Introduction
Oncofetal tumor markers associated with colon cancer
has been extensively studied and elevated levels of
carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9
(CA 19-9) have been found to be associated with a worse prognosis
(1). In clinical practice, CEA and
CA 19-9 levels are usually monitored in patients with colon cancer.
In addition, other serum tumor markers are also associated with a
poor prognosis, including CA 242, CA 72-4 and free β subunit of
human chorionic gonadotropin (β-hCG) in both males and females
(2,3). However, the cut-off value for β-hCG as
a negative prognostic factor for colon cancer in a previous study
was very low (2 pmol/l, 1 IU/l=2.93 pmol/l) (4). Furthermore, the detection of β-hCG in
biopsy samples has also been shown to be associated with deeper
tissue invasion, lymph node and liver metastases, and a lower
survival rate (5-7).
Previously reported β-hCG-positive colon cancer was mostly
identified in elderly patients; however, in females of reproductive
age, positive serum levels of β-hCG pose a unique challenge in
differential diagnosis for clinicians. The present study describes
the case of a young female patient with colon cancer and very high
serum β-hCG levels.
Case report
A 30-year-old female, G2P1, who presented to the
Emergency Department of Jacobi Medical Center in Bronx, New York,
USA, 14 months following delivery, with nausea, vomiting and severe
abdominal pain for 2 weeks. She had occasional bright red blood per
rectum for 4 months and had experience a weight loss of 5 kg in 2
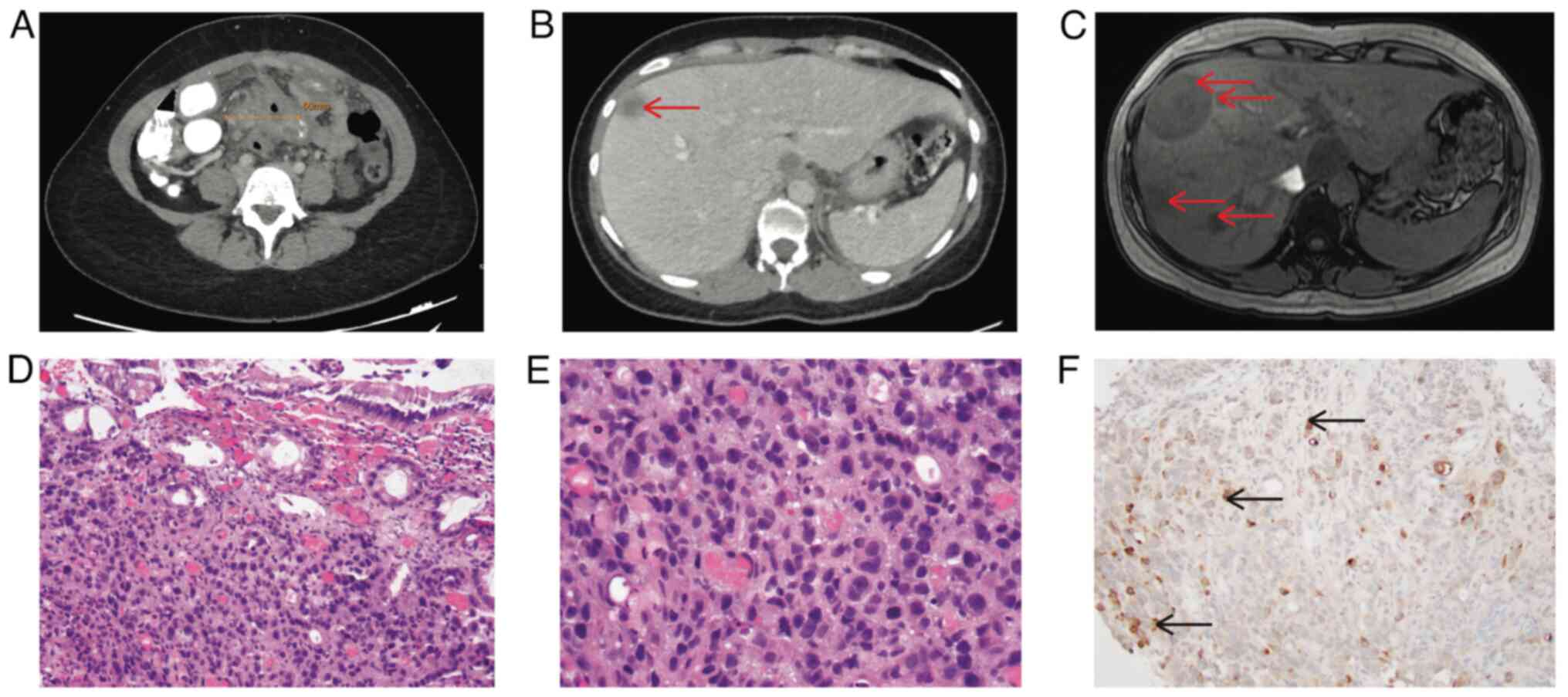
weeks. An abdominal computed Tomography (CT) scan revealed an
ill-defined collection in the mid-abdomen, measuring up to 6 cm,
with adjacent thickened loops of the small bowel, as well as the
sigmoid colon (Fig. 1A) and
hypodense lesions in the liver (Fig.
1B). A colonoscopy revealed mucosal congestion and narrowing in
the sigmoid colon, which could not be traversed with the
colonoscope. However, a biopsy at the narrowing area revealed only
colonic mucosa. She continued to have mild abdominal pain and serum
β-hCG was detectable at 5.9 IU/l (normal range, 0.02 to 0.8 IU/l) 1
month later. An abdominal MRI instead of a CT scan was performed
for concerns of pregnancy. This revealed more severe multiple
hepatic lesions (Fig. 1C). A liver
biopsy, obtained 1.5 months later, revealed moderately
differentiated adenocarcinoma with abundant necrosis. Serum tumor
markers were tested at that time, with borderline CEA levels (5.4
ng/ml; upper limit of normal, 5.0 ng/ml) and significantly elevated
CA19-9 levels (1,168 U/ml; upper limit of normal, 35 U/ml).
Immunohistochemical analysis was performed by GenPath Bioreference
Laboratories (Elmwood Park, NJ, USA). This laboratory is certified
under the Clinical Laboratory Improvement Amendments of 1988
(CLIA-88), CLIA number 33D0668554. Biopsy samples were
paraffin-embedded and the thickness of the sections was 4 µm. The
results of immunohistochemical staining under light microscope were
consistent with colorectal origin (data not shown). Repeat
colonoscopy re-demonstrated severe stenosis in the sigmoid colon
with abnormal appearing mucosa. An endoscopic biopsy revealed
poorly differentiated adenocarcinoma (Fig. 1D and E), with the immunohistochemistry stains
consistent with colonic adenocarcinoma (data not shown).
The patient was scheduled for chemotherapy with the
5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) regimen, at
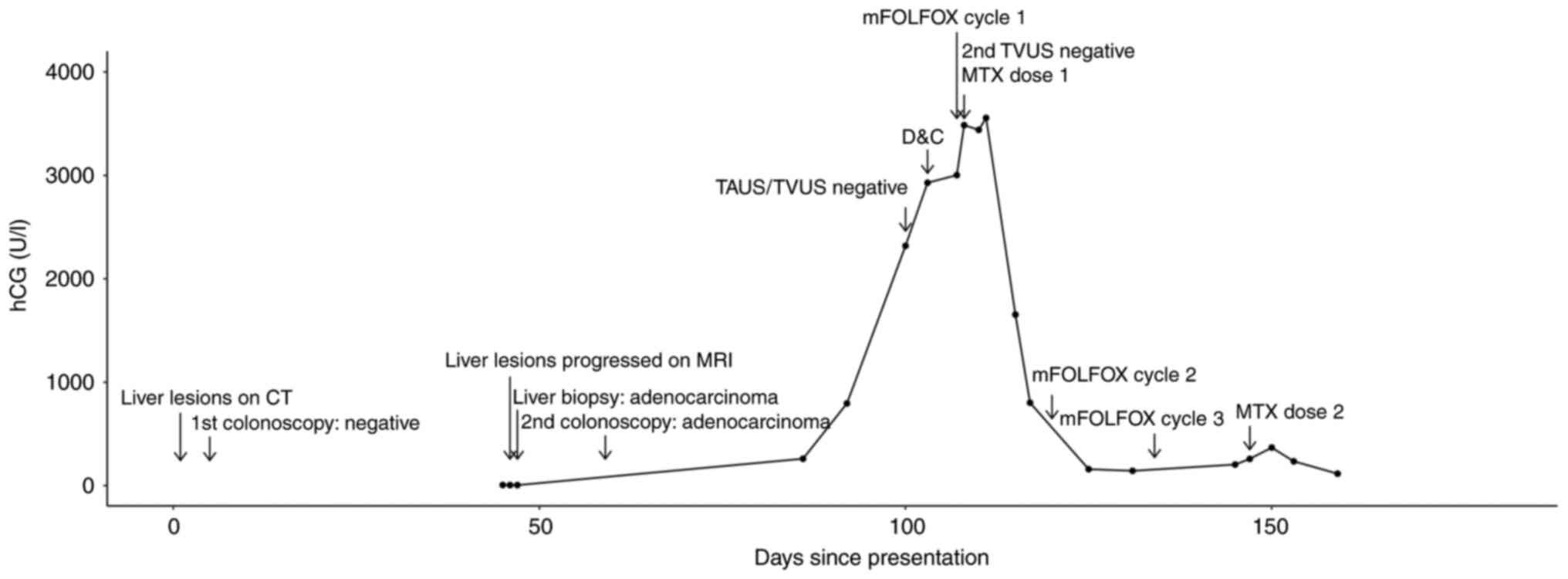
~1 month later. As part of the pre-therapy assessment, the analysis
of β-hCG was repeated and the results were positive at 260.1 U/l.
Transabdominal and transvaginal ultrasound examinations were
performed within 2 weeks and neither found intrauterine or ectopic
pregnancy. A repeat transvaginal ultrasound following suction
dilation and curettage also revealed no intrauterine or ectopic
pregnancy. The first dose of mFOLFOX6 was administered 4 days later
and methotrexate at 50 mg/m2 was also administered
intramuscularly the following day. However, the serum β-hCG levels
continued to increase, reaching a peak level of 3,556 U/l, 3 days
after receiving methotrexate. Previous liver and sigmoid colon
biopsy samples were stained for β-hCG. The liver biopsy sample was
negative for β-hCG staining, but focal positivity for β-hCG was
found in the sigmoid colon sample (data not shown; however, the
sample was processed under the same condition). At ~1 month later,
the serum β-hCG level of the patient had decreased to 204.1 U/l.
The trend of serum β-hCG levels and key events of the patient are
summarized in Fig. 2.
Discussion
Elevated serum β-hCG levels have been reported in
0-20% of patients with colorectal carcinoma, usually at low levels
(8). In the case described herein, a
woman of reproductive age presented with sigmoid colon
adenocarcinoma and was found to have elevated serum β-hCG levels in
a range which was concerning for pregnancy. However, no evidence of
intrauterine or ectopic pregnancy was found on imaging and
curettage, although immunohistochemistry of the colonic
adenocarcinoma identified positive β-hCG staining. This finding
raised the authors' suspicion of tumor-associated β-hCG production
over pregnancy. The patient received mFOLFOX6, as well as
methotrexate at around the same time. Soon after, serum β-hCG level
markedly decreased.
In a previous study involving 10 cases of
β-hCG-positive colorectal adenocarcinoma and 35 β-hCG-negative
counterparts, it was reported that β-hCG-positive adenocarcinomas
were more likely to occur at the rectosigmoid region and that
β-hCG-positive cells were more likely to be distributed at the
periphery of the tumor or arranged in clumps resembling
syncytiotrophoblasts (9). The
expression of β-hCG and themorphology of these cells suggested the
possibility of highly invasive, syncytiotrophoblast-like behavior
of the adenocarcinoma, which could be a manifestation of the
de-differentiation of malignant tissue and could facilitate
progression and metastasis.
Of note, 2 cases with unusually high serum β-hCG
levels (50,000 and 154,000 mIU/ml) in colon cancer were previously
reported in adenocarcinoma of the colon with
syncytiotrophoblast-like cells (10,11).
Rapid progression and metastasis were reported in both cases.
However, no syncytiotrophoblast-like cells were found in the
patient in the present study, although the possibility cannot be
excluded, since there was no primary resection and endoscopic
biopsy may have missed the region with syncytiotrophoblast-like
morphology. Serum β-hCG levels in the thousands were also observed
in other case reports of colon cancer. Tumor-associated β-hCG
production may be associated with sensitivity to chemotherapy and
the serum β-hCG level also decreased following the response to
chemotherapy (12), which is similar
to the response in trophoblastic tumors. Serum β-hCG levels are
associated with immunohistochemical β-hCG staining in the tumor
samples; however, the correlation is relatively weak, indicating
that tumor secretion may not be the only mechanism for elevated
serum β-hCG levels (3). A previous
study also found that β-hCG-derived peptides can stimulate
CD4+ and CD8+ T-lymphocytes in vitro
(13), which could partially explain
its sensitivity to chemotherapy. However, further studies are
required to better understand the underlying mechanisms of such
behavior in β-hCG-positive colon cancer with modern techniques,
such as single-cell sequencing that analyzes the interaction
between cancer cells and their microenvironments (14). Therefore, in patients with colorectal
cancer and high serum β-hCG levels, the monitoring of serum β-hCG
levels can provide additional information on prognosis and response
to therapy. However, larger-scale studies are also required to
determine whether routine β-hCG staining on biopsy samples with
colorectal adenocarcinoma or routine serum β-hCG testing in newly
diagnosed colorectal adenocarcinomas provide additional benefits to
patient care. In female patients of reproductive age with
malignancy, the analysis of β-hCG, on tumor histology as well as
imaging studies for pregnancy need to be obtained to help with
differential diagnosis.
In terms of the clinical management, methotrexate
was administered in the patient in the present study for an assumed
diagnosis of ‘pregnancy of unknown location’ due to a continuously
increasing serum β-hCG level in the absence of evidence for
intrauterine pregnancy, consistent with recommendations by the
American College of Obstetricians and Gynecologists (ACOG)
(15), despite the high probability
of β-hCG from neoplastic source. However, colorectal adenocarcinoma
remains a less known neoplastic cause of elevated serum levels of
β-hCG, and on UpToDate (16), which
is a key reference for clinical practitioners, only gestational
trophoblastic disease is listed on the differential diagnosis as a
neoplastic source of β-hCG. In addition, β-hCG is not one of the
routine immunohistochemical markers of colorectal cancer pathology.
Thus, while waiting for the results of β-hCG staining as an extra
pathological exam, methotrexate was administered to prevent a
potentially life-threatening condition. Future studies are also
required to clarify the benefits and risks of empirical treatment
with methotrexate.
In conclusion, in women of reproductive age with
colorectal carcinoma, β-hCG cannot only serve as a maker for
pregnancy but also as a tumor marker. Positive β-hCG in serum or
histology results in patients with colorectal adenocarcinoma are
associated with a worse prognosis, increased tissue invasion and
metastasis, as well as worse survival outcomes. However, serum
β-hCG levels may be associated with the response to treatment.
Routine serological and histological β-hCG testing as a tumor
marker needs to be considered in patients with suspected or
confirmed colon cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG and FV were involved in the reviewing of the
patient's medical chart, in the processing of images, and in the
writing of the draft of the manuscript and revisions. SW was
involved in pathological examination and in the interpretation of
the patient's results. JS was involved in the reviewing of the
patient's medical chart and in the revision of the manuscript. DK
was involved in the performance of the colonoscopy procedure and
literature review, as well as in the writing and critical reviewing
of the manuscript. All authors have read and approved the final
manuscript. CG, FV, and DK confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Informed consent from the patient for her
participation in the present case report. The present case report
has removed all identifying information to protect patient
privacy.
Patient consent for publication
Informed consent for the publication of the present
case report was obtained from the patient. The present case report
has removed all identifying information to protect patient
privacy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Labianca R, Beretta GD, Kildani B, Milesi
L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et
al: Colon cancer. Crit Rev Oncol Hematol. 74:106–133.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Louhimo J, Carpelan-Holmström M, Alfthan
H, Stenman UH, Järvinen HJ and Haglun C: Serum HCG beta, CA 72-4
and CEA are independent prognostic factors in colorectal cancer.
Int J Cancer. 101:545–548. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Webb A, Scott-Mackie P, Cunningham D,
Norman A, Andreyev J, O'Brien M and Bensted J: The prognostic value
of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG
immunohistochemistry in advanced colorectal cancer. Ann Oncol.
6:581–587. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Korhonen J, Stenman UH and Ylöstalo P:
Serum human chorionic gonadotropin dynamics during spontaneous
resolution of ectopic pregnancy. Fertil Steril. 61:632–636.
1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Campo E, Palacin A, Benasco C, Quesada E
and Cardesa A: Human chorionic gonadotropin in colorectal
carcinoma. An immunohistochemical study. Cancer. 59:1611–1616.
1987.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yamaguchi A, Ishida T, Nishimura G, Kumaki
T, Katoh M, Kosaka T, Yonemura Y and Miyazaki I: Human chorionic
gonadotropin in colorectal cancer and its relationship to
prognosis. Br J Cancer. 60:382–384. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li J, Yin M, Song W, Cui F, Wang W, Wang S
and Zhu H: B subunit of human chorionic gonadotropin promotes tumor
invasion and predicts poor prognosis of early-stage colorectal
cancer. Cell Physiol Biochem. 45:237–249. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Szymendera JJ, Kaminska JA, Nowacki MP,
Szawłowski AW and Gadek A: The serum levels of human
alpha-fetoprotein, AFP, choriogonadotropin, hCG, placental
lactogen, hPL, and pregnancy-specific beta 1-glycoprotein, SP1, are
of no clinical significance in colorectal carcinoma. Eur J Cancer
Clin Oncol. 17:1047–1052. 1981.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shousha S, Chappell R, Matthews J and
Cooke T: Human chorionic gonadotrophin expression in colorectal
adenocarcinoma. Dis Colon Rectum. 29:558–560. 1986.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mashiach R, Kaplan B, Braslavsky D,
Sandbank Y, Neri A, Ovadia J and Schoenfeld A: Carcinoma of the
colon associated with high extragenital production of beta-hCG-a
case report. Acta Obstet Gynecol Scand. 74:845–848. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Metz KA, Richter HJ and Leder LD:
Adenocarcinoma of the colon with syncytiotrophoblastic
differentiation: Differential diagnosis and implications. Pathol
Res Pract. 179:419–424. 1985.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hainsworth JD and Greco FA: Human
chorionic gonadotropin production by colon carcinoma. Biochemical
heterogeneity and identification of a chemotherapy-sensitive cell
subpopulation. Cancer. 56:1337–1340. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dangles V, Halberstam I, Scardino A,
Choppin J, Wertheimer M, Richon S, Quelvennec E, Moirand R, Guillet
JG, Kosmatopoulos K, et al: Tumor-associated antigen human
chorionic gonadotropin beta contains numerous antigenic
determinants recognized by in vitro-induced CD8+ and CD4+ T
lymphocytes. Cancer Immunol Immunother. 50:673–681. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li PH, Zhang X, Yan H, Xia X, Deng Y, Miao
Q, Luo Y, Liu G, Luo H, Zhang Y, et al: Contribution of crosstalk
of mesothelial and tumoral epithelial cells in pleural metastasis
of lung cancer. Transl Lung Cancer Res. 13:965–985. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
American College of Obstetricians and
Gynecologists' Committee on Practice Bulletins-Gynecology. ACOG
practice bulletin no. 193: Tubal ectopic pregnancy. Obstet Gynecol.
131:e91–e103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tulandi T: Ectopic pregnancy: Clinical
manifestations and diagnosis. UpToDate 2024. https://www.uptodate.com/contents/ectopic-pregnancy-clinical-manifestations-and-diagnosis?search=ectopic%20pregnancy&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1#H3573597546.
Accessed September 11, 2024.
|