Introduction
Patent foramen ovale (PFO) is a prevalent defect in
the interatrial septum, occurring in 15-35% of the adult population
(1). While the majority of
individuals with a PFO remain asymptomatic throughout their lives,
this condition has increasingly been recognized as a potential
contributor to cryptogenic stroke, particularly in children and
young adults without classic risk factors for atheroembolic stroke
(2). Cryptogenic stroke accounts for
25-40% of all ischemic stroke cases, posing diagnostic and
therapeutic challenges due to the absence of a clearly identifiable
cause (3). In young patients with
cryptogenic stroke, the prevalence of PFO can be as high as 50%
(4).
The role of PFO in the development of cryptogenic
stroke may involve promoting thrombus formation or acting as a
pathway for paradoxical embolism, where a thrombus bypasses lung
filtration and moves from venous to arterial circulation through
the foramen ovale, potentially leading to cerebral ischemia.
However, establishing a direct causal link between PFO and stroke
can be complex, and the available evidence is often speculative and
less well documented, necessitating a comprehensive diagnostic
workup (1,5). Additionally, treatment options for PFO
remain controversial due to the lack of clear practice guidelines
and the risks associated with both invasive and non-invasive
procedures.
The present study describes the case of a
14-year-old male patient who suffered an acute ischemic stroke
without conventional risk factors. Following a thorough diagnostic
workup that identified a marked right-to-left shunt through the
PFO, the patient received dual antiplatelet therapy and underwent
successful percutaneous PFO closure. Notably, the patient achieved
substantial recovery at the 6-month follow-up, underscoring the
importance of a multidisciplinary approach in diagnosing and
managing PFO-related cryptogenic stroke in young patients.
Case report
Patient history
A 14-year-old male presented to the Emergency
Department of GMERS Medical College (Gandhinagar, India) with
sudden onset of right-sided weakness, slurred speech and facial
drooping while playing soccer. His medical history was
unremarkable, with no prior neurological deficits or cardiovascular
issues. As a healthy, active teenager, he had no history of
smoking, drug use, or a notable family history of cardiovascular or
neurological diseases. Upon admission, his vital signs were within
normal limits, and there were no signs of trauma or infection.
Clinical examination
A physical examination revealed that the patient was
alert and oriented; however, he exhibited right-sided hemiparesis
(strength of 2/5 in the right upper and lower limbs), dysarthria
and a right facial droop, leading to a National Institutes of
Health Stroke Scale (NIHSS) score of 6. An urgent non-contrast
computed tomography (CT) scan of the head revealed no evidence of
intracranial hemorrhage or mass lesion. Given the age of the
patient and absence of traditional stroke risk factors, a
cryptogenic stroke was suspected. He was admitted to the pediatric
intensive care unit (PICU) of GMERS Medical College (Gandhinagar,
India) for monitoring and further workup.
Diagnostic workup
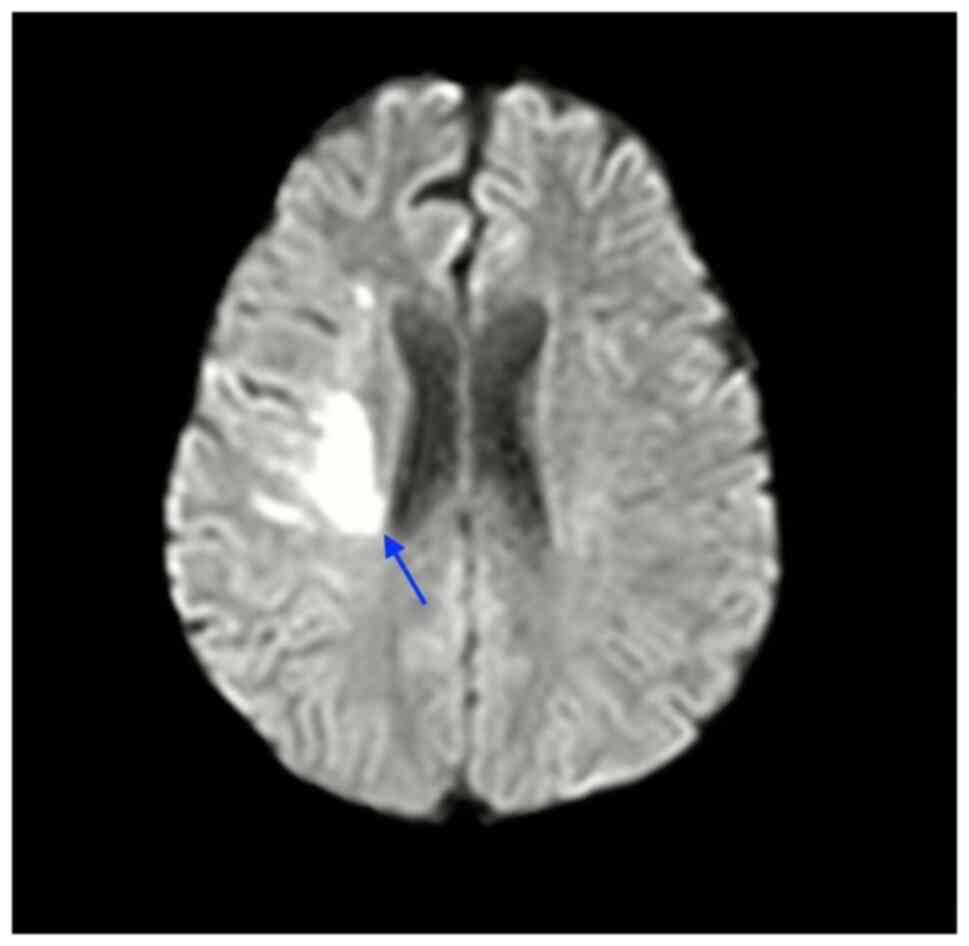
Magnetic resonance imaging (MRI) of the brain with
diffusion-weighted imaging (DWI) confirmed an acute ischemic
infarct in the left middle cerebral artery (MCA) territory. A
thorough diagnostic workup was initiated to identify potential
underlying causes. Initial evaluation with a transthoracic
echocardiogram (TTE) was normal; however, due to persistent stroke
symptoms and the lack of identifiable risk factors, a
transesophageal echocardiogram (TEE) was performed. This revealed a
PFO with a marked right-to-left shunt, confirmed by a bubble study,
while no other structural cardiac abnormalities were detected
(Fig. 1 and Video S1).
A further evaluation included a hypercoagulability
panel, which yielded negative results for any clotting disorders.
Laboratory tests for autoimmune and infectious etiologies,
including antiphospholipid antibodies and tests for vasculitis,
also yielded negative results. The patient's lipid profile, fasting
glucose (85 mg/dl), and hemoglobin A1c (5.4%) were all within
normal limits, indicating no metabolic abnormalities contributing
to his condition.
Management
Throughout the diagnostic and treatment process, a
multidisciplinary team consisting of specialists from cardiology,
neurology and pediatrics played a pivotal role. Their collaborative
discussions were instrumental in shaping the treatment management
plan of the patient. i) Diagnostic collaboration: Neurologists were
integral in interpreting the imaging studies and determining the
acute ischemic nature of the stroke. Their insight prompted further
cardiac evaluations, ultimately leading to the identification of
the PFO through TEE. ii) Risk assessment and treatment planning:
After confirming the diagnosis of a PFO and cryptogenic stroke, the
team convened to discuss the treatment options. Neurologists
advocated for immediate dual antiplatelet therapy with aspirin (81
mg daily) and clopidogrel (75 mg daily), while cardiologists
emphasized the long-term benefits of PFO closure. This
collaborative dialogue was critical in evaluating the risks and
benefits of each option in the context of the age and clinical
presentation of the patient. iii) Procedure decision: The consensus
among the team was to proceed with percutaneous PFO closure to
reduce the risk of recurrent stroke. This decision was made after
weighing the potential complications of the procedure against the
high risk of future stroke due to the identified shunt.
Outcome
Following the multidisciplinary discussions, the
patient underwent successful percutaneous closure of the PFO
without complications. His neurological symptoms gradually improved
with rehabilitation, and at the 6-month follow-up, he demonstrated
a near-complete recovery, with only mild residual weakness in the
right hand. Repeat imaging confirmed no new infarcts, and he
continued on dual antiplatelet therapy with aspirin and clopidogrel
for an additional 6 months postoperatively.
The case described herein highlights the critical
need for a multidisciplinary approach in diagnosing and managing
cryptogenic stroke in young patients. By integrating the expertise
of various specialists, the team was able to provide a
comprehensive assessment and implement timely interventions,
ultimately improving the outcomes of the patient, and significantly
reducing the risk of recurrent stroke.
Discussion
The present case report describes the clinical
features, diagnosis and treatment of a 14-year-old male patient who
experienced a cryptogenic stroke, later attributed to a PFO. The
clinical presentation of the patient was atypical, considering his
age, the absence of traditional cardiovascular risk factors and the
lack of a notable family history. Pediatric stroke cases are rare,
with incidence rates ranging from 2.5 to 13 per 100,000 individuals
per year (6). The case described
herein offers a unique opportunity to explore the potential
etiologies of pediatric stroke, including congenital heart defects,
such as PFO, even in the absence of obvious predisposing
factors.
A PFO is a congenital heart abnormality caused by
the failure of antenatal interatrial communication to close,
rendering it the most common cause of right-to-left shunting in
adults. Autopsy studies report a prevalence of PFO between 14 and
35%, with a median of 26% and a weighted mean of 25% (7). This suggests that approximately two
billion individuals worldwide have a persistent right-to-left
communication (8). PFO is associated
with various clinical syndromes, including cryptogenic stroke,
decompression sickness, migraines and platypnea-orthodeoxia
syndrome (9).
A cryptogenic stroke is defined as a stroke for
which no clear cause can be identified following a thorough medical
evaluation, accounting for 15-40% of all ischemic stroke cases
(10). The association between PFO
and cryptogenic stroke has been a matter of debate for several
years, with some studies suggesting that nearly half of patients
with cryptogenic strokes have a PFO (11). Estimates indicate that 40 to 50% of
individuals experiencing a cryptogenic stroke may have this
condition (11,12). While the literature indicates a
significant link between PFO and stroke in older adults,
particularly those >55 years of age, its association with
pediatric populations has been less established (13-15).
The present case report supports the need for a thorough cardiac
evaluation in young patients with unexplained stroke (9,16).
The role of PFO in stroke remains unclear, with a
continuing debate over whether it is a direct cause, a contributing
risk factor, or merely an incidental finding. The previous
meta-analysis by Alsheikh-Ali et al (17) estimated that approximately one-third
of PFOs identified in patients with cryptogenic stroke were likely
incidental and unrelated to the stroke. Despite this fact, the
exact contribution of PFO to the pathogenesis of stroke,
particularly in the pediatric populations, remains an active area
of research, affecting clinical treatment approaches.
In the case that PFO is pathogenic, potential
mechanisms for stroke include paradoxical embolism (where a venous
thrombus crosses the PFO), in situ thrombus formation within the
PFO, and atrial arrhythmias due to disrupted electrical signaling.
The key risk factors for PFO-related stroke include young age, PFO
size, the extent of the right-to-left shunt, PFO morphology and the
presence of an atrial septal aneurysm. Additional factors, such as
coagulation imbalances and other atrial anomalies (e.g., right
atrial septal pouch, Eustachian valve, or Chiari network), can
further increase the risk of embolic events, acting either
independently or synergistically. Notably, the PFO in Cryptogenic
Stroke Study (PICSS) found a higher prevalence of large PFOs in
patients who suffered a cryptogenic stroke compared to those with
identified stroke causes, suggesting that larger PFOs may be an
independent risk factor for recurrent cerebrovascular events
(5).
The diagnostic process in the case described herein
highlights the challenges of identifying the underlying cause of
stroke in young patients without clear risk factors, particularly
regarding whether PFO is incidental or pathogenic, and the concerns
related to deep vein thrombosis (DVT) and pulmonary thromboembolism
(PTE) associated with PFO (18-20).
Clergeau et al (18) reported
that PFO independently increased the risk of silent brain ischemia
(small, often undetected areas of brain damage) in patients who
have experienced PTE.
However, the case in the present study is unique
compared to others, such as the one presented by Park et al
(19), which involved a massive PTE
followed by DVT. In this instance, a CT scan of the head revealed
no evidence of intracranial hemorrhage or mass lesion, while an MRI
with DWI revealed an acute ischemic infarct in the left MCA
territory. Further evaluation included a hypercoagulability panel,
which yielded negative results for any clotting disorders.
Additionally, tests for autoimmune and infectious etiologies, such
as antiphospholipid antibodies, lupus anticoagulant and vasculitis,
yielded negative results. The lipid profile, and fasting glucose
and hemoglobin A1c levels of the patient were normal. This
complexity suggests that while PFO may be a contributing factor, it
does not conclusively establish a pathogenic role in this specific
case. Further evidence is required to definitively prove PFO as the
direct cause of the stroke.
TTE and TEE with saline contrast injection are
commonly used to diagnose PFO. A PFO is confirmed if microbubbles
appear in the left-sided cardiac chambers within three cardiac
cycles after the right atrium reaches peak opacification (13). In the study by Pearson et al
(20), transesophageal
echocardiography detected a potential cardiac source of embolism in
57% of the participants, significantly higher than the 15%
detection rate for TTE (P<0.0005). This was evident in the case
in the present study, where initial normal findings on TTE could
have led to an incomplete evaluation; however, subsequent TEE
revealed the PFO. This underscores the importance of using advanced
imaging techniques when initial assessments are inconclusive,
particularly in cases where PFO is suspected (20,21).
The treatment approach for PFO in patients with
cryptogenic stroke remains a matter of debate. Antithrombotic
treatment options include antiplatelet drugs (e.g., aspirin and
clopidogrel) and anticoagulants (warfarin, heparin and direct oral
anticoagulants), with no notable difference in efficacy observed in
the absence of atrial fibrillation (12). However, the recurrence rate for
neurological events is higher with medication (5.0 events per 100
person-years) compared to PFO closure (0.8 events), indicating the
superiority of the latter, particularly in patients <60 years of
age (22,23).
Long-term follow-up data on PFO closure indicate
significant benefits in preventing recurrent stroke and improving
the quality of life of patients. The meta-analysis by Agarwal et
al (22) demonstrated that
patients who underwent transcatheter closure had a notably lower
risk of recurrent neurological events compared to those receiving
medical therapy alone, underscoring the efficacy of the procedure
in the prevention of secondary stroke. Similarly, Lee et al
(12) reported that patients with
cryptogenic stroke and high-risk PFOs experienced reduced stroke
recurrence rates post-closure, highlighting the importance of
addressing this defect in affected individuals. Furthermore, Yaghi
et al (3) emphasized
improvements in the overall quality of life among patients
following PFO closure, suggesting not only clinical benefits, but
also enhancements in daily functioning and well-being.
Additionally, the comprehensive analysis conducted by Sposato et
al (8) in a multi-center
registry confirmed the safety and sustained efficacy of PFO closure
procedures over the long term, reinforcing the role of the
procedure in the management of stroke. Collectively, these studies
provide compelling evidence that PFO closure can lead to favorable
long-term outcomes, advocating for its consideration in appropriate
patient populations.
Neurologists often recommend closure for large
shunts and atrial septal aneurysms, while cardiologists focus on
thrombophilia cases. Post-closure, neurologists typically prefer
long-term single antiplatelet therapy, whereas cardiologists may
suggest dual therapy or no long-term treatment, highlighting the
need for collaborative decision-making among specialties (24). Surgical intervention is rare,
reserved for larger PFOs or when percutaneous closure fails
(9,23). In the case in the present study, the
patient began treatment with dual antiplatelet drugs, with aspirin
and clopidogrel. Following multidisciplinary discussions, it was
determined that PFO closure would aid in the prevention of future
strokes.
In conclusion, the present case report demonstrates
the crucial role of PFO as a potential cause of cryptogenic stroke
in a young, otherwise healthy individual. Despite the absence of
traditional stroke risk factors, the identification of a
substantial right-to-left shunt through the PFO was crucial in
diagnosing the underlying cause of the ischemic event. The
successful percutaneous closure of the PFO, combined with dual
antiplatelet therapy, resulted in a notable neurological recovery
for the patient. This outcome emphasizes the importance of
considering PFO in the differential diagnosis of cryptogenic stroke
in younger patients and suggests that early intervention, including
PFO closure, can be an effective strategy to prevent stroke
recurrence and improve patient outcomes.
Supplementary Material
2D echocardiographyillustrating blood
flow through the patent foramen ovale.
Supplementary Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DVP, CAN, RH, HB, SM, OPB and MS contributed to the
conception, design, data collection, analysis, and writing of the
present case report. DVP was responsible for the treatment and
management of the patient. CAN and DVP confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient's parents for his participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient's parents for the publication of the present case report
and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Romano V, Gallinoro CM, Mottola R, Serio
A, Di Meglio F, Castaldo C, Sirico F and Nurzynska D: Patent
foramen ovale-a not so innocuous septal atrial defect in adults. J
Cardiovasc Dev Dis. 8(60)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khan R, Chan AK, Mondal TK and Paes BA:
Thrombosis and Hemostasis in Newborns (THIN) Group. Corporate
author: Patent foramen ovale and stroke in childhood: A systematic
review of the literature. Eur J Paediatr Neurol. 20:500–511.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yaghi S, Bernstein RA, Passman R, Okin PM
and Furie KL: Cryptogenic stroke: Research and practice. Circ Res.
120:527–540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan K and Kasner SE: Patent foramen ovale
and cryptogenic stroke: Diagnosis and updates in secondary stroke
prevention. Stroke Vasc Neurol. 26:84–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ioannidis SG and Mitsias PD: Patent
foramen ovale in cryptogenic ischemic stroke: Direct cause, risk
factor, or incidental finding? Front Neurol. 11(567)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hollist M, Au K, Morgan L, Shetty PA, Rane
R, Hollist A, Amaniampong A and Kirmani BF: Pediatric stroke:
Overview and recent updates. Aging Dis. 12:1043–1055.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pristipino C, Sievert H, D'Ascenzo F,
Louis Mas J, Meier B, Scacciatella P, Hildick-Smith D, Gaita F,
Toni D, Kyrle P, et al: European position paper on the management
of patients with patent foramen ovale General approach and left
circulation thromboembolism. Eur Heart J. 40:3182–3195.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sposato LA, Albin CSW, Elkind MSV, Kamel H
and Saver JL: Patent foramen ovale management for secondary stroke
prevention: State-of-the-art appraisal of current evidence. Stroke.
55:236–247. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saharan S, Vettukattil J, Bhat A, Amula V,
Bansal M, Chowdhury D, Dyamenahalli U, Gupta S, Das B, Susheel
Kumar TK, et al: Patent foramen ovale in children: Unique pediatric
challenges and lessons learned from adult literature. Ann Pediatr
Cardiol. 15:44–52. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang C and Kasner S: Diagnosis,
prognosis, and management of cryptogenic stroke. F1000 Research.
12;5(F1000 Faculty Rev-168)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mojadidi MK, Zaman MO, Elgendy IY, Mahmoud
AN, Patel NK, Agarwal N, Tobis JM and Meier B: Cryptogenic stroke
and patent foramen ovale. J Am Coll Cardiol. 71:1035–1043.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim
DH, Song JM, Kang DH, Kwon SU, Kang DW, et al: Cryptogenic stroke
and high-risk patent foramen ovale. J Am Coll Cardiol.
71:2335–2342. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Davis JT, Hay MW, Hardin AM, White MD and
Lovering AT: Effect of a patent foramen ovale in humans on thermal
responses to passive cooling and heating. J Appl Physiol (1985).
123:1423–1432. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huber R, Grittner U, Weidemann F, Thijs V,
Tanislav C, Enzinger C, Fazekas F, Wolf M, Hennerici MG, McCabe
DJH, et al: Patent foramen ovale and cryptogenic strokes in the
stroke in young fabry patients study. Stroke. 48:30–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miranda B, Fonseca AC and Ferro JM: Patent
foramen ovale and stroke. J Neurol. 265:1943–1949. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hubail Z, Lemler M, Ramaciotti C, Moore J
and Ikemba C: Diagnosing a patent foramen ovale in children: Is
transesophageal echocardiography necessary? Stroke. 42:98–101.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alsheikh-Ali AA, Thaler DE and Kent DM:
Patent foramen ovale in cryptogenic stroke: Incidental or
pathogenic? Stroke J Cereb Circ. 40:2349–2355. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clergeau MR and Hamon M, Morello R, Saloux
E, Viader F and Hamon M: Silent cerebral infarcts in patients with
pulmonary embolism and a patent foramen ovale: A prospective
diffusion-weighted MRI study. Stroke. 40:3758–3762. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Park MS, Park JP, Yun SH, Lee JU, Kim JK,
Lee NE, Song JE, Lee SE, John SH, Lim JH and Rhew JY: A case of
cryptogenic stroke associated with patent foramen ovale coexisting
with pulmonary embolisms, deep vein thromboses, and renal artery
infarctions. Korean Circ J. 42:853–856. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pearson AC, Labovitz AJ, Tatineni S and
Gomez CR: Superiority of transesophageal echocardiography in
detecting cardiac source of embolism in patients with cerebral
ischemia of uncertain etiology. J Am Coll Cardiol. 17:66–72.
1991.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hur J, Hong YJ, Im DJ, Lee HJ, Kim YJ and
Choi BW: Technological improvements in cardiac thrombus diagnosis.
Cardiovasc Imaging Asia. 1:166–176. 2017.
|
|
22
|
Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM
and Kapadia SR: Meta-analysis of transcatheter closure versus
medical therapy for patent foramen ovale in prevention of recurrent
neurological events after presumed paradoxical embolism. JACC
Cardiovasc Interv. 5:777–789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hampton T, Alsaleem M and Murphy-Lavoie
HM: Patent foramen ovale. Statpearls. Treasure Island (FL):
StatPearls Publishing. 2024.
|
|
24
|
Khan F, Derbas LA, Messé SR, Kavinsky C,
Kasner SE and Favilla CG: Management of patients with patent
foramen ovale and stroke: A national survey of interventional
cardiologists and vascular neurologists. J Am Heart Assoc.
12(e029451)2023.PubMed/NCBI View Article : Google Scholar
|