed tomography (CT)
scan of the head, which illustrates the hematoma and provides brain
compression (2,3).
Surgical hematoma evacuation constitutes the gold
standard for the recurrence of CSDH (2,3,13,14).
Drugs such as steroids, statins and tranexamic acid have been used
as adjunctive therapy to diminish the risk of reappearance.
Εqually, middle meningeal artery embolization (MMAE) recently gave
hopeful outcomes in recurrent cases (13,15-19).
Neuroedoscopy helps determine the adhesions in compartmentalized
lesions (20,21), while the role of membranectomy
remains to be established (22).
The present study describes the case of an elderly
male patient with refractory CSDH who was treated on an escalated
basis. In addition, after reviewing the relevant literature, the
complexity of refractory CSDH and all major available treatment
alternatives are discussed. Finally, the present study attempted to
identify the patient's ‘point of no return’, if any.
Case report
An 85-year-old male patient presented to the
University Hospital of Larissa (Larissa, Greece) in April, 2023 for
the first time complaining of increasing headaches and instability
while walking. The medical records of the patient mentioned
anticoagulant therapy (clopidogrel, 75 mg per day) for stroke
prevention by atrial arrhythmias (propafenone, 150 mg per day) and
diabetes type II (gliclazide, 60 mg per day). A clinical
examination revealed mild left hemiparesis [4/5 muscle strength
according to the Medical Research Council (MRC) Scale for Muscle
Strength], slow thinking ability, and mild disorientation. Magnetic
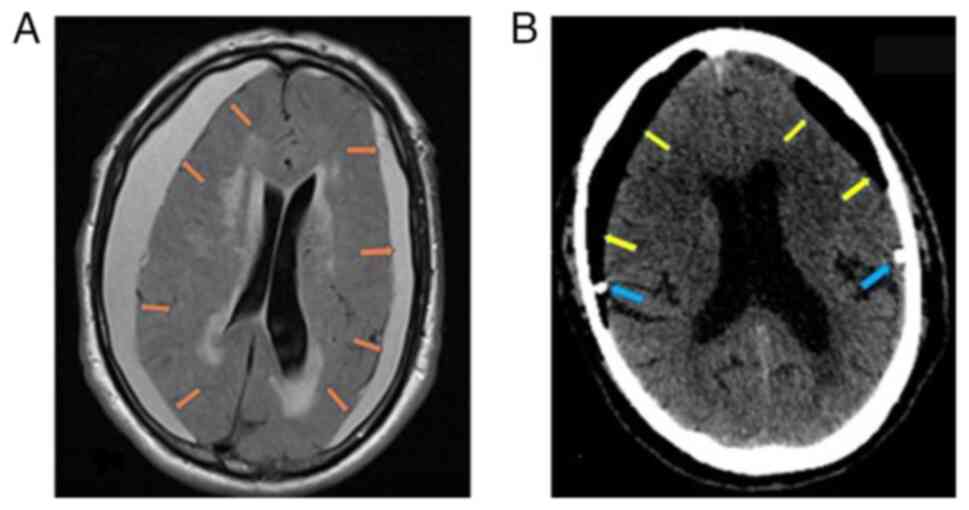
resonance imaging (MRI) of the brain reealed bilateral subdural
hematomas that compressed the brain parenchyma (Fig. 1A). The patient underwent hematoma
evacuation through burr holes and a closed drainage system. The
post-operative head CT scan revealed complete hematoma evacuation,
and on the 2nd post-operative day, the patient could walk
unassisted without any neurological deficit (Fig. 1B).
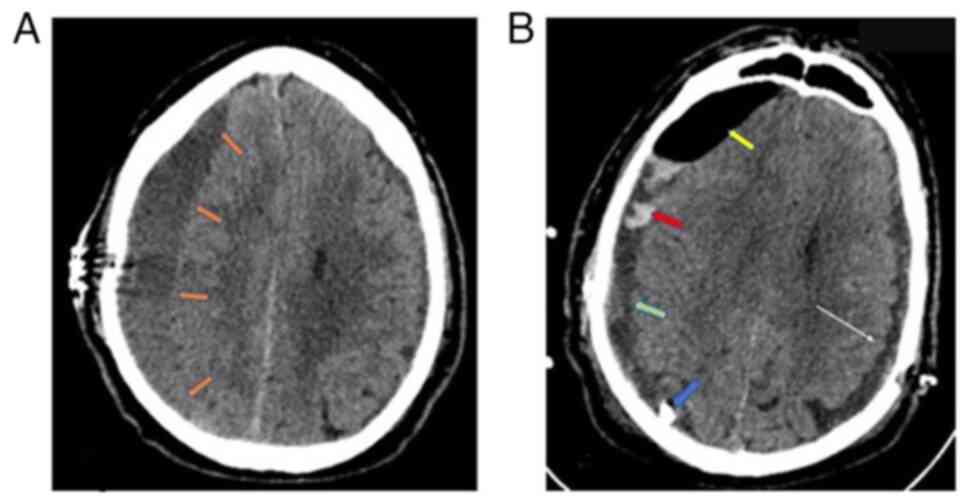
After 2 weeks, the patient returned to the hospital
with a recurrence of symptoms and a Glasgow Coma Scale (GCS) score
of 13/15 (M:5, V:5, E:3), left hemiparesis and profound
disorientation. The new head CT scan revealed a recurrence of the
right subdural hematoma, which was again evacuated through a new
burr hole, now placed rostrally, and a closed drainage system
(Fig. 2A). In addition, in order to
prevent recurrence intraoperatively, the thick neomembranes that
were removed and a small amount of CSDH that was entrapped were
identified. The neurological status of the patient again completely
improved, while the post-operative head CT scan revealed partial
hematoma evacuation, which was treated conservatively with steroids
[dexamethasone was administered orally in a dose of 80 mg three
times a day for 1 week starting at the end of the first
post-operative week, and then gradual reduction of the dose (20 mg
at a time) every 5 days]. (Fig. 2B).
Based on a personalized management, it was decided to administer
dexamethasone as an add-on treatment for hematoma recurrence and
the option for MMAE was discussed; however, the patient did not
attend his regular follow-up.
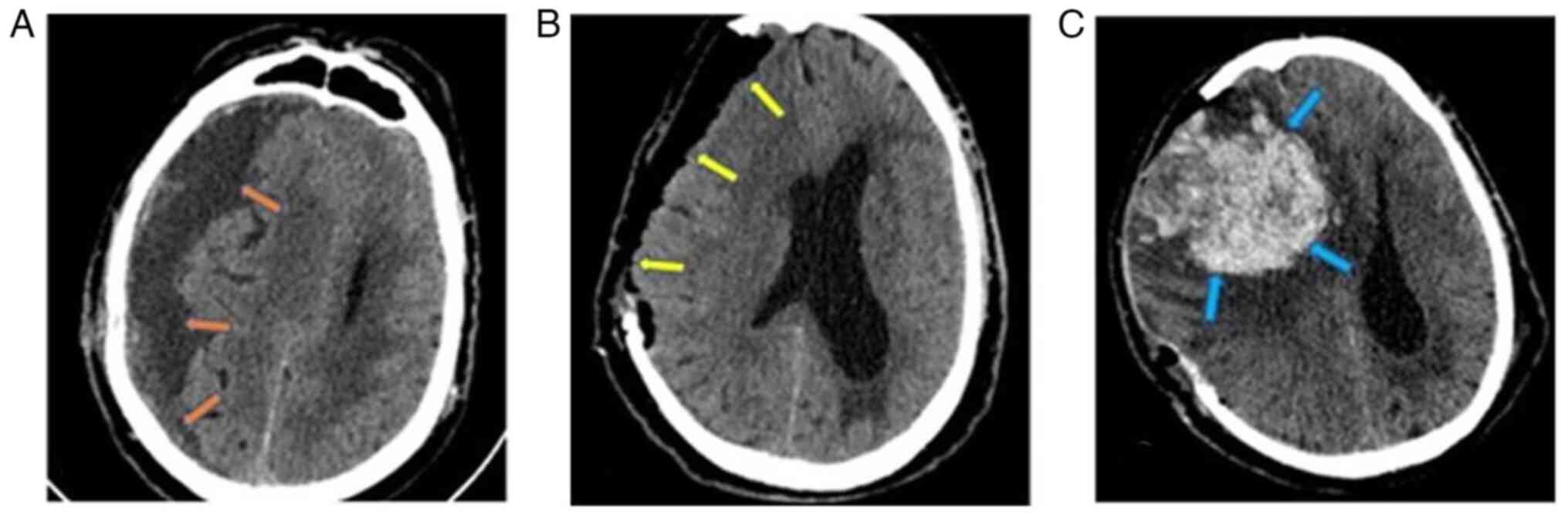
Subsequently, 1 month later, the patient was
admitted to the Emergency Room of the University Hospital of
Larissa comatose with a GCS score of 6/15 (motor response, 4;
verbal response, 1; eye-opening, 1). A new head CT scan revealed a
large recurrent subdural hematoma with a significant midline shift
(1.45 cm) (Fig. 3A). Considering the
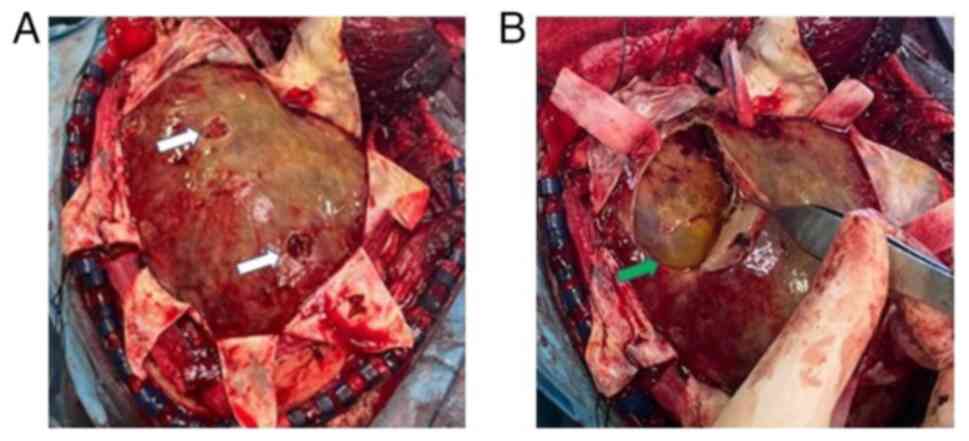
history of the patient, he underwent a decompressive craniectomy
for hematoma removal. After opening the dura, multiple layers of
hard neomembranes trapping a small amount of yellowish fluid in
numerous pockets were found (Fig.
4). Therefore, the neomembranes were removed and appropriate
hemostasis was performed, followed by layer-by-layer surgical wound
closure. An immediate post-operative CT scan revealed complete
hematoma removal and midline shift improvement (0.8 cm) (Fig. 3B), and the patient was transferred to
the intensive care unit for gradual awakening.
On the 2nd post-operative day, the patient exhibited
anisocoria (right, 5 mm; left, 2 mm), which soon changed to
fixed-and-dilated pupils and a tense skin flap of the craniectomy.
The final head CT scan revealed an extensive intraparenchymal
hemorrhage on the right side with a midline shift of 2 cm and a
trapped ipsilateral ventricle (Fig.
3C). At the request of the legal representative of the patient,
no further surgical intervention was performed, and the patient
succumbed within 48 h.
Discussion
The present case report demonstrates that a
relatively benign lesion, such as CSDH, may occasionally exhibit
very malignant behavior despite adequate treatment. The malignant
nature of CSDH in the case described herein became apparent with
the repeated recurrences, the subsequent intraparenchymal
hemorrhage, and eventually, the demise of the patient. It is
essential to re-consider several clinical and radiological
parameters throughout the disease course to identify the ‘point of
no return’, if any.
Risk factors
Zhu et al (12) performed a network meta-analysis on
the patient-related risk factors that are associated with an
increased risk of hematoma recurrence. The patient had several of
these predictors. Epidemiological risk factors included an advanced
age [standardized mean difference, 0.10; 95% confidence interval
(CI), 0.01-0.18], the male sex [relative risk (RR), 1.32; 95% CI,
1.50-1.51] and bilateral location (1.41; 1.20-1.67) (12). The radiological characteristics of
the original hematoma did not warn of an increased risk of
recurrence, as it was a type 1 lesion (hypodense; RR, 0.79;
0.59-1.05) and not a type 2 lesion (hyperdense, laminar, separated
and graded) (12).
Primary management
Neurosurgeons may opt between single burr hole
craniostomy (BHC), double BHC, twist drill craniostomy (TDC) and
minicraniotomy to remove CSDH; however, each of these has its own
recurrence and reoperation profiles (12,14).
There is evidence to suggest that double BHC is the most effective
approach (12). In addition, the
recurrence rate after BHC has been shown to be lower than after
minicraniotomy [odds ratio (OR), 0.58; 95% CI, 0.35-0.97] (15). Another meta-analysis by Yagnik et
al (14) revealed no difference
in the recurrence rate between BHC and TDC (OR, 1.16; 95% CI,
0.84-1.62); however, TDC was associated with a higher reoperation
rate, particularly when negative suction drainage was not used
(14). Zhu et al (12) highlighted the importance of
intraoperative saline irrigation (RR, 0.35; 95% CI, 0.19-0.63) and
the use of a closed drainage system (RR, 0.45; 0.33-0.60) in
reducing the risk of hematoma recurrence. In the patient in the
present study, a single BHC was used with warm saline irrigation
and a closed drainage system on both sides. Han et al
(23) reported that the lack of
brain re-expansion was the strongest predictor of hematoma
recurrence (OR, 25.91; 95% CI, 7.11-94.35) and was strongly
associated with senile brain atrophy (OR, 2.36; 1.36-4.11)
(23,24). However, the post-operative head CT
scan revealed that the brain of the patient never re-expanded
sufficiently.
Management of the first
recurrence
According to Henry et al (13), when the symptoms recurred, he
hematoma was more complex, with a width >20 mm (OR, 2.37; 95%
CI, 1.56-3.60) and a midline shift >10 mm (OR, 1.61; 95% CI,
1.17-2.22). Therefore, it was decided to re-evacuate the hematoma
through a second BHC, which was connected to a closed drainage
system.
Steroids, statins and their combinations have been
proposed as medical adjuncts to reduce hematoma recurrence
(17,25). The effect of statins and steroids is
hypothesized to be mediated by their immunomodulatory properties
(25). According to Zhu et al
(12), patients receiving
atorvastatin (OR, 0.31; 95% CI, 0.14-0.69) and corticosteroids (OR
0.41; 95% CI, 0.24-0.70) had a lower hematoma recurrence rate. On
the contrary, Monteiro et al (16), based on a meta-analysis of seven
studies, found insufficient evidence to recommend the regular use
of statins (OR, 0.8; 95% CI, 0.35-1.81) in CSDH. In two previous
meta-analyses of 12 and five trials, respectively, the authors
reported a lower recurrence rate with steroids (OR, 0.39; 95% CI,
0.19-0.79 and RR, 0.4; 95% CI, 0.28-0.58), but at a higher
incidence of adverse events (RR, 2.7; 95% CI, 1.71-4.28), including
psychiatric symptoms (RR, 3.22; 95% CI, 1.83-5.64), and no
difference in neurological outcomes (RR, 1; 95% CI, 0.93-1.08),
infection rate (RR, 1.86; 95% CI, 0.56-6.14) and all-cause
mortality (RR, 0.66; 95% CI, 0.2-2.18) (26,27).
Considering all the available evidence, based on a personalized
management, in the present study, it was decided to administer
dexamethasone as an add-on treatment for hematoma recurrence and
discussed the option for MMAE.
MMAE is considered to reduce the risk of recurrence
by interrupting blood supply to the dura, thereby minimizing
leakage through the high permeability neomembranes (18,19). It
can be used in patients with previously untreated CSDH (upfront
MMAE), following surgical hematoma evacuation in cases without any
evidence of recurrence (prophylactic MMAE), and for recurrent CSDH
after prior surgical excision (18,19).
Currently, there is insufficient evidence to indicate that MMAE
reduces the risk of recurrence (18,19).
Management of the second
recurrence
In the present study, in the second recurrence, the
poor neurological status, significant mass effect, and the failure
of previous attempts mandated an urgent decompressive craniectomy.
Intraoperatively, the thick neomembranes that were removed and a
small amount of CSDH that was entrapped were identified. The role
of membranectomy, both inner and outer, or only outer, is still
debated (22). An outer
membranectomy allows for the uninhibited expansion of the brain by
eliminating the mass effect from the neomembranes and reducing the
risk of re-bleeding, while inner membranectomy allows for the
unimpeded circulation of cerebrospinal fluid through dural
lymphatics (22). Hacıyakupoğlu
et al (22) used craniotomy
and membranectomy to treat 13 patients with recurrent CSDH with
good results and no recurrence after 3 months.
Endoscopically-assisted burr hole hematoma
evacuation offers a safe and effective alternative to craniotomy
(20,21,28). A
flexible neuroendoscope is inserted through one or two burr holes
in the frontal and/or occipital regions (28). Under direct vision, the trabeculae
are transected, the compartments of the hematoma cavity are united,
and the contents are flushed out with body-warm saline (28). If microhemorrhages occur, a bipolar
microcatheter is used for hemostasis (28). Any residual hematoma is drained
through a closed-tube system (28).
There is recent evidence to indicate that neuroendoscopy reduces
recurrence rates compared with conventional treatment (13.1 vs.
3.1% with plain BHC, P<0.001); however, the mortality, morbidity
and functional rates remain relatively unaltered (21). In addition, the need for special
instruments and training in the use of neuroendoscopy seems to
limit its broad usage, as in the case described herein.
Intraparenchymal hemorrhage
The patient in the present study experienced a
massive intraparenchymal hemorrhage (IPH) with a fatal outcome on
the 2nd post-operative day. IPH is a rare, yet serious complication
following CSDH evacuation (29). In
a previous literature review by Krueger et al (29), 48 cases were described. IPH
frequently occurs in males (85%), with CSDH causing a significant
midline shift (54%), at ~1.9 days (±3 days) after surgery (29). The hemorrhage is usually located in
the hemisphere ipsilateral (P=0.02) to the hematoma (29). Several interrelated mechanisms,
including altered venous circulation, rapid re-expansion of the
brain and local edema, have been implicated in the pathogenesis of
hemorrhage (29). A second
intervention is required in ~27% of cases, with mortality rates
reaching as high as 25% (29).
Point of no return
Table I estimates the
probability of hematoma recurrence for each of the aforementioned
parameters. OR values were derived from the literature, and the
relevant reference citations are cited in the last column. The
probability of recurrence was calculated based on the OR values.
The most influential factor in the patient described herein was the
lack of brain re-expansion (recurrence probability, 96%), followed
by hematoma width (70%) and the presence of senile atrophy (70%).
The first modifiable factor, the type of surgical evacuation,
ranked seventh (54%). Retrospectively, it appears that the
craniocerebral mismatch determined the serial recurrences and, as a
result, the fate of the patient in the present case report. In
theory, MMAE could reduce the risk of recurrence to 13%. However,
the available evidence was derived from studies on primary CSDH
without focusing on high-recurrence-risk patients, as in the case
described herein. The efficacy of MMAE remains to be determined in
this population in future studies.
 | Table ISummary table of the characteristics
of the patient in the present study according to the evidence on
hematoma recurrence from the literature. |
Table I
Summary table of the characteristics
of the patient in the present study according to the evidence on
hematoma recurrence from the literature.
| Authors | Parameter | Odds ratio | Probability of
recurrence (%) | Modifiable
factor | (Refs.) |
|---|
| Han et al | Failure of brain
re-expansion | 25.0 | 96 | No | (23) |
| Zhu et al | Hematoma width
(>20 mm) | 2.37 | 70 | No | (12) |
| Han et al | Senile brain
atrophy | 2.36 | 70 | No | (23) |
| Zhu et al | Midline shift (>10
mm) | 1.61 | 62 | No | (12) |
| Zhu et al | Bilateral
location | 1.41 | 59 | No | (12) |
| Zhu et
al | Male gender | 1.32 | 57 | No | (12) |
| Yagnik et
al | Single burr
hole | 1.16 | 54 | Yes | (14) |
| Zhu et
al | Type 1
lesiona | 0.79 | 44 | No | (12) |
| Zhu et
al | Closed drainage
system | 0.45 | 41 | Yes | (12) |
| Shrestha et
al | Steroids | 0.39 | 28 | Yes | (26) |
| Zhu et
al | Irrigation | 0.35 | 26 | Yes | (12) |
| Ironside et
al, Jumah et al | No MMAE | 6.66 | 87 | Yes | (18,19) |
In conclusion, contrary to the common belief, the
management of a CSDH is a complex and challenging task.
Furthermore, despite advances in the primary treatment of CSDH, the
outcomes remain suboptimal in certain cases. Therefore, the
literature has extensively explored potential predictors of
treatment failure. Recurrent CSDH, characterized by multiple
compartments and thick neomembranes, poses even greater challenges.
In fact, effective treatment alternatives for such cases are
extremely limited, as highlighted herein, including the patient in
the present study. Consequently, a more individualized approach is
required, which may involve more aggressive treatment options such
as craniectomy and membranectomy. In addition, future research is
required with the use of more advanced approaches, such as
endovascular embolization of the meningeal artery, which may reduce
the recurrence rate and lead to improved outcomes in patients with
CSDH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AGB and GF conceptualized the study. AGB, VEG, GF,
II, AK, TS and KNF made a substantial contribution to the
interpretation and analysis of the patient's data, and wrote and
prepared the draft of the manuscript. GF and AGB treated the
patient and performed the surgical procedures. GF and KNF analyzed
the data and provided critical revisions. GF and AGB confirm the
authenticity of all the data. All authors contributed to manuscript
revision, and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed was obtained from the patient for
his participation in the present case report. The patient had
provided written informed consent for publication after the second
intervention.
Patient consent for publication
Written informed was obtained from the patient for
the publication of the present case report and any related images.
The patient had provided written informed consent for publication
after the second intervention.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang CL, Sim JL, Delgardo MW, Ruan DT and
Connolly ES Jr: Predicting chronic subdural hematoma resolution and
time to resolution following surgical evacuation. Front Neurol.
11(677)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nouri A, Gondar R, Schaller K and Meling
T: Chronic subdural hematoma (cSDH): A review of the current state
of the art. Brain Spine. 1(100300)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tommiska P, Lönnrot K, Raj R, Luostarinen
T and Kivisaari R: Transition of a clinical practice to use of
subdural drains after burr hole evacuation of chronic subdural
hematoma: The Helsinki experience. World Neurosurg. 129:e614–e626.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cofano F, Pesce A, Vercelli G, Mammi M,
Massara A, Minardi M, Palmieri M, D'Andrea G, Fronda C, Lanotte MM,
et al: Risk of recurrence of chronic subdural hematomas after
surgery: A multicenter observational cohort study. Front Neurol.
11(560269)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hamou H, Alzaiyani M, Pjontek R, Kremer B,
Albanna W, Ridwan H, Clusmann H, Hoellig A and Veldeman M: Risk
factors of recurrence in chronic subdural hematoma and a proposed
extended classification of internal architecture as a predictor of
recurrence. Neurosurg Rev. 45:2777–2786. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shen J, Yuan L, Ge R, Wang Q, Zhou W,
Jiang XC and Shao X: Clinical and radiological factors predicting
recurrence of chronic subdural hematoma: A retrospective cohort
study. Injury. 50:1634–1640. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Greuter L, Lutz K, Fandino J, Mariani L,
Guzman R and Soleman J: Drain type after burr-hole drainage of
chronic subdural hematoma in geriatric patients: A subanalysis of
the cSDH-Drain randomized controlled trial. Neurosurg Focus.
49(E6)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pahatouridis D, Alexiou GA, Fotakopoulos
G, Mihos E, Zigouris A, Drosos D and Voulgaris S: Chronic subdural
haematomas: A comparative study of an enlarged single burr hole
versus double burr hole drainage. Neurosurg Rev. 36:151–154.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fountas K, Kotlia P, Panagiotopoulos V and
Fotakopoulos G: The outcome after surgical vs nonsurgical treatment
of chronic subdural hematoma with dexamethasone. Interdisciplinary
Neurosurgery. 16:70–74. 2019.
|
|
10
|
Bartek J Jr, Sjåvik K, Schaible S, Gulati
S, Solheim O, Förander P and Jakola AS: The role of
angiotensin-converting enzyme inhibitors in patients with chronic
subdural hematoma: A Scandinavian population-based multicenter
study. World Neurosurg. 113:e555–e560. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang JJY, Aw NMY, Tan CH, Lee KS, Chen
VHE, Wang S, Dinesh N, Foo ASC, Yang M, Goh CP, et al: Impact of
time to resumption of antithrombotic therapy on outcomes after
surgical evacuation of chronic subdural hematoma: A multicenter
cohort study. J Clin Neurosci. 89:389–396. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu F, Wang H, Li W, Han S, Yuan J, Zhang
C, Li Z, Fan G, Liu X, Nie M and Bie L: Factors correlated with the
postoperative recurrence of chronic subdural hematoma: An umbrella
study of systematic reviews and meta-analyses. EClinicalMedicine.
43(101234)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Henry J, Amoo M, Kissner M, Deane T,
Zilani G, Crockett MT and Javadpour M: Management of chronic
subdural hematoma: A systematic review and component network
meta-analysis of 455 studies with 103 645 cases. Neurosurgery.
91:842–855. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yagnik KJ, Goyal A and Van Gompel JJ:
Twist drill craniostomy vs burr hole drainage of chronic subdural
hematoma: A systematic review and meta-analysis. Acta Neurochir
(Wien). 163:3229–3241. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miah IP, Holl DC, Blaauw J, Lingsma HF,
den Hertog HM, Jacobs B, Kruyt ND, van der Naalt J, Polinder S,
Groen RJM, et al: Dexamethasone versus surgery for chronic subdural
hematoma. N Engl J Med. 388:2230–2240. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Monteiro A, Housley SB, Kuo CC, Donnelly
BM, Khawar WI, Khan A, Waqas M, Cappuzzo JM, Snyder KV, Siddiqui
AH, et al: The effect of statins on the recurrence of chronic
subdural hematomas: A systematic review and meta-analysis. World
Neurosurg. 166:244–250. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu W, Chen W, Jiang Y, Ma M, Zhang W,
Zhang X and Cheng Y: Effectiveness comparisons of drug therapy on
chronic subdural hematoma recurrence: A Bayesian network
meta-analysis and systematic review. Front Pharmacol.
13(845386)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ironside N, Nguyen C, Do Q, Ugiliweneza B,
Chen CJ, Sieg EP, James RF and Ding D: Middle meningeal artery
embolization for chronic subdural hematoma: A systematic review and
meta-analysis. J Neurointerv Surg. 13:951–957. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jumah F, Osama M, Islim AI, Jumah A, Patra
DP, Kosty J, Narayan V, Nanda A, Gupta G and Dossani RH: Efficacy
and safety of middle meningeal artery embolization in the
management of refractory or chronic subdural hematomas: A
systematic review and meta-analysis. Acta Neurochir (Wien).
162:499–507. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ichimura S, Takahara K, Nakaya M, Yoshida
K and Fujii K: Neuroendoscopic Technique for recurrent chronic
subdural hematoma with small craniotomy. Turk Neurosurg.
30:701–706. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu L, Guo X, Ou Y, Yu X, Zhu B, Yang C and
Liu W: Efficacy analysis of neuroendoscopy-assisted burr-hole
evacuation for chronic subdural hematoma: A systematic review and
meta-analysis. Neurosurg Rev. 46(98)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hacıyakupoğlu E, Yılmaz DM, Kınalı B,
Arpacı T, Akbaş T and Hacıyakupoğlu S: Recurrent chronic subdural
hematoma: Report of 13 cases. Open Med (Wars). 13:520–527.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han S, Feng Y, Xu C, Li X, Zhu F, Li Z,
Zhang C and Bie L: Brain re-expansion predict the recurrence of
unilateral CSDH: A clinical grading system. Front Neurol.
13(908151)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miki K, Abe H, Morishita T, Hayashi S,
Yagi K, Arima H and Inoue T: Double-crescent sign as a predictor of
chronic subdural hematoma recurrence following burr-hole surgery. J
Neurosurg. 131:1905–1911. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J: Chinese Society of Neurosurgery,
Chinese Medical Association, Chinese Neurosurgical Critical Care
Specialist Council, Collaborational Group of Chinese Neurosurgical
Translational and Evidence-based Medicine. Expert consensus on drug
treatment of chronic subdural hematoma. Chin Neurosurg J.
7(47)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shrestha DB, Budhathoki P, Sedhai YR, Jain
S, Karki P, Jha P, Mainali G and Ghimire P: Steroid in chronic
subdural hematoma: An updated systematic review and meta-analysis
post DEX-CSDH trial. World Neurosurg. 158:84–99. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao Y, Xiao Q, Tang W, Wang R and Luo M:
Efficacy and safety of glucocorticoids versus placebo as an
adjuvant treatment to surgery in chronic subdural hematoma: A
systematic review and meta-analysis of randomized controlled
clinical trials. World Neurosurg. 159:198–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hellwig D, Heinze S, Riegel T and Benes L:
Neuroendoscopic treatment of loculated chronic subdural hematoma.
Neurosurg Clin N Am. 11:525–534. 2000.PubMed/NCBI
|
|
29
|
Krueger EM, Gustin AJ, Gustin PJ, Jaffa Z
and Farhat H: Intraparenchymal hemorrhage after evacuation of
chronic subdural hematoma: A case series and literature review.
World Neurosurg. 155:160–170. 2021.PubMed/NCBI View Article : Google Scholar
|