1. Introduction
Atrial fibrillation (AF) is a common cardiac
arrhythmia that affects millions of individuals worldwide. The
prevalence of AF has exhibited a 3-fold increase over the past 50
years according to data from the Framingham Heart Study and the
American Heart Association (AHA) (1,2). It is
characterized by an irregular and often rapid heartbeat, which can
lead to several serious complications, such as stroke, heart
failure and a decreased quality of life (3). The condition is caused by abnormal
electrical signals in the heart that disrupt the normal rhythm of
the atria (4). Several risk factors
have been linked to the development of AF, many of which are
modifiable and include hypertension, obesity, obstructive sleep
apnea (OSA) and alcohol consumption (4).
The primary goals of treating patients with AF
include managing symptoms, controlling the heart rate or rhythm,
and reducing the risk of stroke (5).
Catheter ablation (CA) is an established treatment method for
improving symptoms, rate or rhythm control and is the most common
procedure performed in electrophysiology. Evidence from randomized
controlled trials (RCTs) also supports the effectiveness of CA in
patients with comorbid AF and heart failure (5-8).
The present review discusses the current and evolving indications
for CA in patients with AF, summarizing the safety and efficacy of
CA procedures, and reviewing the data that support the significance
of lifestyle modifications for improving outcomes after
ablation.
2. Current indications and evidence in the
literature
CA, a highly sophisticated and complex procedure,
requires a thorough and rigorous assessment of its potential risks
and benefits prior to its implementation as a therapeutic strategy
in AF (9). To ensure optimal
outcomes, it is imperative that the procedure be executed by highly
qualified and experienced electrophysiologists or surgeons
operating in specialized centers. Furthermore, the indications for
CA should be determined by the specific subtype of AF of the
patient's and the previous response of the patient to class I or
III antiarrhythmic medications, as established by the current
literature. These considerations are of paramount importance in
ensuring the safe and effective utilization of CA as a therapeutic
option for AF and are vital for achieving successful long-term
outcomes for patients with AF (1-9).
In addition to these considerations, patient
characteristics such as the presence of comorbid conditions such as
heart disease, obesity, and sleep apnea, as well as factors such as
left atrial size, age, frailty, and duration of continuous AF, may
predict a lower success rate or higher complication rate in the
management of AF (9). Therefore, it
is essential to take these variables into account when managing and
treating patients with AF to ensure the best possible outcomes.
Table I summarizes the indications
with the level of evidence.
 | Table ISummary of indications and
recommendations for catheter ablation in atrial fibrillation
(9,25). |
Table I
Summary of indications and
recommendations for catheter ablation in atrial fibrillation
(9,25).
| Indication | Level of
evidence |
Recommendations |
|---|
| Catheter ablation
as a first-line therapeutic option | IIa | Symptomatic
paroxysmal AF: Reasonable as a primary treatment option without a
trial of antiarrhythmic agents. |
| | Ia | In selected
patients with symptomatic paroxysmal AF: Consider as a first- line
treatment option, particularly in younger patients with few comor-
bidities and where rhythm control is desired. |
| | IIa | Symptomatic
persistent AF: Reasonable as a primary treatment option without a
trial of antiarrhythmic agents. |
| | IIb | Long-standing
symptomatic AF: Can be considered as a primary treatment option
without a trial of antiarrhythmic agents. |
| | IIa | Symptomatic pauses
(tachy-brady syndrome): Reasonable as a primary treatment option.
Highly competitive athletes: Recommended as a primary treatment
option. |
| Catheter ablation
as second- line treatment | Ia | Recommended for
patients who have failed one or more antiarrhythmic medications as
a second-line treatment option. |
| Catheter ablation
in patients with heart failure and reduced ejection fraction
(HFrEF) | IIb | Can be considered
as a treatment option for patients with heart failure with reduced
ejection fraction. Reasonable indications for patients with heart
failure: AF ablation in selected patients with heart failure is
reasonable, following similar indications to those without heart
failure. Associated with a lower risk of all-cause mortality and
improved left ventricular ejection fraction (LVEF) compared to
medical therapy. |
| Catheter ablation
in patients with heart failure and pre- served ejection fraction
(HFpEF) | IIb | Can be considered
as a treatment option for patients with HFpEF. Similar indications
as for patients without heart failure: AF ablation is reasonable in
selected patients with HFpEF. Effective in maintaining sinus
rhythm, reducing heart failure rehospita- lizations, and improving
quality of life. |
| Catheter ablation
in elderly patients | IIa | Reasonable to
consider for selected older individuals with AF, following similar
indications to younger patients. Higher complication rates,
including cerebrovascular accidents, bleeding, and mortality,
compared to younger patients. |
| Catheter ablation
in asymp- tomatic patients | IIb | May be considered
for selected patients with persistent or paroxysmal AF; however,
the benefit in asymptomatic patients remains uncertain, and further
research is required. |
| Catheter ablation
to reduce stroke risk | | Clinical trials and
meta-analyses do not support a significant reduction in stroke risk
compared to medical therapy. |
CA as a first-line therapeutic
option
First-line therapy with AF ablation, prior to the
administration of class I or class III antiarrhythmic agents, has
been extensively evaluated in the literature concerning both
symptomatic paroxysmal and persistent AF (9-22).
This approach is linked to a considerable decrease in the
recurrence of arrhythmias, significant enhancements in symptoms
related to arrhythmia, and improved quality of life. Moreover,
ablation is associated with a reduced frequency of adverse events.
Additionally, CA is associated with markedly lower rates of disease
progression, indicating its role as a disease-modifying
intervention (23).
A recent meta-analysis conducted by Turagam et
al (24), which included six
randomized controlled trials and a total of 1,212 participants,
demonstrated that the ablation group exhibited a significantly
lower recurrent atrial arrhythmia rate [risk ratio (RR), 0.61; 95%
CI, 0.51-0.74], and a reduced incidence of symptoms and
hospitalization (RR, 0.44; 95% CI, 0.27-0.72 and RR, 0.32; 95% CI,
0.19-0.53, respectively). Additionally, there was no statistically
significant difference in the incidence of adverse events between
the two groups (RR, 1.52; 95% CI, 0.81-2.85). The most commonly
observed adverse effect in the ablation group was cardiac effusion,
while bradycardia was observed in the medication group.
These findings are consistent with the 2016 European
Society of Cardiology (ESC) guidelines for the management of AF and
the 2014 American College of Cardiology (ACC)/AHA/Heart Rhythm
Society (HRS) guidelines for the management of AF. A consensus
statement on AF ablation in 2017 established that the level of
evidence for utilizing ablation as a primary line of treatment
without a trial of antiarrhythmic agents is class IIa indication in
both paroxysmal and persistent symptomatic AF, and class IIb in
long-standing symptomatic AF (9-11).
In the 2023 ACC/AHA/ACCP/HRS guidelines CA is recommended as
first-line treatment in selected symptomatic patients with
paroxysmal AF who is generally young with fewer comorbidities to
improve symptoms and reduce progression to persistent AF (class 1a
recommendation) (25).
In patients with symptomatic pauses (tachy-brady
syndrome), CA is considered the preferred treatment option. In
these cases, initiation of medical therapy in the absence of a
permanent pacemaker has been demonstrated to increase morbidity and
mortality. Several studies have reported that CA is an effective
treatment option, resulting in the resolution of symptoms without
the need for a pacemaker (26-28).
Furthermore, CA is recommended as a primary line of
treatment in highly competitive athletes as several studies have
demonstrated favorable outcomes in this patient population
(29-31).
CA as second-line treatment
In the 2017 consensus and 2023 ACC/AHA/ACCP/HRS
guidelines, CA is indicated as a treatment option for patients who
have failed one or more antiarrhythmic medications, did not
tolerate the medications or the antiarrhythmic medication is not
preferred and continued rhythm control is desired and classified as
a class IA recommendation (9,25). This
classification is based on a significant body of clinical research,
with >16 randomized trials having been conducted to evaluate the
effectiveness of CA in this population (9,25,31). A
recent meta-analysis by Deshpande et al (32) in 2022 included a total of 4,822
patients and found that the risk of arrhythmia recurrence was
significantly lower in the ablation group, with an odds ratio of
0.25 (95% CI, 0.18-0.36). Additionally, all-cause mortality was
also found to be significantly lower in the ablation group, with an
odds ratio of 0.33 (95% CI, 0.17-0.63). There were no significant
differences between the two groups in terms of stroke/transient
ischemic attacks (TIAs), bleeding and cardiovascular mortality.
CA in patients with heart failure and
reduced ejection fraction
The loss of atrial contraction in AF impairs left
ventricular filling, potentially reducing cardiac output by up to
25%. The irregular and rapid conduction of impulses in AF may lead
to left ventricular dysfunction (33,34). The
restoration of sinus rhythm can improve stroke volume and may
reverse cardiac remodeling. This may explain the rapid improvements
in hemodynamics observed in patients following the re-establishment
of sinus rhythm, and it also clarifies why some patients with heart
failure exhibit rapid hemodynamic improvements after returning to
sinus rhythm (33-35).
In the 2019 2019 AHA/ACC/HRS Focused Update
guidelines, CA as a treatment option for heart failure patients was
classified as a class IIb recommendation (18). This classification is based on a
significant body of clinical research, including numerous clinical
trials and meta-analyses, which have evaluated the safety and
outcomes of this treatment in this patient population (9,36-38).
A recent meta-analysis conducted by Şaylık et al (37) in 2022, which included a total of
2,187 patients, demonstrated that CA was associated with a lower
risk of all-cause mortality with a relative risk of 0.64 (CI: 0.5,
0.82). Additionally, this study found that patients who underwent
CA had greater improvement in left ventricular ejection fraction
(LVEF) with a mean difference of 5.38 (CI: 1.80, 8.97), as well as
an improved quality of life, as demonstrated by a greater reduction
in scores on the Minnesota Living with Heart Failure Questionnaire
[MD=-9.59 (CI: -16.72, -2.45), P<0.01], and longer 6-min walking
distances compared to patients in the medical therapy group
[MD=20.3; (CI: -4.37, 44.9)]. Furthermore, a meta-analysis by Chang
et al (38) demonstrated
similar findings and reported lower rates of heart failure
hospitalization and AF recurrence among patients who underwent
CA.
CA in patients with heart failure with
preserved ejection fraction (HFpEF)
AF is commonly observed in patients diagnosed with
HFpEF, characterized by an LVEF of 50% or greater. The prevalence
of AF in this patient population ranges from approximately 40 to
60%, and both conditions are typically associated with advancing
age (39,40).
Patients with HFpEF who also have AF exhibit a
poorer prognosis compared to those in sinus rhythm. The hazard
ratio for combined all-cause mortality or heart failure
hospitalizations in patients with HFpEF with AF was 1.365 (95% CI,
1.152-1.619; P<0.001) (39).
A recent meta-analysis conducted by Gu et al
(41) evaluated the effectiveness of
CA in the treatment of HFpEF and without heart failure. Their study
included a total of 1,696 patients and found that CA was effective
in maintaining sinus rhythm in the HFpEF group and was non-inferior
to those without heart failure with a relative risk of 0.92 (95%
CI, 0.76-1.10; P=0.34) (41).
Additionally, their study found that CA significantly improved the
maintenance of sinus rhythm with a relative risk of 4.73 (95% CI,
1.86-12.03; P=0.001) and reduced rehospitalization for heart
failure compared with medical therapy with a relative risk of 0.36
(95% CI, 0.19-0.71; P=0.003). However, their study found no
significant differences between the two groups in terms of
mortality rate (P=.59). Overall, that study provides evidence
supporting the effectiveness of CA as a treatment option for
patients with HFpEF and without heart failure (41). Other studies also support these
findings (9,42).
CA in the elderly
AF is a common condition among older adults (≥65
years), and there have been numerous studies that have specifically
focused on evaluating the outcomes of AF ablation in this
population. However, the safety and efficacy of CA as a treatment
for AF in older individuals is a topic of ongoing debate in the
medical community. Meta-analyses have yielded conflicting results,
with some studies suggesting non-inferiority in terms of recurrence
rate compared to younger populations, while others have reported
inferior efficacy. Despite these inconsistencies, there is a
consensus among medical experts that the complication rate,
including cerebrovascular accidents, bleeding, and mortality, is
higher in older patients who undergo CA (9,41-46).
In view of these findings, the 2017 consensus and
2023 ACC/AHA/ACCP/HRS guidelines recommend that CA be considered as
a treatment option for selected older individuals with AF, with
similar indications as for younger patients (class IIa
recommendation) (9,25). However, it is should be noted that
older patients may have a higher need for concomitant
antiarrhythmic medication after CA compared to younger patients
(46).
CA in asymptomatic patients
The prevalence of asymptomatic AF in the literature
ranges from 10 to 40%, with higher prevalence in males and in older
age groups (47). Some studies have
reported conflicting data regarding the cardiovascular risk and
stroke in asymptomatic patients, with some studies reporting less
cardiovascular disease and a lower long-term risk overall in
comparison to symptomatic patients (47,48),
while other studies have reported higher or similar risk to
symptomatic patients (47,49,50).
Several studies have compared the results of CA in
asymptomatic patients to those of symptomatic patients. The study
by Mohanty et al (51)
included 61 asymptomatic patients with longstanding persistent AF
and reported that 57% of the patients maintained sinus rhythm at 20
months of follow-up, with significant improvement in quality of
life and exercise capacity. In total, 25 patients had a recurrence
of AF after ablation; among these, 21 patients (34% of the total
number of patients included) were symptomatic after the recurrence.
The study by Wu et al (52)
included 66 asymptomatic patients with asymptomatic persistent AF,
and 35% of the patients maintained sinus rhythm at 1-year
follow-up. Symptoms scores improved significantly across 6 of 8
measures, suggesting that the patients were not truly asymptomatic.
43 (65.15%) patients had a recurrence; among these, 16 patients
(24% of all patients) became symptomatic.
The study by Pak et al (53) included a total of 5,013 patients from
the Kansai Plus Atrial Fibrillation (KPAF) Registry, 64.4% of the
patients had paroxysmal AF, 22.7% had persistent AF and 13% had
long-standing AF. They reported no significant difference between
symptomatic and asymptomatic patients as regards the recurrence of
the supraventricular arrhythmia in 4 years of follow-up; 37.5 vs.
40.6% (P=0.6) in paroxysmal AF, 45.2 vs. 55.1% (P=0.09) in
persistent AF and 59.3 vs. 63.6% (P=1.0) in long-standing AF. In
addition, there was no significant difference between the two
groups in cardiovascular, cerebral and gastrointestinal events
during the 4 years of follow-up following CA; 7.1 vs. 6.8% (P=0.7)
in paroxysmal AF, 5.4 vs. 8.7% (P=0.3) in persistent AF and 4.4 vs.
5.1% (P=0.5) in long-standing AF (53).
Another study by Kawaji et al (54) compared CA to conservative management
in patients with symptomatic and asymptomatic AF. They included 537
AF patients, and the median follow-up period was 5.3 years. The CA
group was associated with a significantly lower incidence of
composite cardiovascular death, heart failure hospitalization,
ischemic stroke, or major bleeding, (14.7 vs. 25.4% at 8 years;
log-rank P=0.008). However, this advantage was significant only in
patients who had previous AF-related complications (19.2 vs. 55.6%
at 8 years; log-rank P=0.006), but not among those without any
complication (13.9 and 17.3%; P=0.08). On the other hand, among
symptomatic patients, the benefit was regardless of previous
AF-related complications (54).
The 2017 consensus and 2023 ACC/AHA/ACCP/HRS
guidelines recommend that AF ablation can be considered in selected
(younger patients with minimal comorbidities and a moderate to high
burden of AF or persistent AF) patients with symptomatic or
minimally symptomatic AF as a class IIb recommendation. The
procedure required discussion with the patient regarding risks, and
benefits (9,25). However, it should be noted that the
benefit of the procedure in asymptomatic patients may be uncertain,
and further research is required to establish the optimal course of
action for these patients.
CA to reduce stroke risk
Patients undergoing CA often hope to avoid long-term
oral anticoagulation therapy. A sub-study from the AFFIRM trial
(55) reported that maintenance of
sinus rhythm and oral anticoagulation are associated with an
improved survival and a 60% reduction in the risk of stroke. Hence,
it is inferred that CA, with its higher rates of patients
maintained in sinus rhythm, would be associated with a reduced risk
of stroke. However, CA was not used as a strategy for rhythm
control in the AFFIRM trial. Clinical trials and meta-analyses from
randomized controlled trials do not support this theory (9,32,56-59).
To date, 20 clinical trials have compared CA to medical therapy.
The overall risk of stroke was low in both the CA group (0.85) and
the medical therapy group (57). The
incidence of stroke did not differ significantly between the two
groups in all randomized control trials. Meta-analyses of clinical
trials, such as those by Deshpande et al (32), Barra et al (56), Mao et al (58) and Shi et al (59), all reported no difference in stroke
incidence between the two groups. However, the study by Barra et
al (56) included a separate
analysis of cohort studies and registries, in which a significant
difference was found, with CA being associated with a reduced risk
of stroke and cerebrovascular accident (2.3 vs. 5.5%; RR, 0.57; 95%
CI, 0.46-0.70; P<0.001; I2=62%) (56).
One possible explanation for the lack of significant
impact of CA on the risk of stroke in randomized controlled trials
is the low incidence of stroke and low baseline risk in these
trials. Out of 44 strokes reported in all trials, 29 were reported
in two trials [CASTLE-AF (8) and
CABANA trials (6)], with the
remaining trials reporting only 15 stroke cases (32,56-59).
The annual incidence of stroke among patients undergoing CA is
typically low, regardless of the outcomes of the procedure, due to
the inherently low baseline risk. The study by Freedman et
al (60) reported that the risk
of stroke in patients with AF who were on anticoagulation therapy
was similar to that of patients without AF and was primarily
related to patient risk factors, such as age, sex and
comorbidities, rather than anticoagulation failure.
Therefore, it would be challenging to demonstrate a
significant additional benefit of CA in reducing residual stroke
risk beyond that of proper anticoagulation. Even if ablation were
to lower the risk of stroke by 50%, the number of patients required
to undergo the procedure to prevent one stroke event would still be
high, given the low baseline risk of 1.0% in patients receiving
medical treatment, as seen in randomized studies. This is why no
randomized study has yet been able to demonstrate a meaningful
impact of AF ablation on stroke rates, and why even combined data
lacks sufficient statistical power. The low incidence of stroke in
randomized trials is indeed reassuring, although one must exercise
caution when generalizing these results to real-world patients.
Although outcomes in randomized trials may not always reflect
real-world patient populations, some studies have shown that the
incidence of stroke among real-world patients aligns with the rates
reported in landmark trials such as ROCKET-AF and RE-LY (61,62).
3. Outcomes of the procedure
Success rate
The success rates of percutaneous AF CA vary
considerably, ranging from 50 to 80% (9-47).
However, accurately estimating the success of CA for AF is
challenging due to inconsistencies in the definitions of procedural
success and post-procedural recurrences, variations in the
intensity of post-procedural rhythm monitoring, and differences in
outcome analyses after single or multiple AF ablation procedures
(9,11,25).
The academic community has established a definition
for AF recurrence, which refers to the occurrence of any
symptomatic or asymptomatic atrial tachyarrhythmia lasting >30
sec after the AF-CA procedure (9,11,25).
However, it is important to recognize that this definition should
be understood within the broader clinical context, which includes
factors, such as the improvement of symptoms, the reduction of
heart failure symptoms and improvements in LVEF, and overall
quality of life. These broader clinical outcomes should be
considered when assessing the success of AF-CA, beyond the strict
definition of AF recurrence alone. A summary of the outcomes of the
procedure is presented in Table
II.
 | Table IISummary of the outcomes following
catheter ablation for atrial fibrillation. |
Table II
Summary of the outcomes following
catheter ablation for atrial fibrillation.
| Outcome | Success rate (no
recurrence) | Quality of
life | Mortality |
|---|
| Single
procedure | 65% at 1 year, 56%
at 3 years, 51% at 5 years | Improved in both
symptomatic and asymptomatic patients | No significant
difference compared to medical therapy |
| Multiple
procedures | 86% at 12 months,
79% at 3 years, 78% at 5 years | Sustained
improvements in quality of life scores | Significantly lower
in patients with stable sinus rhythm and no recurrencea |
Success rate after single and multiple
procedures
There is increasing evidence from the literature to
suggest that the long-term success rates for AF ablation are
significantly improved following repeated procedures compared to a
single procedure (63-65).
A previous meta-analysis of 19 studies involving patients with
6,167 AF with an average age ranging from 51 to 65 years
demonstrated that repeated AF ablation procedures were associated
with significantly improved long-term success rates compared to a
single procedure (63). The results
of that study revealed that following a single CA procedure, the
rate of freedom from AF was 65% at 1 year, 56% at 3 years, and 51%
at 5 years. However, following multiple ablation procedures, AF was
successfully suppressed in 86% of patients at 12 months, 79% of
patients at 3 years, and 78% at 5 years (63).
Moreover, studies have shown that the success rate
for a single-procedure CA is higher in patients with paroxysmal AF
than in those with persistent AF (63-65).
It was found that following 3-5 years of follow-up, only 54% of
patients with paroxysmal AF and 42% with persistent AF were found
to be arrhythmia-free (63).
Nevertheless, following multiple procedures, long-term success
rates were similar in patients with paroxysmal and persistent AF
(79 and 78%, respectively), although the number of procedures per
patient was significantly higher in patients with persistent AF
than in those with paroxysmal arrhythmia (63). These findings highlight the potential
benefits of repeated ablation procedures for achieving better
long-term outcomes in AF patients, especially those with persistent
AF.
Quality of life
The primary goal of treating patients with AF is to
improve their symptoms. Thus, making formal evaluations of quality
of life is important in determining the success of ablation
procedures (66,67). These evaluations offer a more
comprehensive view of symptom change, arrhythmia burden, health and
function, compared to only monitoring rhythm status. For this
purpose, various tools are used, such as the SF-36 health survey,
which can be utilized for a wide range of health conditions
(68), and AF-specific
questionnaires (67,69). However, it should be noted that both
types of quality of life instruments depend on the subjective
experience of the patient and there is no agreement on which is
superior. Consequently, utilizing a standard instrument, such as
the SF-36 in the assessment of AF can still yield valuable insight
into the quality of life of an individual.
Numerous studies have demonstrated that CA can
significantly improve quality of life in patients with AF compared
to antiarrhythmic drug (AAD) therapy (67,70-75).
Non-randomized studies have consistently shown sustained
improvements in quality of life scores following 12 months of AF
ablation (76), while in randomized
clinical trials comparing ablation as first-line therapy, quality
of life improved to a greater extent with ablation than with AAD
treatment (72-76).
However, the duration of improvement in quality of
life following CA vs. AAD therapy remains a topic of discussion. A
previous meta-analysis of 12 randomized controlled trials involving
1,707 patients with symptomatic AF, which compared CA as first or
second-line therapy with AAD treatment, found that at a 3-month
follow-up interval, CA led to greater improvements in various
aspects of the SF-36 questionnaire and in the symptom frequency
score compared to AAD treatment (77). Although the differences between the
two treatments decreased over time, beyond 9 months, there were no
significant differences in any of the quality of life metrics,
symptom frequency, or severity scores (77).
Notably, patients who remain AF-free following
ablation exhibit greater improvements in quality of life than those
with recurrent arrhythmia (70-77).
However, even patients with AF recurrence exhibit significant
improvements in quality of life following ablation compared to
pre-ablation values, possibly due to a higher proportion of
asymptomatic arrhythmia episodes, reduction in AF burden, left
atrial denervation, increased AAD effectiveness, or a placebo
effect post-procedure (78). These
findings highlight the complexity of the association between AF
ablation and quality of life outcomes, and suggest the need for the
careful consideration of patient-specific factors when discussing
the potential benefits and risks of ablation.
Mortality
Randomized trials and metanalyses of the trials have
not demonstrated the superiority of ablation compared to medical
therapy in reducing mortality, with the exception of patients with
heart failure with reduced ejection fraction as aforementioned,
most probably due to the limited duration of post-procedural
follow-up and the selection of relatively younger patients with a
low prevalence of structural heart disease and low thromboembolic
risk (9,22,35-38,79).
However, several non-randomized studies have demonstrated the
advantages of CA over medical therapy with respect to survival in
the subgroups of ‘sicker’ and older patients with AF with
significant heart disease, HF, and a high CHA2DS2VASc score
(80-85).
These studies included patients with a mean age between 57 and 69
years and more than one risk factor for thromboembolism; more than
half of the patients had significant structural heart or lung
disease (80-85).
During the mean follow-up of 2.5-4.4 years, a significantly lower
mortality rate (3-6 vs. 7-14%) was observed following ablation
compared to AAD therapy. Moreover, a stable sinus rhythm after the
procedure was strongly associated with a reduction in mortality
[hazard ratio (HR), 0.14; 95% CI, 0.06 to 12.36], while an AF
recurrence was an independent predictor of mortality (HR, 2.52; 95%
CI, 1.05 to 6.06) (81,83).
4. Complications
As with any medical procedure, CA for AF is not
without complications. Studies have estimated that the incidence of
major complications associated with CA ranges between 1 to 6.29%
(86-88).
In this section of the review, the specific complications
associated with CA for AF that should be regularly considered by
clinicians involved in its management are discussed, with the aim
of effectively monitoring, preventing and managing any such
complications that may arise. A summary of the complications
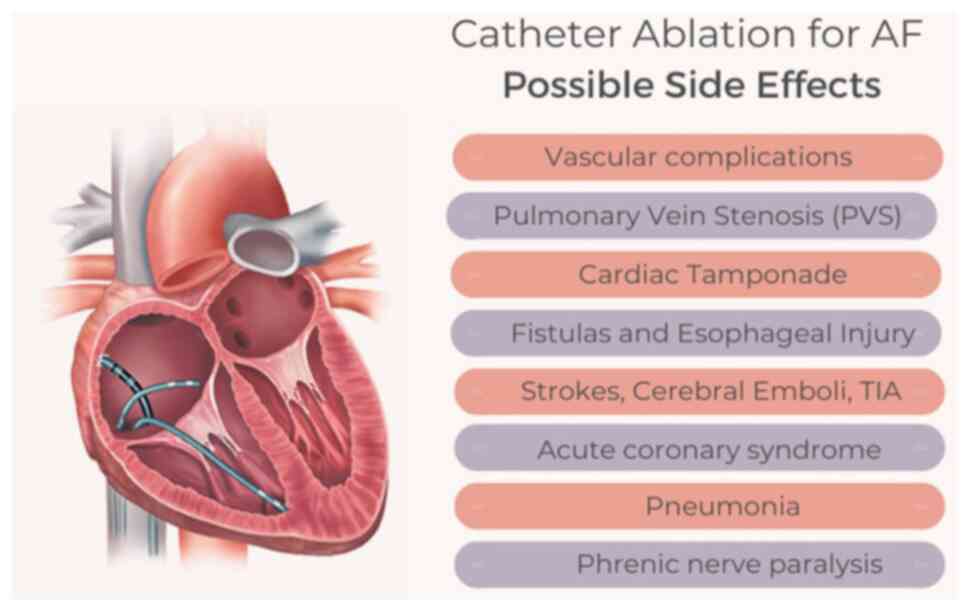
associated with the procedure is illustrated in Fig. 1.
Vascular complications
Vascular complications, such as access site
complications, bleeding, hematoma, arteriovenous fistula and
pseudoaneurysm are well-documented as the most common complications
following CA. With that being said, the findings of previous
studies vary when mentioning the exact rate of vascular
complications (86,87,89,90).
Steinbeck et al (89), in
their study in 2018, attributed this variation to numerous reasons
such as sex, an age >75 years and other comorbidities such as
hypertension. Prudente et al (91) found that patients with an increasing
age and those with AF at the time of CA were associated with a
greater number of femoral vascular complications. Notably, catheter
sheath size and procedure duration did not affect the complication
rate (91). One interesting finding
was the use of ultrasound when guiding the access to the vascular
compartment and was associated with lower rates of major vascular
complications (92). As expected,
procedures performed in high-volume centers were associated with
lower rates of complications (87).
Pulmonary vein stenosis (PVS)
It is well-known that almost 90% of AF cases arise
from pulmonary veins, which is the location where CA of
arrhythmogenic foci of AF takes place. As a consequence, it was
observed that weeks or months post-procedure, the narrowing of the
pulmonary vein lumen can develop (93). The most commonly accepted proposed
mechanism is neointimal proliferation and myocardial fibrosis
(88). Generally, PVS classification
depends on the degree of stenosis, where 20-50% is considered as
mild, 50-69% is considered moderate, and ≥70% is considered severe
(88,93). The prevalence of PVS varies in the
literature. However, the majority of studies agree on a prevalence
of ~40% (88,93,94) with
a percentage of 0.3-3.4 for severe PVS (94). The most common presenting symptoms
are shortness of breath, cough, fatigue, exercise intolerance,
chest pain on exertion and hemoptysis (94). As regards diagnosis, contrast
computed tomography (CT) scans and magnetic resonance imaging (MRI)
remain the preferred method of choice. Perfusion scans,
transesophageal echocardiograms (TEEs) and pulmonary venography are
other modalities that can be utilized for diagnosis (88,94).
Cardiac tamponade
Out of the numerous complications associated with CA
for AF, cardiac tamponade remains the most common life-threatening
complication (88,89). The reported incidents of cardiac
tamponade range between 1-1.6% (88,89,95). On
the other hand, it was previously found that an older age (>65
years), redo-procedures (95), and
using two or more trans-septal punctures were associated risk
factors for developing cardiac tamponade (88). Usually, the mechanism involved in
this process is when puncturing occurs in the posterior right
atrial wall just before entering the septum or when exiting from
the left atrium towards the left atrial appendage, roof, or lateral
wall (88). However, it is worth
mentioning that the risk of increased bleeding into the pericardial
space can be augmented, particularly when the patient is on large
doses of anticoagulation medications, as is the case in numerous
patients (96).
Fistulas and esophageal injury;
esophageal hematoma, atrial-esophageal fistula and atrial
pericardial fistula
Esophageal perforation is an unfortunate
complication that ensues during catheter manipulation in the close
proximity of the posterior atrial wall and the esophagus. Although
uncommon, previous research has reported that it can be detected by
the use of intraprocedural TEE that is commonly utilized in most
facilities during the procedures (97). Reported symptoms are dysphagia, food
regurgitation and hoarseness occurring within 12 h of the the
procedure. A chest CT scan and upper endoscopy can further confirm
the diagnosis (87).
Similarly, atrio-esophageal and atrio-pericardial
fistulas most commonly occur upon using any energy-exerting method
when used against the posterior atrial wall (87). Due to its severe sequelae,
atrio-esophageal fistula is known to be the most lethal
complication of CA of AF (96) with
a mortality rate that reaches 50%, according to the study by Sra
(88). It usually presents within
2-4 weeks following CA (88) and can
present with a number of symptoms, such as fever, neurological
symptoms, septic shock (87), chest
pain, heartburn, dysphagia, anorexia, hematemesis (96) and mortality (87), with a chest CT scan being the
preferred diagnostic method (87).
Other complications
The aforementioned complications were not the only
ones mentioned in the literature. Other not uncommon complications
include peri-esophageal vagal nerve injury, phrenic nerve injury,
strokes, cerebral emboli, TIAs, air embolism, acute coronary artery
syndrome, recurrent laryngeal nerve injury, mitral valve mechanical
injury, arrhythmias, pericarditis, stiff left atrial syndrome and
mortality (87,88,96,98).
5. Optimization of risk factors prior to the
procedure
It is crucial to realize the modifiable risk factors
of AF, such as hypertension, diabetes, obesity, OSA and alcohol
consumption (9). Addressing these
risk factors through lifestyle changes, medication, or other
interventions can improve the effectiveness of CA and reduce the
risk of AF recurrence (9). Moreover,
modifying these risk factors can improve overall health and reduce
the risk of other cardiovascular diseases, reducing morbidity and
mortality. Therefore, identifying and optimizing the modifiable
risk factors of AF is crucial in managing the condition and
improving patient outcomes. A summary of the strategies that can be
used to improve the outcomes of patients is presented in Table III.
 | Table IIIStrategies used to improve the
outcomes of patients undergoing CA for AF. |
Table III
Strategies used to improve the
outcomes of patients undergoing CA for AF.
| Risk factor | Modification | Effect on AF
ablation outcomes |
|---|
| Hypertension | Aggressive blood
pressure control | Improved long-term
AF suppression |
| Diabetes
mellitus | Improved glycemic
control | Improved long-term
AF suppression |
| Smoking | Smoking
cessation | Improved long-term
AF suppression |
| Obesity | Weight loss | Improved long-term
AF suppression |
| Obstructive sleep
apnea | Continuous positive
airway pressure (CPAP) therapy | Improved long-term
AF suppression |
| Alcohol | Alcohol
abstinence | Improved long-term
AF suppression |
| Dyslipidemia | Statin therapy | May improve
long-term AF suppression |
| Polyunsaturated
fatty acids (PUFAs) |
Supplementation | May improve early,
but not late AF recurrence rate |
| Aggressive risk
reductions | Lifestyle
modification and risk factor management | Improved long-term
AF suppression |
| Imaging | Ultrasound and
MRI | Improved long-term
AF suppression |
Hypertension
Preprocedural hypertension is one of the risk
factors that have been found to be associated with an increased
risk of AF recurrence following CA (99). Aggressive blood pressure (BP) control
vs. standard BP control was examined in the SMAC-AF trial and the
results revealed that aggressive BP control was not beneficial in
reducing AF recurrence following CA (100).
In patients with resistant hypertension, renal
artery denervation performed in conjunction with pulmonary vein
isolation has been shown to provide better long-term AF suppression
than pulmonary vein isolation alone (101).
Diabetes mellitus (DM)
While long-term freedom from AF following CA
appears to be similar in patients with and without DM (102), a higher baseline glycated
hemoglobin (HbA1c) level has been linked to an increased risk of
late AF recurrence in patients with DM following ablation. It is
worth noting that the quality of glycemic control in the year
leading up to the CA procedure for AF was significantly associated
with the occurrence of AF recurrence within the 12 months following
the procedure (103).
The role of pioglitazone in protecting patients
with type 2 DM and paroxysmal AF from recurrent AF following CA has
been investigated (104), with
promising results. In patients who used pioglitazone, the success
rate of a single pulmonary vein isolation procedure was
significantly higher following a 2-year follow-up compared with
non-users (86.3 vs. 70.7%, respectively), while the need for
redo-ablation was significantly lower when compared to non-users
(9.8 vs. 24.2%, respectively) (104).
Smoking
Among patients with recurrent AF, smokers have a
higher risk of arrhythmia relapse following CA compared with
non-smokers. A previous study demonstrated that the 1-year AF
recurrence rate following pulmonary vein isolation was
significantly higher in smokers than in non-smokers (43 vs. 14%;
HR, 3.19). However, the results did not reveal a significant
difference in the risk of recurrence following CA between current
and past smokers (105).
Obesity
A previous meta-analysis of 23 studies identified a
27% increase in the relative risk of AF recurrence following CA in
overweight or obese patients (106). Additionally, in overweight and
obese patients with cardiovascular risk factors, a structured risk
factor modification including weight reduction significantly
improved long-term AF-free survival after ablation (87 vs. 17.8%,
respectively) (107).
OSA
Patients with OSA have a 31% higher risk of AF
recurrence following CA compared to those without OSA (108). Severe OSA, defined as an
apnea-hypopnea index of ≥10, was identified as an independent
predictor of ablation failure (109,110).
Furthermore, as previously demonstrated, the risk
of AF recurrence in patients with OSA undergoing CA increased by
57% if they did not receive concurrent continuous positive airway
pressure (CPAP) therapy (108). It
is noteworthy that the post-ablation recurrence rates of AF in OSA
patients receiving CPAP were similar to those of patients with AF
who did not have OSA (108,111).
Alcohol consumption
Multiple studies have reported that alcohol
abstinence following AF ablation can improve outcomes. Abstaining
from alcohol consumption following the procedure was found to be
associated with a significantly lower AF recurrence rate in
comparison to patients who previously consumed alcohol or those who
were currently consuming alcohol (34.1 vs. 41.9%) (112).
In another study, in obese individuals with more
than one cardiometabolic risk factor, a post-ablation lifestyle
intervention that included reducing alcohol intake to <30 g per
week was shown to lead to improved long-term rhythm outcomes
following the procedure (107).
Dyslipidemia
Dyslipidemia has been identified as a risk factor
for very late AF recurrence following CA. In a previous long-term
study, hyperlipidemia was independently associated with a 4-fold
higher risk of very late arrhythmia recurrence occurring more than
one year after AF ablation (113).
A previous randomized study demonstrated that the
short-term use of statins does not affect the outcome of AF
ablation. Patients treated with 80 mg atorvastatin and those who
received a placebo following the procedure had similar rates of
early and late AF recurrence (114).
It was also previously demonstrated that
supplementation with 1 to 4 g/day of polyunsaturated fatty acids
(PUFAs) for 6 to 12 months did not significantly improve the
clinical course of paroxysmal AF (115,116).
However, combining 2 to 6 g/day of PUFAs with AAD therapy was
associated with a significant reduction in the recurrence rate of
AF following cardioversion, from 77.5 to 38.5% (117,118).
Furthermore, PUFAs significantly reduced the early, but not the
late recurrence rate of AF post-ablation (119).
Effect of aggressive risk reductions
in clinical trials and cohort studies
Pathak et al (107) conducted a cohort study to evaluate
the impact of risk factors and weight management on AF ablation
outcomes. Of the 281 consecutive patients undergoing AF ablation,
149 with a body mass index ≥27 kg/m2 and ≥1 cardiac risk
factor were offered risk factor management (RFM) according to the
AHA/ACC guidelines (107). The
lifestyle treatment program included special assistance with weight
management, an exercise prescription of 200 min of moderate
exercise per week, advice regarding salt restriction, lipid
management, glucose monitoring and treatment, smoking and alcohol
counseling, and evaluation for sleep apnea (107). There were no differences in
baseline characteristics, the number of procedures, or follow-up
duration between the groups (P-value, not significant). RFM
resulted in greater reductions in weight (P=0.002) and blood
pressure (P=0.006), and improved glycemic control (P=0.001) and
lipid profiles (P=0.01) (107). At
follow-up, AF frequency, duration, symptoms and symptom severity
decreased to a greater extent in the RFM group compared with the
control group (all P<0.001) (107). Single-procedure drug-unassisted
arrhythmia-free survival was greater in patients offered RFM
compared with the control subjects (P<0.001) (107). Multiple-procedure arrhythmia-free
survival was markedly better in patients offered RFM compared with
the control subjects (P<0.001), at 16 and 42.4%, respectively,
using AADs (P=0.004) (107).
Overall, aggressive RFM improved the long-term success of AF
ablation (107).
Utilization of imaging for improving
the outcomes of patients undergoing CA
The mapping and preparation for AF ablation is
crucial to ensure successful treatment outcomes. In recent years,
advances in imaging techniques, such as ultrasound and MRI, have
played a crucial role in identifying arrhythmogenic substrates
prior to electrophysiology studies (120,121).
Ultrasound imaging, also known as echocardiography,
is a non-invasive tool that utilizes high-frequency sound waves to
produce images of the heart. The use of ultrasound in AF ablation
procedures has increased in recent years, with the development of
three-dimensional (3D) imaging techniques. 3D ultrasound can
provide a comprehensive view of the heart, including the atria and
surrounding structures, allowing for more precise mapping of the
arrhythmogenic substrates (120,122).
MRI is another non-invasive imaging tool that uses
a magnetic field and radio waves to produce images of the heart and
surrounding structures. MRI can provide detailed images of the
heart's anatomy, including the location and extent of fibrous
tissue and scarring, which can contribute to AF. This information
can be useful in planning AF ablation procedures and in determining
the appropriate ablation strategy (120,121).
Gimelli et al (121) conducted a study to evaluate the use
of multi-modality imaging in the identification of arrhythmogenic
substrates prior to electrophysiology studies. Their study found
that the combination of ultrasound and MRI provided a comprehensive
understanding of the underlying causes of AF, including the
location and extent of fibrous tissue and scarring. This
information allowed for a more precise and effective ablation
strategy.
In conclusion, the use of imaging techniques, such
as ultrasound and MRI, has greatly improved the mapping and
preparation for AF ablation procedures. These tools provide
critical information on the arrhythmogenic substrates, allowing for
more precise and effective ablation strategies. The findings from
the study by Gimelli et al (121) demonstrate the importance of
multi-modality imaging in the identification of arrhythmogenic
substrates prior to electrophysiology studies.
6. Use of antiarrhythmic and anticoagulation
agents following the procedure
Use of AADs
Conflicting data exist regarding the optimal
strategy for AAD therapy following CA in patients with AF (9,25). A
previous multicenter, registry-based prospective study was
performed in Germany on 3,275 patients undergoing CA (123). That study compared the outcomes of
patients who were started on AADs vs. those who were not, at the
time of discharge. The results were further analyzed in subgroups
of patients with paroxysmal AF and those with persistent AF
(123). That study found that at 12
months following the procedure, patients who were discharged and
treated with AADs exhibited similar rates of recurrence,
rehospitalization and cardiovascular events compared to those who
were not on AADs. However, in the subgroup of patients with
paroxysmal AF, the use of AAD at discharge was linked to lower
treatment satisfaction and a higher rate of repeat ablation
(123). The lower treatment
satisfaction was concluded by the patients rating the procedure as
‘non-successful’ more often when they are discharged on AADs. This
finding can be explained by the desire of the patients to terminate
antiarrhythmic medications to consider the procedure
successful.
On the other hand, a multicenter RCT was conducted
on a total 153 patients with paroxysmal AF who underwent pulmonary
vein isolation (124). That study
included only 153 patients who had continued taking the previously
ineffective AADs during a 3-month ‘blanking period’. Patients who
developed recurrence during this period were excluded from the
study. At the end of the blanking period, patients who remained
free of AF were randomly assigned to one of two groups: A group in
which AADs were continued after the procedure and another group in
which AADs were discontinued (124). The results revealed that continuing
the use of AADs significantly reduced the recurrence of atrial
tachyarrhythmias in the first year after pulmonary vein isolation
(124). However, it should be noted
that patients who had a recurrence during the blanking period were
not studied. By the nature of the study protocol, only patients who
were continued on AADs in the first 3 months were recruited, which
may have increased their dependence on AADs increasing the
possibility of recurrence after discontinuation. Additionally, that
study was not blinded which may have been a source of bias.
Use of anticoagulants
The safety of discontinuing oral anticoagulant
(OAC) therapy following AF ablation remains controversial. Current
practical clinical guidelines (9,25)
recommend continuing OAC therapy for stroke/thromboembolism for at
least 2 months in all patients, regardless of stroke risk factors.
Beyond this time, the decision to continue or terminate OAT should
not be based on the apparent success or failure of CA for AF or the
pattern of AF, but on the stroke (CHA2DS2-VASc score) and bleeding
risks (HAS-BLED score) and comorbidities of the patient (9,25).
However, the estimated bleeding risk, in the absence of absolute
contraindications to OAC therapy, is not recommended to guide the
decision to use OAC therapy for stroke prevention. Patients who are
at a high risk of stroke (i.e., CHA2DS2-VASc score ≥2 for males or
≥3 for females, prior history of stroke), in whom the reduction in
the risk of a disabling stroke may outweigh the risk of bleeding.
For patients with an intermediate risk of stroke (CHA2DS2-VASc
score 1 in males or 2 in females), long-term OAC therapy is also
recommended. Finally, in patients who are at a low risk of stroke
(CHA2DS2-VASc score of 0 in males or 1 in females), the risk of
stroke/systemic embolism in observational studies is very low
(0-0.2%), and the risk of bleeding associated with long-term OAC
therapy outweighs the benefits of stroke prevention; thus, the
discontinuation of OAC therapy should be considered 2 months
post-CA for AF, regardless of AF recurrence (9,25).
The current evidence evaluating the safety of
discontinuing anticoagulation in patients with AF following
successful CA is limited to observational studies. The large
observational study conducted by Karasoy et al (125) reported the outcomes of 4,050
patients with AF undergoing first-time radiofrequency CA. Among the
1,507 patients with n increased risk of stroke
(CHA2DS2VASc score ≥2), OAC was discontinued
in 30% of patients at 1 year. The overall rate of thromboembolism
was low and comparable between patients with discontinued OAC (0.93
per 100 patient-years) and continued OAC use (0.97 per 100
patient-years).
Proietti et al (126) conducted a systematic review of 10
prospective cohort and 6 retrospective cohort studies (25,177
patients) that reported cerebrovascular events (CVE) following CA
for AF and compared patients treated with OACs vs. those who were
not treated with OACs. There were no significant differences in the
incidence of CVE between patients treated or not with OACs
following CA for AF. Patients not treated with OACs suffered
significantly less bleeding than those on-OAT with a relative risk
of 0.17 (CI, 0.09-0.34); however, they had lower CHADS2 scores than
those treated with OACs, probably reflecting the reluctance of
clinicians to discontinue OAC therapy in patients who were at a
high thromboembolic risk and the influence of current
guidelines.
Additionally, a recent meta-analysis of prospective
studies was conducted by Liu et al (127) to assess the safety and feasibility
of discontinuing OAC therapy following successful AF ablation.
Their study included 11,148 patients (7,160 in the off-OAC group
and 3,988 in the on-OAC group) where no significant difference in
thromboembolism between both groups was observed with an odds ratio
of 0.73 (95% CI, 0.51-1.05; I2=0.0%) (127). In addition, their study concluded
that the risk of major bleeding in the off-OAC group was
significantly lower compared to the on-OAC group with an odds ratio
of 0.18 (95% CI, 0.07-0.51; I2=51.7%). Overall, that
study provides evidence suggesting that it may be safe to
discontinue OAC therapy in patients following successful AF
ablation. Additionally, an increased risk of major bleeding was
observed in patients on OACs (127).
Regrettably, there is still a lack of prospective
randomized clinical trials to recommend whether it is safe or not
to discontinue OAC therapy following successful CA for AF in
patients with intermediate-to-high stroke risk and the decision of
whether OAC can be safely discontinued post-CA for AF remains
controversial. Currently, continued long-term OAC guided by the
stroke risk factor profile is the only proven strategy to prevent
stroke. A summary of the recommendations regarding the use of
anticoagulant and antiarrhythmic agents following the procedure is
presented in Table IV.
 | Table IVSummary of the recommendations
regarding the use of anticoagulant and antiarrhythmic agents
following the procedure. |
Table IV
Summary of the recommendations
regarding the use of anticoagulant and antiarrhythmic agents
following the procedure.
| Factor | Recommendation | Evidence | Risks | Benefits |
|---|
| Antiarrhythmic
drugs | Continue or
discontinue based on individual patient factors | Conflicting
data | - | May reduce the risk
of the recurrence of atrial fibrillation |
| Anticoagulation
therapy | Continue for at
least 2 months, then consider discontinuation based on individual
patient factors | Conflicting
data | Bleeding | May reduce the risk
of stroke |
7. Cost-effectiveness of the procedure
The determination of cost-effectiveness relies
heavily on deduced results from clinical trials that are
characterized by limited durations of follow-up and inadequate
sample sizes, necessitating the formulation of conjectures
regarding critical clinical outcomes (128,129).
In order to enhance the effectiveness of these estimations, it is
imperative to secure comprehensive data from more extensive and
prolonged studies. This will provide a more nuanced and informed
perception of the long-term costs and benefits associated with
treatment enabling the refinement of cost-effectiveness
calculations.
Furthermore, the costs associated with AF ablation
procedures can fluctuate significantly based on the settings of
treatment and the type of equipment utilized (130,131).
Estimations of the cost-effectiveness of AF ablation can be further
affected by a multitude of factors, such as the patient
demographics, the magnitude of symptoms, the duration of analysis,
and the assumptions made regarding the effect of AF ablation on
QOL, strokes and other clinical outcomes.
The argument of the cost-effectiveness of AF
ablation is based on the premise that over time, the expenses
incurred from the procedure can be partially compensated by the
reduction of healthcare resource utilization that stems from the
long-term management of arrhythmias in patients who do not receive
ablation. This notion is supported by some empirical evidence
(132-134).
However, the majority of formal cost-effectiveness studies have not
been able to establish that AF ablation is cost-neutral or
cost-saving in the short to intermediate term. This highlights the
need for longer-term studies when considering the
cost-effectiveness of AF ablation.
The cost-effectiveness of AF ablation has largely
been evaluated in comparison to AAD therapy as a second-line
treatment option for patients with paroxysmal AF (131,135-138).
These studies have generally produced favorable cost-effectiveness
ratios (136,138). However, the cost-effectiveness of
AF ablation as a first-line therapy, particularly in patients with
persistent or long-term persistent AF, is not as well established.
On the other hand, one study based on the MANTRA-PAF trial
population suggests that AF ablation may only be cost-effective as
a first-line treatment in younger patients (139).
8. Conclusion
CA holds promise as an effective therapeutic
strategy for the management of AF. However, careful patient
selection, procedural expertise and the consideration of individual
patient characteristics are crucial for optimizing outcomes.
Further research is required in order to clarify the role of CA in
specific patient populations and its impact on stroke
prevention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
OO and MFI conceptualized the review, wrote the
initial draft of the manuscirpt, and reviewed the manuscript. SA,
HAA, MT, LA, AlO, AbO, QA, MAD, HA, MAH, WA, AT and MA contributed
to the writing and reviewing of the manuscript. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schnabel RB, Yin X, Gona P, Larson MG,
Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW,
Ellinor PT, et al: 50 Year trends in atrial fibrillation
prevalence, incidence, risk factors, and mortality in the
Framingham heart study: A cohort study. Lancet. 386:154–162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart disease and stroke statistics-2019
update: A report from the american heart association. Circulation.
139:e56–e528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kornej J, Börschel CS, Benjamin EJ and
Schnabel RB: Epidemiology of atrial fibrillation in the 21st
century: Novel methods and new insights. Circ Res. 127:4–20.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Staerk L, Sherer JA, Ko D, Benjamin EJ and
Helm RH: Atrial fibrillation: Epidemiology, pathophysiology, and
clinical outcomes. Circ Res. 120:1501–1517. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mark DB, Anstrom KJ, Sheng S, Piccini JP,
Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg
Y, et al: Effect of catheter ablation vs medical therapy on quality
of life among patients with atrial fibrillation: the CABANA
randomized clinical trial. JAMA. 321:1275–1285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Packer DL, Mark DB, Robb RA, Monahan KH,
Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N,
Mitchell LB, et al: Effect of catheter ablation vs antiarrhythmic
drug therapy on mortality, stroke, bleeding, and cardiac arrest
among patients with atrial fibrillation: The CABANA randomized
clinical trial. JAMA. 321:1261–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prabhu S, Taylor AJ, Costello BT, Kaye DM,
McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB,
Pathik B, et al: Catheter ablation versus medical rate control in
atrial fibrillation and systolic dysfunction: The CAMERA-MRI study.
J Am Coll Cardiol. 70:1949–1961. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marrouche NF, Brachmann J, Andresen D,
Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders
P, Proff J, et al: Catheter ablation for atrial fibrillation with
heart failure. N Engl J Med. 378:417–427. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Calkins H, Hindricks G, Cappato R, Kim YH,
Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al:
2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on
catheter and surgical ablation of atrial fibrillation. Europace.
20:e1–e160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
January CT, Wann LS, Alpert JS, Calkins H,
Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD,
Field ME, et al: 2014 AHA/ACC/HRS guideline for the management of
patients with atrial fibrillation: A report of the American college
of cardiology/American heart association task force on practice
guidelines and the heart rhythm society. J Am Coll Cardiol.
64:e1–e76. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kirchhof P, Benussi S, Kotecha D, Ahlsson
A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks
J, et al: 2016 ESC guidelines for the management of atrial
fibrillation developed in collaboration with EACTS. Eur J
Cardiothorac Surg. 50:e1–e88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Camm AJ, Kirchhof P, Lip GY, Schotten U,
Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G,
Prendergast B, et al: Guidelines for the management of atrial
fibrillation: The task force for the management of atrial
fibrillation of the European society of cardiology (ESC). Europace.
12:1360–1420. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Camm AJ, Lip GY, De Caterina R, Savelieva
I, Atar D, Hohnloser SH, Hindricks G and Kirchhof P: ESC Committee
for Practice Guidelines (CPG). 2012 Focused update of the ESC
guidelines for the management of atrial fibrillation: An update of
the 2010 ESC guidelines for the management of atrial fibrillation.
Developed with the special contribution of the European heart
rhythm association. Eur Heart J. 33:2719–2747. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wyse DG, Waldo AL, DiMarco JP, Domanski
MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC,
Dalquist JE, et al: A comparison of rate control and rhythm control
in patients with atrial fibrillation. N Engl J Med. 347:1825–1833.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Van Gelder IC, Hagens VE, Bosker HA,
Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ,
Tijssen JG, et al: A comparison of rate control and rhythm control
in patients with recurrent persistent atrial fibrillation. N Engl J
Med. 347:1834–1840. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Carlsson J, Miketic S, Windeler J, Cuneo
A, Haun S, Micus S, Walter S and Tebbe U: STAF Investigators.
Randomized trial of rate-control versus rhythm-control in
persistent atrial fibrillation: The strategies of treatment of
atrial fibrillation (STAF) study. J Am Coll Cardiol. 41:1690–1696.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kirchhof P, Camm AJ, Goette A, Brandes A,
Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM,
et al: Early rhythm-control therapy in patients with atrial
fibrillation. N Engl J Med. 383:1305–1316. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
January CT, Wann LS, Calkins H, Chen LY,
Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME,
Furie KL, et al: 2019 AHA/ACC/HRS focused update of the 2014
AHA/ACC/HRS guideline for the management of patients with atrial
fibrillation: A report of the American College of
cardiology/american heart association task force on clinical
practice guidelines and the heart rhythm society in collaboration
with the society of thoracic surgeons. Circulation. 140:e125–e151.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hindricks G, Potpara T, Dagres N, Arbelo
E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA,
Dilaveris PE, et al: 2020 ESC guidelines for the diagnosis and
management of atrial fibrillation developed in collaboration with
the European association for cardio-thoracic surgery (EACTS): The
task force for the diagnosis and management of atrial fibrillation
of the European Society of Cardiology (ESC) developed with the
special contribution of the European heart rhythm association
(EHRA) of the ESC. Eur Heart J. 42:373–498. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Doyle JF and Ho KM: Benefits and risks of
long-term amiodarone therapy for persistent atrial fibrillation: A
meta-analysis. Mayo Clin Proc. 84:234–242. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Roy D, Talajic M, Dorian P, Connolly S,
Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagné P, et al:
Amiodarone to prevent recurrence of atrial fibrillation. Canadian
trial of atrial fibrillation investigators. N Engl J Med.
342:913–920. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khan AR, Khan S, Sheikh MA, Khuder S,
Grubb B and Moukarbel GV: Catheter ablation and antiarrhythmic drug
therapy as first- or second-line therapy in the management of
atrial fibrillation: Systematic review and meta-analysis. Circ
Arrhythm Electrophysiol. 7:853–860. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Andrade JG: Ablation as first-line therapy
for atrial fibrillation. Eur Cardiol. 18(e46)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Turagam MK, Musikantow D, Whang W, Koruth
JS, Miller MA, Langan MN, Sofi A, Choudry S, Dukkipati SR and Reddy
VY: Assessment of catheter ablation or antiarrhythmic drugs for
first-line therapy of atrial fibrillation: A meta-analysis of
randomized clinical trials. JAMA Cardiol. 6:697–705.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Joglar JA, Chung MK, Armbruster AL,
Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger
ZD, Gopinathannair R, et al: 2023 ACC/AHA/ACCP/HRS guideline for
the diagnosis and management of atrial fibrillation: A report of
the american college of cardiology/American heart association joint
committee on clinical practice guidelines. Circulation.
149:e1–e156. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hocini M, Sanders P, Deisenhofer I, Jaïs
P, Hsu LF, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, et
al: Reverse remodeling of sinus node function after catheter
ablation of atrial fibrillation in patients with prolonged sinus
pauses. Circulation. 108:1172–1175. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen YW, Bai R, Lin T, Salim M, Sang CH,
Long DY, Yu RH, Tang RB, Guo XY, Yan XL, et al: Pacing or ablation:
Which is better for paroxysmal atrial fibrillation-related
tachycardia-bradycardia syndrome? Pacing Clin Electrophysiol.
37:403–411. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Inada K, Yamane T, Tokutake K, Yokoyama K,
Mishima T, Hioki M, Narui R, Ito K, Tanigawa S, Yamashita S, et al:
The role of successful catheter ablation in patients with
paroxysmal atrial fibrillation and prolonged sinus pauses: Outcome
during a 5-year follow-up. Europace. 16:208–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koopman P, Nuyens D, Garweg C, La Gerche
A, De Buck S, Van Casteren L, Alzand B, Willems R and Heidbuchel H:
Efficacy of radiofrequency catheter ablation in athletes with
atrial fibrillation. Europace. 13:1386–1393. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Furlanello F, Lupo P, Pittalis M, Foresti
S, Vitali-Serdoz L, Francia P, De Ambroggi G, Ferrero P, Nardi S,
Inama G, et al: Radiofrequency catheter ablation of atrial
fibrillation in athletes referred for disabling symptoms preventing
usual training schedule and sport competition. J Cardiovasc
Electrophysiol. 19:457–462. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pathak RK, Elliott A, Middeldorp ME,
Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM,
Abhayaratna WP, et al: Impact of CARDIOrespiratory fitness on
arrhythmia recurrence in obese individuals with atrial
fibrillation: The CARDIO-FIT study. J Am Coll Cardiol. 66:985–996.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deshpande R, AlKhadra Y, Singanallur P,
Botchway A and Labedi M: Outcomes of catheter ablation versus
antiarrhythmic therapy in patients with atrial fibrillation: A
systematic review and meta-analysis. J Interv Card Electrophysiol.
65:773–802. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Deedwania PC and Lardizabal JA: Atrial
fibrillation in heart failure: A comprehensive review. Am J Med.
123:198–204. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nerheim P, Birger-Botkin S, Piracha L and
Olshansky B: Heart failure and sudden death in patients with
tachycardia-induced cardiomyopathy and recurrent tachycardia.
Circulation. 110:247–252. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Raymond RJ, Lee AJ, Messineo FC, Manning
WJ and Silverman DI: Cardiac performance early after cardioversion
from atrial fibrillation. Am Heart J. 136:435–442. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
January CT, Wann LS, Calkins H, Chen LY,
Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME,
Furie KL, et al: 2019 AHA/ACC/HRS focused update of the 2014
AHA/ACC/HRS guideline for the management of patients with atrial
fibrillation: A report of the American college of
cardiology/American heart association task force on clinical
practice guidelines and the heart rhythm society. J Am Coll
Cardiol. 74:104–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Şaylık F, Çınar T, Akbulut T and Hayıroğlu
Mİ: Comparison of catheter ablation and medical therapy for atrial
fibrillation in heart failure patients: A meta-analysis of
randomized controlled trials. Heart Lung. 57:69–74. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chang TY, Chao TF, Lin CY, Lin YJ, Chang
SL, Lo LW, Hu YF, Chung FP and Chen SA: Catheter ablation of atrial
fibrillation in heart failure with impaired systolic function: An
updated meta-analysis of randomized controlled trials. J Chin Med
Assoc. 86:11–18. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zafrir B, Lund LH, Laroche C, Ruschitzka
F, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic
PM, Maggioni AP, et al: Prognostic implications of atrial
fibrillation in heart failure with reduced, mid-range, and
preserved ejection fraction: A report from 14 964 patient in the
European society of cardiology heart failure long-term registry.
Eur Heart J. 39:4277–4284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sartipy U, Dahlström U, Fu M and Lund LH:
Atrial fibrillation in heart failure with preserved, mid-range, and
reduced ejection fraction. JACC Heart Fail. 5:565–574.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gu G, Wu J, Gao X, Liu M, Jin C and Xu Y:
Catheter ablation of atrial fibrillation in patients with heart
failure and preserved ejection fraction: A meta-analysis. Clin
Cardiol. 45:786–793. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kawamura I, Aikawa T, Yokoyama Y, Takagi H
and Kuno T: Catheter ablation for atrial fibrillation in elderly
patients: Systematic review and a meta-analysis. Pacing Clin
Electrophysiol. 45:59–71. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee WC, Wu PJ, Chen HC, Fang HY, Liu PY
and Chen MC: Efficacy and safety of ablation for symptomatic atrial
fibrillation in elderly patients: A meta-analysis. Front Cardiovasc
Med. 8(734204)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li F, Zhang L, Wu LD, Zhang ZY, Liu HH,
Zhang ZY, Zhang J, Qian LL and Wang RX: Do elderly patients with
atrial fibrillation have comparable ablation outcomes compared to
younger ones? Evidence from pooled clinical studies. J Clin Med.
11(4468)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Spragg DD, Dalal D, Cheema A, Scherr D,
Chilukuri K, Cheng A, Henrikson CA, Marine JE, Berger RD, Dong J
and Calkins H: Complications of catheter ablation for atrial
fibrillation: Incidence and predictors. J Cardiovasc
Electrophysiol. 19:627–631. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Leong-Sit P, Zado E, Callans DJ, Garcia F,
Lin D, Dixit S, Bala R, Riley MP, Hutchinson MD, Cooper J, et al:
Efficacy and risk of atrial fibrillation ablation before 45 years
of age. Circ Arrhythm Electrophysiol. 3:452–457. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kalman JM, Sanders P, Rosso R and Calkins
H: Should we perform catheter ablation for asymptomatic atrial
fibrillation? Circulation. 136:490–499. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rienstra M, Vermond RA, Crijns HJ, Tijssen
JG and Van Gelder IC: RACE Investigators. Asymptomatic persistent
atrial fibrillation and outcome: Results of the RACE study. Heart
Rhythm. 11:939–945. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boriani G, Laroche C, Diemberger I,
Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L,
Tavazzi L, Maggioni AP and Lip GYH: Asymptomatic atrial
fibrillation: Clinical correlates, management, and outcomes in the
EORP-AF pilot general registry. Am J Med. 128:509–518.e2.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Santhanakrishnan R, Wang N, Larson MG,
Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS,
Lee DS, et al: Atrial fibrillation begets heart failure and vice
versa: Temporal associations and differences in preserved versus
reduced ejection fraction. Circulation. 133:484–492.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Mohanty S, Santangeli P, Mohanty P, Di
Biase L, Holcomb S, Trivedi C, Bai R, Burkhardt D, Hongo R, Hao S,
et al: Catheter ablation of asymptomatic longstanding persistent
atrial fibrillation: impact on quality of life, exercise
performance, arrhythmia perception, and arrhythmia-free survival. J
Cardiovasc Electrophysiol. 25:1057–1064. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wu L, Lu Y, Zheng L, Qiao YU, Chen G, Ding
L, Hou B, Sun W, Liew R, Zhang S and Yao Y: Comparison of
radiofrequency catheter ablation between asymptomatic and
symptomatic persistent atrial fibrillation: A propensity score
matched analysis. J Cardiovasc Electrophysiol. 27:531–535.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pak M, Kobori A, Shizuta S, Sasaki Y,
Toyota T, Yoshizawa T, Inoue K, Kaitani K, Kurotobi T, Morishima I,
et al: The impact of catheter ablation for patients with
asymptomatic atrial fibrillation: Subanalysis of kansai plus atrial
fibrillation (kpaf) registry. Eur Heart J. 41 (Suppl
2)(ehaa946.0442)2020.
|
|
54
|
Kawaji T, Shizuta S, Tanaka M, Nishiwaki
S, Aizawa T, Yamagami S, Komasa A, Yoshizawa T, Kato M, Yokomatsu
T, et al: Prognostic impact of catheter ablation in patients with
asymptomatic atrial fibrillation. PLoS One.
17(e0279178)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Corley SD, Epstein AE, DiMarco JP,
Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein
RC, Krahn AD, et al: Relationships between sinus rhythm, treatment,
and survival in the Atrial Fibrillation follow-up investigation of
rhythm management (AFFIRM) study. Circulation. 109:1509–1513.
2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Barra S, Baran J, Narayanan K, Boveda S,
Fynn S, Heck P, Grace A, Agarwal S, Primo J, Marijon E and
Providência R: Association of catheter ablation for atrial
fibrillation with mortality and stroke: A systematic review and
meta-analysis. Int J Cardiol. 266:136–142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Barra S, Narayanan K, Boveda S, Primo J,
Gonçalves H, Baran J, Agarwal S, Marijon E and Providência R:
Atrial Fibrillation ablation and reduction of stroke events:
Understanding the paradoxical lack of evidence. Stroke.
50:2970–2976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mao YJ, Wang H, Chen JX and Huang PF:
Meta-analysis of medical management versus catheter ablation for
atrial fibrillation. Rev Cardiovasc Med. 21:419–432.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shi LZ, Heng R, Liu SM and Leng FY: Effect
of catheter ablation versus antiarrhythmic drugs on atrial
fibrillation: A meta-analysis of randomized controlled trials. Exp
Ther Med. 10:816–822. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Freedman B, Martinez C, Katholing A and
Rietbrock S: Residual risk of stroke and death in
anticoagulant-treated patients with atrial fibrillation. JAMA
Cardiol. 1:366–368. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Balsam P, Tymińska A, Ozierański K,
Zaleska M, Żukowska K, Szepietowska K, Maciejewski K, Peller M,
Grabowski M, Lodziński P, et al: Randomized controlled clinical
trials versus real-life atrial fibrillation patients treated with
oral anticoagulants. Do we treat the same patients? Cardiol J.
27:590–599. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Camm AJ, Amarenco P, Haas S, Hess S,
Kirchhof P, Lambelet M, Bach M and Turpie AGG: Real-world vs
randomized trial outcomes in similar populations of
rivaroxaban-treated patients with non-valvular atrial fibrillation
in ROCKET AF and XANTUS. Europace. 21:421–427. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ganesan AN, Shipp NJ, Brooks AG, Kuklik P,
Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC and Sanders P:
Long-term outcomes of catheter ablation of atrial fibrillation: A
systematic review and meta-analysis. J Am Heart Assoc.
2(e004549)2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gaita F, Caponi D, Scaglione M, Montefusco
A, Corleto A, Di Monte F, Coin D, Di Donna P and Giustetto C:
Long-term clinical results of 2 different ablation strategies in
patients with paroxysmal and persistent atrial fibrillation. Circ
Arrhythm Electrophysiol. 1:269–275. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ouyang F, Tilz R, Chun J, Schmidt B,
Wissner E, Zerm T, Neven K, Köktürk B, Konstantinidou M, Metzner A,
et al: Long-term results of catheter ablation in paroxysmal atrial
fibrillation: Lessons from a 5-year follow-up. Circulation.
122:2368–2377. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wijffels MC, Kirchhof CJ, Dorland R and
Allessie MA: Atrial fibrillation begets atrial fibrillation. A
study in awake chronically instrumented goats. Circulation.
92:1954–1968. 1995.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wokhlu A, Monahan KH, Hodge DO, Asirvatham
SJ, Friedman PA, Munger TM, Bradley DJ, Bluhm CM, Haroldson JM and
Packer DL: Long-term quality of life after ablation of atrial
fibrillation the impact of recurrence, symptom relief, and placebo
effect. J Am Coll Cardiol. 55:2308–2316. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ware JE, Snow KK, Kosinski M and Gandek B:
SF-36 Health Survey: Manual and Interpretation Guide. New England
Medical Center, The Health Institute, Boston, 1993.
|
|
69
|
Kirchhof P, Auricchio A, Bax J, Crijns H,
Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger
L, et al: Outcome parameters for trials in atrial fibrillation:
Executive summary. Eur Heart J. 28:2803–2817. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Reynolds MR, Ellis E and Zimetbaum P:
Quality of life in atrial fibrillation: Measurement tools and
impact of interventions. J Cardiovasc Electrophysiol. 19:762–768.
2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jaïs P, Cauchemez B, Macle L, Daoud E,
Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P,
et al: Catheter ablation versus antiarrhythmic drugs for atrial
fibrillation: The A4 study. Circulation. 118:2498–2505.
2008.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wazni OM, Marrouche NF, Martin DO, Verma
A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther
J, et al: Radiofrequency ablation vs antiarrhythmic drugs as
first-line treatment of symptomatic atrial fibrillation: A
randomized trial. JAMA. 293:2634–2640. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Cosedis Nielsen J, Johannessen A,
Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S,
Englund A, Hartikainen J, Mortensen LS and Hansen PS:
Radiofrequency ablation as initial therapy in paroxysmal atrial
fibrillation. N Engl J Med. 367:1587–1595. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Morillo CA, Verma A, Connolly SJ, Kuck KH,
Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS and Natale A:
RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic
drugs as first-line treatment of paroxysmal atrial fibrillation
(RAAFT-2): A randomized trial. JAMA. 311:692–700. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Walfridsson H, Walfridsson U, Nielsen JC,
Johannessen A, Raatikainen P, Janzon M, Levin LA, Aronsson M,
Hindricks G, Kongstad O, et al: Radiofrequency ablation as initial
therapy in paroxysmal atrial fibrillation: Results on
health-related quality of life and symptom burden. The MANTRA-PAF
trial. Europace. 17:215–221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Maesen B, van der Heijden CAJ, Bidar E,
Vos R, Athanasiou T and Maessen JG: Patient-reported quality of
life after stand-alone and concomitant arrhythmia surgery: A
systematic review and meta-analysis. Interact Cardiovasc Thorac
Surg. 34:339–348. 2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Siontis KC, Ioannidis JPA, Katritsis GD,
Noseworthy PA, Packer DL, Hummel JD, Jais P, Krittayaphong R, Mont
L, Morillo CA, et al: Radiofrequency ablation versus antiarrhythmic
drug therapy for atrial fibrillation: Meta-analysis of quality of
life, morbidity, and mortality. JACC Clin Electrophysiol.
2:170–180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Raine D, Langley P, Shepherd E, Lord S,
Murray S, Murray A and Bourke JP: Effect of catheter ablation on
quality of life in patients with atrial fibrillation and its
correlation with arrhythmia outcome. Open Heart.
2(e000302)2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Dagres N, Varounis C, Flevari P,
Piorkowski C, Bode K, Rallidis LS, Tsougos E, Leftheriotis D,
Sommer P, Hindricks G and Kremastinos DT: Mortality after catheter
ablation for atrial fibrillation compared with antiarrhythmic drug
therapy. A meta-analysis of randomized trials. Am Heart J.
158:15–20. 2009.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Pappone C, Rosanio S, Augello G, Gallus G,
Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A,
Santinelli V, et al: Mortality, morbidity, and quality of life
after circumferential pulmonary vein ablation for atrial
fibrillation: Outcomes from a controlled nonrandomized long-term
study. J Am Coll Cardiol. 42:185–197. 2003.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Nademanee K, Schwab MC, Kosar EM, Karwecki
M, Moran MD, Visessook N, Michael AD and Ngarmukos T: Clinical
outcomes of catheter substrate ablation for high-risk patients with
atrial fibrillation. J Am Coll Cardiol. 51:843–849. 2008.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Hunter RJ, McCready J, Diab I, Page SP,
Finlay M, Richmond L, French A, Earley MJ, Sporton S, Jones M, et
al: Maintenance of sinus rhythm with an ablation strategy in
patients with atrial fibrillation is associated with a lower risk
of stroke and death. Heart. 98:48–53. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Lin YJ, Chao TF, Tsao HM, Chang SL, Lo LW,
Chiang CE, Hu YF, Hsu PF, Chuang SY, Li CH, et al: Successful
catheter ablation reduces the risk of cardiovascular events in
atrial fibrillation patients with CHA2DS2-VASc risk score of 1 and
higher. Europace. 15:676–684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Friberg L, Tabrizi F and Englund A:
Catheter ablation for atrial fibrillation is associated with lower
incidence of stroke and death: Data from Swedish health registries.
Eur Heart J. 37:2478–2487. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Saliba W, Schliamser JE, Lavi I,
Barnett-Griness O, Gronich N and Rennert G: Catheter ablation of
atrial fibrillation is associated with reduced risk of stroke and
mortality: A propensity score-matched analysis. Heart Rhythm.
14:635–642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Cappato R, Calkins H, Chen SA, Davies W,
Iesaka Y, Kalman J, Kim YH, Klein G, Packer D and Skanes A:
Worldwide survey on the methods, efficacy, and safety of catheter
ablation for human atrial fibrillation. Circulation. 111:1100–1105.
2005.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Patel NJ, Deshmukh A, Pau D, Goyal V,
Patel SV, Patel N, Agnihotri K, Asirvatham S, Noseworthy P, Di
Biase L, Natale A and Viles-Gonzalez JF: Contemporary utilization
and safety outcomes of catheter ablation of atrial flutter in the
United States: Analysis of 89,638 procedures. Heart Rhythm.
13:1317–1325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Sra J: Atrial fibrillation ablation
complications. J Interv Card Electrophysiol. 22:167–172.
2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Steinbeck G, Sinner MF, Lutz M,
Müller-Nurasyid M, Kääb S and Reinecke H: Incidence of
complications related to catheter ablation of atrial fibrillation
and atrial flutter: A nationwide in-hospital analysis of
administrative data for Germany in 2014. Eur Heart J. 39:4020–4029.
2018.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Gupta A, Perera T, Ganesan A, Sullivan T,
Lau DH, Roberts-Thomson KC, Brooks AG and Sanders P: Complications
of catheter ablation of atrial fibrillation: A systematic review.
Circ. Arrhythmia Electrophysiol. 6:1082–1088. 2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Prudente LA, Moorman JR, Lake D, Xiao Y,
Greebaum H, Mangrum JM, DiMarco JP and Ferguson JD: Femoral
vascular complications following catheter ablation of atrial
fibrillation. J Interv Card Electrophysiol. 26:59–64.
2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Sharma PS, Padala SK, Gunda S, Koneru JN
and Ellenbogen KA: Vascular complications during catheter ablation
of cardiac arrhythmias: A comparison between vascular ultrasound
guided access and conventional vascular access. J Cardiovasc
Electrophysiol. 27:1160–1166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Edriss H, Denega T, Test V and Nugent K:
Pulmonary vein stenosis complicating radiofrequency catheter
ablation for atrial fibrillation: A literature review. Respir Med.
117:215–222. 2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Fender EA, Widmer RJ, Hodge DO, Cooper GM,
Monahan KH, Peterson LA, Holmes DR Jr and Packer DL: Severe
pulmonary vein stenosis resulting from ablation for atrial
fibrillation: Presentation, management, and clinical outcomes.
Circulation. 134:1812–1821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Maclean E, Mahtani K, Roelas M, Vyas R,
Butcher C, Ahluwalia N, Honarbakhsh S, Creta A, Finlay M, Chow A,
et al: Transseptal puncture for left atrial ablation: Risk factors
for cardiac tamponade and a proposed causative classification
system. J Cardiovasc Electrophysiol. 33:1747–1755. 2022.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sorgente A, Chierchia GB, de Asmundis C,
Sarkozy A, Capulzini L and Brugada P: Complications of atrial
fibrillation ablation: When prevention is better than cure.
Europace. 13:1526–1532. 2011.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kumar S, Ling LH, Halloran K, Morton JB,
Spence SJ, Joseph S, Kistler PM, Sparks PB and Kalman JM:
Esophageal hematoma after atrial fibrillation ablation: Incidence,
clinical features, and sequelae of esophageal injury of a different
sort. Circ Arrhythm Electrophysiol. 5:701–705. 2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mujović N, Marinković M, Lenarczyk R, Tilz
R and Potpara TS: Catheter ablation of atrial fibrillation: An
overview for clinicians. Adv Ther. 34:1897–1917. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Letsas KP, Weber R, Bürkle G, Mihas CC,
Minners J, Kalusche D and Arentz T: Pre-ablative predictors of
atrial fibrillation recurrence following pulmonary vein isolation:
The potential role of inflammation. Europace. 11:158–163.
2009.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Parkash R, Wells GA, Sapp JL, Healey JS,
Tardif JC, Greiss I, Rivard L, Roux JF, Gula L, Nault I, et al:
Effect of aggressive blood pressure control on the recurrence of
atrial fibrillation after catheter ablation: A randomized,
open-label clinical trial [SMAC-AF (substrate modification with
aggressive blood pressure control)]. Circulation. 135:1788–1798.
2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Pokushalov E, Romanov A, Katritsis DG,
Artyomenko S, Bayramova S, Losik D, Baranova V, Karaskov A and
Steinberg JS: Renal denervation for improving outcomes of catheter
ablation in patients with atrial fibrillation and hypertension:
Early experience. Heart Rhythm. 11:1131–1138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Anselmino M, Matta M, D'Ascenzo F, Pappone
C, Santinelli V, Bunch TJ, Neumann T, Schilling RJ, Hunter RJ,
Noelker G, et al: Catheter ablation of atrial fibrillation in
patients with diabetes mellitus: A systematic review and
meta-analysis. Europace. 17:1518–1525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gorenek B, Pelliccia A, Benjamin EJ,
Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M,
Kudaiberdieva G, Lane DA, et al: European heart rhythm association
(EHRA)/European association of cardiovascular prevention and
rehabilitation (EACPR) position paper on how to prevent atrial
fibrillation endorsed by the heart rhythm society (HRS) and Asia
pacific heart rhythm society (APHRS). Europace. 19:190–225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Gu J, Liu X, Wang X, Shi H, Tan H, Zhou L,
Gu J, Jiang W and Wang Y: Beneficial effect of pioglitazone on the
outcome of catheter ablation in patients with paroxysmal atrial
fibrillation and type 2 diabetes mellitus. Europace. 13:1256–1261.
2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Fukamizu S, Sakurada H, Takano M, Hojo R,
Nakai M, Yuba T, Komiyama K, Tatsumoto A, Maeno K, Mizusawa Y, et
al: Effect of cigarette smoking on the risk of atrial fibrillation
recurrence after pulmonary vein isolation. J Arrhythm. 26:21–29.
2010.
|
|
106
|
Lin KJ, Cho SI, Tiwari N, Bergman M, Kizer
JR, Palma EC and Taub CC: Impact of metabolic syndrome on the risk
of atrial fibrillation recurrence after catheter ablation:
Systematic review and meta-analysis. J Interv Card Electrophysiol.
39:211–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Pathak RK, Middeldorp ME, Lau DH, Mehta
AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD,
et al: Aggressive risk factor reduction study for atrial
fibrillation and implications for the outcome of ablation: The
ARREST-AF cohort study. J Am Coll Cardiol. 64:2222–2231.
2014.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y,
Guo WH, Jiang CX and Ma CS: Efficacy of catheter ablation of atrial
fibrillation in patients with obstructive sleep apnoea with and
without continuous positive airway pressure treatment: A
meta-analysis of observational studies. Europace. 16:1309–1314.
2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Matiello M, Nadal M, Tamborero D, Berruezo
A, Montserrat J, Embid C, Rios J, Villacastín J, Brugada J and Mont
L: Low efficacy of atrial fibrillation ablation in severe
obstructive sleep apnoea patients. Europace. 12:1084–1089.
2010.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Naruse Y, Tada H, Satoh M, Yanagihara M,
Tsuneoka H, Hirata Y, Ito Y, Kuroki K, Machino T, Yamasaki H, et
al: Concomitant obstructive sleep apnea increases the recurrence of
atrial fibrillation following radiofrequency catheter ablation of
atrial fibrillation: Clinical impact of continuous positive airway
pressure therapy. Heart Rhythm. 10:331–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Neilan TG, Farhad H, Dodson JA, Shah RV,
Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R,
Steigner M, et al: Effect of sleep apnea and continuous positive
airway pressure on cardiac structure and recurrence of atrial
fibrillation. J Am Heart Assoc. 2(e000421)2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Takigawa M, Takahashi A, Kuwahara T,
Takahashi Y, Okubo K, Nakashima E, Watari Y, Nakajima J, Yamao K,
Osaka Y, et al: Impact of alcohol consumption on the outcome of
catheter ablation in patients with paroxysmal atrial fibrillation.
J Am Heart Assoc. 5(e004149)2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Shah AN, Mittal S, Sichrovsky TC, Cotiga
D, Arshad A, Maleki K, Pierce WJ and Steinberg JS: Long-term
outcome following successful pulmonary vein isolation: Pattern and
prediction of very late recurrence. J Cardiovasc Electrophysiol.
19:661–667. 2008.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Suleiman M, Koestler C, Lerman A,
Lopez-Jimenez F, Herges R, Hodge D, Bradley D, Cha YM, Brady PA,
Munger TM, et al: Atorvastatin for prevention of atrial
fibrillation recurrence following pulmonary vein isolation: A
double-blind, placebo-controlled, randomized trial. Heart Rhythm.
9:172–178. 2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Macchia A, Grancelli H, Varini S, Nul D,
Laffaye N, Mariani J, Ferrante D, Badra R, Figal J, Ramos S, et al:
Omega-3 fatty acids for the prevention of recurrent symptomatic
atrial fibrillation: Results of the FORWARD (randomized trial to
assess efficacy of PUFA for the maintenance of sinus rhythm in
persistent atrial fibrillation) trial. J Am Coll Cardiol.
61:463–468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Kowey PR, Reiffel JA, Ellenbogen KA,
Naccarelli GV and Pratt CM: Efficacy and safety of prescription
omega-3 fatty acids for the prevention of recurrent symptomatic
atrial fibrillation: A randomized controlled trial. JAMA.
304:2363–2372. 2010.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Kumar S, Sutherland F, Morton JB, Lee G,
Morgan J, Wong J, Eccleston DE, Voukelatos J, Garg ML and Sparks
PB: Long-term omega-3 polyunsaturated fatty acid supplementation
reduces the recurrence of persistent atrial fibrillation after
electrical cardioversion. Heart Rhythm. 9:483–491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Nodari S, Triggiani M, Campia U, Manerba
A, Milesi G, Cesana BM, Gheorghiade M and Dei Cas L: n-3
polyunsaturated fatty acids in the prevention of atrial
fibrillation recurrences after electrical cardioversion: A
prospective, randomized study. Circulation. 124:1100–1106.
2011.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Patel D, Shaheen M, Venkatraman P,
Armaganijan L, Sanchez JE, Horton RP, Di Biase L, Mohanty P, Canby
R, Bailey SM, et al: Omega-3 polyunsaturated fatty acid
supplementation reduced atrial fibrillation recurrence after
pulmonary vein antrum isolation. Indian Pacing Electrophysiol J.
9:292–298. 2009.PubMed/NCBI
|
|
120
|
Kolandaivelu A: Role of cardiac imaging
(CT/MR) before and after RF catheter ablation in patients with
atrial fibrillation. J Atr Fibrillation. 5(523)2012.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Gimelli A, Ernst S and Liga R:
Multi-modality imaging for the identification of arrhythmogenic
substrates prior to electrophysiology studies. Front Cardiovasc
Med. 8(640087)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Szili-Torok T, Kimman GJ, Scholten M,
Thornton A, Ten Cate F, Roelandt J and Jordaens L: Ablation lesions
in Koch's triangle assessed by three-dimensional myocardial
contrast echocardiography. Cardiovasc Ultrasound.
2(27)2004.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Schleberger R, Metzner A, Kuck KH,
Andresen D, Willems S, Hoffmann E, Deneke T, Eckardt L, Brachmann
J, Hochadel M, et al: Antiarrhythmic drug therapy after catheter
ablation for atrial fibrillation-Insights from the German ablation
registry. Pharmacol Res Perspect. 9(e00880)2021.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Duytschaever M, Demolder A, Phlips T,
Sarkozy A, El Haddad M, Taghji P, Knecht S, Tavernier R,
Vandekerckhove Y and De Potter T: PulmOnary vein isolation with vs
without continued antiarrhythmic drug treatment in subjects with
recurrent atrial fibrillation (POWDER AF): Results from a
multicentre randomized trial. Eur Heart J. 39:1429–1437.
2018.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Karasoy D, Gislason GH, Hansen J,
Johannessen A, Køber L, Hvidtfeldt M, Özcan C, Torp-Pedersen C and
Hansen ML: Oral anticoagulation therapy after radiofrequency
ablation of atrial fibrillation and the risk of thromboembolism and
serious bleeding: Long-term follow-up in nationwide cohort of
Denmark. Eur Heart J. 36:307–314a. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Proietti R, AlTurki A, Di Biase L, China
P, Forleo G, Corrado A, Marras E, Natale A and Themistoclakis S:
Anticoagulation after catheter ablation of atrial fibrillation: An
unnecessary evil? A systematic review and meta-analysis. J
Cardiovasc Electrophysiol. 30:468–478. 2019.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Liu XH, Xu Q, Luo T, Zhang L and Liu HJ:
Discontinuation of oral anticoagulation therapy after successful
atrial fibrillation ablation: A systematic review and meta-analysis
of prospective studies. PLoS One. 16(e0253709)2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Chan P, Vijan S, Morady F and Oral H:
Cost-effectiveness of left atrial catheter ablation, antiarrhythmic
therapy, and rate control therapy for paroxysmal and chronic atrial
fibrillation. Circulation. 111(e318)2005.
|
|
129
|
Eckard N, Davidson T, Walfridsson H and
Levin LÅ: Cost-effectiveness of catheter ablation for patients with
symptomatic atrial fibrillation. J Atr Fibrillation.
2(195)2009.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Winkle RA, Mead RH, Engel G, Kong MH and
Patrawala RA: Discontinuing anticoagulation following successful
atrial fibrillation ablation in patients with prior strokes. J
Interv Card Electrophysiol. 38:147–153. 2013.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Winkle RA, Mead RH, Engel G, Kong MH and
Patrawala RA: Physician-controlled costs: The choice of equipment
used for atrial fibrillation ablation. J Interv Card
Electrophysiol. 36:157–165. 2013.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Weerasooriya R, Jaïs P, Scavée C, Macle L,
Shah DC, Arentz T, Salerno JA, Raybaud F, Choi KJ, Hocini M, et al:
Dissociated pulmonary vein arrhythmia: Incidence and
characteristics. J Cardiovasc Electrophysiol. 14:1173–1179.
2003.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Vijayaraman P, Dandamudi G, Naperkowski A,
Oren J, Storm R and Ellenbogen KA: Assessment of exit block
following pulmonary vein isolation: Far-field capture masquerading
as entrance without exit block. Heart Rhythm. 9:1653–1659.
2012.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Khaykin Y, Wang X, Natale A, Wazni OM,
Skanes AC, Humphries KH, Kerr CR, Verma A and Morillo CA: Cost
comparison of ablation versus antiarrhythmic drugs as first-line
therapy for atrial fibrillation: An economic evaluation of the
RAAFT pilot study. J Cardiovasc Electrophysiol. 20:7–12.
2009.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Reynolds MR, Zimetbaum P, Josephson ME,
Ellis E, Danilov T and Cohen DJ: Cost-effectiveness of
radiofrequency catheter ablation compared with antiarrhythmic drug
therapy for paroxysmal atrial fibrillation. Circ Arrhythm
Electrophysiol. 2:362–369. 2009.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Assasi N, Blackhouse G, Xie F, Gaebel K,
Robertson D, Hopkins R, Healey J, Roy D and Goeree R: Ablation
procedures for rhythm control in patients with atrial fibrillation:
Clinical and cost-effectiveness analyses. CADTH Technol Overv.
2(e2101)2012.PubMed/NCBI
|
|
137
|
Reynolds MR, Lamotte M, Todd D, Khaykin Y,
Eggington S, Tsintzos S and Klein G: Cost-effectiveness of
cryoballoon ablation for the management of paroxysmal atrial
fibrillation. Europace. 16:652–659. 2014.PubMed/NCBI View Article : Google Scholar
|
|
138
|
McKenna C, Palmer S, Rodgers M, Chambers
D, Hawkins N, Golder S, Van Hout S, Pepper C, Todd D and Woolacott
N: Cost-effectiveness of radiofrequency catheter ablation for the
treatment of atrial fibrillation in the United Kingdom. Heart.
95:542–549. 2009.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Aronsson M, Walfridsson H, Janzon M,
Walfridsson U, Nielsen JC, Hansen PS, Johannessen A, Raatikainen P,
Hindricks G, Kongstad O, et al: The cost-effectiveness of
radiofrequency catheter ablation as first-line treatment for
paroxysmal atrial fibrillation: Results from a MANTRA-PAF substudy.
Europace. 17:48–55. 2015.PubMed/NCBI View Article : Google Scholar
|