Introduction
Immune checkpoint inhibitors (ICIs) have emerged as
critical tools in the treatment of various malignancies by
harnessing the potential of the immune system to combat cancer
cells (1,2), transforming cancer treatment in the era
of precision medicine (2).
FDA-approved drugs, such as cemiplimab, ipilimumab, nivolumab and
pembrolizumab, targeting cytotoxic T-lymphocyte-associated protein
4 (CTLA-4) and programmed cell death protein 1 (PD-1) checkpoints,
have had a profound effect on patients with various types of
cancer, including melanoma, non-small cell lung cancer, renal cell
cancer and classical Hodgkin lymphoma (3,4).
Nivolumab and cemiplimab, monoclonal antibodies targeting PD-1, are
notable ICIs that have demonstrated notable therapeutic efficacy.
However, the use of ICIs is associated with the risk of developing
immune-related adverse events (irAEs), which can affect diverse
organ systems. These potentially include conditions, such as
diabetes, hypothyroidism, adrenal insufficiency, interstitial
pneumonia, colitis, renal impairment and liver dysfunction
(5). Neurological adverse effects
are rare, occurring in <3% of patients treated with ICIs,
including axonal polyneuropathies, Guillain-Barré syndrome,
myositis, myasthenia gravis (MG), posterior reversible
encephalopathy syndrome, aseptic meningitis, enteric neuropathy,
transverse myelitis, and autoimmune encephalitis (5,6). MG, an
autoimmune neuromuscular junction transmission disorder
characterized by fatigable muscle weakness, is a commonly reported
irAE associated with ICI therapy (1). Nivolumab and cemiplimab-induced
myositis/MG presents a clinical challenge due to its rarity and the
need for prompt recognition and management to prevent
life-threatening outcomes (2).
The present study describes the cases of 2 patients
who developed myositis/MG following nivolumab and cemiplimab
therapy, a 71-year-old male and a 66-year-old female. In addition,
a brief review of the literature was performed. The present study
aims to shed light on the clinical presentation,
diagnostic-associated difficulties and therapeutic interventions
encountered with this unique clinical condition.
Case report
Case 1
Case 1 involves a 71-year-old male with a previous
medical history of hypertension, hyperlipidemia, obstructive sleep
apnea, acid reflux disease and esophageal adenocarcinoma that had
metastasized to the liver. He received a treatment regimen
consisting of leucovorin, oxaliplatin and 5-fluorouracil (FOLFOX),
along with nivolumab and radiotherapy, to address the esophageal
cancer. At 5 weeks after commencing treatment with nivolumab, he
presented to the authors' oncology service at the University of
Alabama (UAB; Birmingham, AL, USA) with chief complaints of acute
onset double vision, fatigable weakness and shortness of breath.
His social history entailed the consumption of 1 beer weekly. He
had no history of illicit drug abuse. He had quit smoking 33 years
prior. His family history was positive for colon cancer in an aunt
on his father's side of the family. His father had a history of
heart valve disease and ischemic stroke. His mother had a history
of coronary artery disease. He was empirically treated with 100 mg
prednisone for elevated hepatic transaminases. Upon a physical
examination, his vital signs were found to be stable and his oxygen
saturation was 98% in room air. Upon a neurological examination,
his speech was clear with good naming and repetition. A cranial
nerve examination revealed right eye ptosis, impaired extraocular
muscle movements in all directions, and proximal more than distal
limb weakness. Deep tendon reflexes were preserved all over with
down-going toes. An examination of his sensory and cerebellar
functions did not reveal any notable findings. A further evaluation
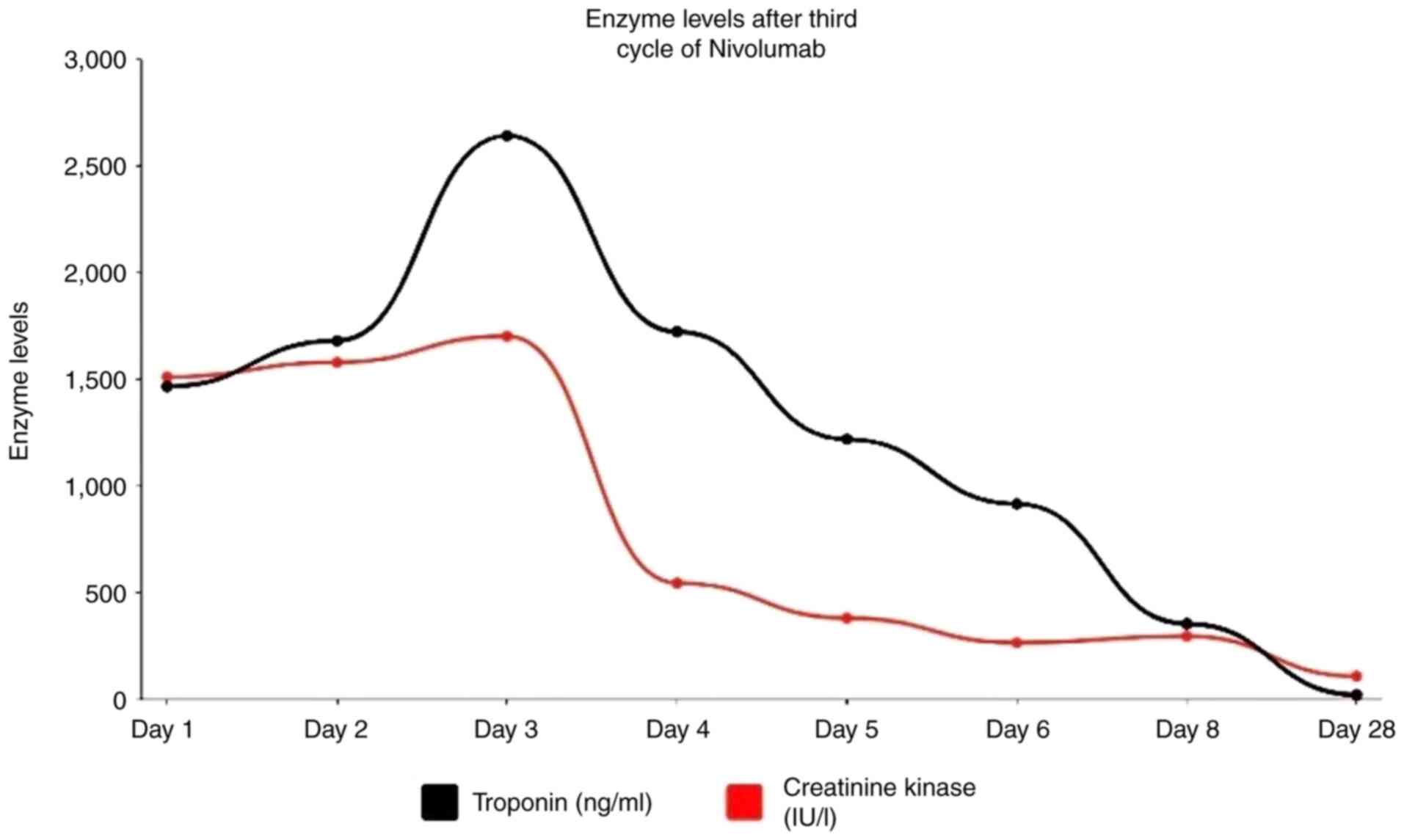
revealed elevated troponin levels peaking at 2,641 ng/ml (Fig. 1) with electrocardiogram changes
suggestive of non-ST-elevated myocardial infarction (NSTEMI) and
creatine kinase (CK) at 1,511 IU/l (Fig.
1). He was admitted to the hospital, and cardiology was
consulted for his NSTEMI.
The new and acute onset symptoms during chemotherapy
made it more likely to be immunotherapy-induced myositis/MG rather
than a steroid induced myopathy case. The neurology and
rheumatology teams were consulted thereafter due to a concern for
ICI-induced myositis/MG. His Myasthenia Gravis Foundation of
America (MGFA) classification was deemed to be class IIIa. His
laboratory test results are listed in Table I. A nerve conduction study (NCS) and
an electromyography (EMG) revealed evidence of mild axonal sensory
and motor neuropathy with no evidence of myopathy. Repetitive nerve
stimulation (RNS) testing did not reveal any decrement or
facilitation response. A brain MRI with contrast did not reveal any
notable findings or acute changes and did not reveal any brain
metastases. The patient was commenced on high-dose prednisone at 1
g/kg/day for 3 days and then transitioned to prednisone at 2
mg/kg/day on day 3, and subsequently to methylprednisolone at 1,000
mg daily for 3 days due to a concern for immunotherapy-induced
cardiotoxicity. The patient was also commenced on intravenous
immunoglobulin (IVIG) as per the neurological recommendation due to
the concern for generalized MG and/or myositis, despite negative
EMG and serological testing. The condition of the patient
clinically improved with decreased levels CK of 295 U/dl and
troponin upon discharge (Fig. 1). He
was discharged on 1 mg/kg/day (100 mg) of prednisone daily until
his follow-up appointment with his oncologist, following which
prednisone was tapered slowly over a period of 6 to 8 weeks.
 | Table ILaboratory test results for patients 1
and 2. |
Table I
Laboratory test results for patients 1
and 2.
| Laboratory test | Patient 1 | Patient 2 |
|---|
| Serum
electrolytes | Normal | Normal |
| Liver function
tests | Low albumen (3.1
g/dl) | Low albumen (3.4
g/dl) and mildly elevated ALT (58 U/l) and AST (42 U/l) |
| CK | Shown in Fig. 1 | Normal (55-69
U/l) |
| Differential blood
count | Iron deficiency
anemia and neutrophiliaa |
Neutrophiliaa |
| Thyroid function
tests | Normal | Normal |
| SPEP and IFE | Normal | Normal |
| Urine IFE | Normal | Normal |
| AChR antibody | Negative | Negative |
| MuSK antibody | Negative | Negative |
| VGCC antibody | Negative | Negative |
| Serum myositis
panel | Negative | Not tested |
| Serum paraneoplastic
panel | Negative | Positive low titer
VGKC antibody (0.05 nMol/l) |
| Cerebrospinal
fluid | | |
|
WBCs | 0 | Not tested |
|
Glucose | Elevated (78
mg/dl) | |
|
Protein | Elevated (65
mg/dl) | |
|
Paraneoplastic
panel | Negative | |
After 2 weeks, he presented again to the UAB Medical
Center for the third time in 2 months with worsening symptoms,
including drooping of the right eyelid, double vision, shallow
breathing, difficulty swallowing, proximal limb weakness,
dysarthria and failure to thrive. A neurological examination
revealed right eye ptosis, nasal speech, impaired extraocular
muscle movement and fatigable proximal muscle weakness. These
clinical findings raised a strong suspicion of an ongoing
myasthenic process, particularly in the setting of continual
decreasing levels of CK (98 U/dl). His hospital course was
complicated, and his condition rapidly deteriorated. He was placed
on intermittent bi-level-positive airway pressure support to
improve his breathing effort (now MGFA class IVb, 8 weeks from the
onset of his myositis/MG). He was commenced on plasmapheresis.
However, the clinical condition of the patient only minimally
improved in terms of truncal strength, swallowing and diaphragmatic
weakness. The palliative care team engaged with the patient and
family to address the goals of care. The patient and family desired
to forgo further chemotherapy and return home with hospice care to
maximize the quality of life in the remaining time. The patient
passed away 1 month thereafter.
Case 2
Case 2 involves a 66-year-old female patient with a
previous medical history of hypertension and hyperlipidemia who was
commenced on cemiplimab therapy for metastatic squamous cell
carcinoma at an outside cancer clinic. She received her first dose
and within 10 days; she had generalized weakness, difficulty rising
from a chair and walking with an unsteady gait. Over the following
2 weeks, she began to suffer from weakness in the proximal upper
limbs, swallowing difficulties, dysarthria, diplopia and right eye
ptosis. Her clinical image was consistent with MG, although tests
for acetylcholine receptor (AChR) and muscle-specific kinase (MuSK)
antibodies yielded negative results. She was empirically commenced
on an immediate course of prednisone and pyridostigmine, although
the latter was terminated due to diarrhea. Additionally, the
patient received five doses of IVIG, without exhibiting a notable
improvement in her symptoms. She was then referred to the UAB
Medical Center for oncologic and neurologic management at 2 months
from the initial dose of cemiplimab. Of note, she did not have any
history of smoking, alcohol consumption, or illicit drug abuse. Her
family history was negative for cancer or neurological
disorders.
Upon an initial examination, she endorsed continued
generalized weakness, a 28-pound weight loss due to dysphagia,
voice changes and a difficulty with deep breathing. Her only
therapeutic at the time was prednisone which had been tapered from
60 to 20 mg. A neurological examination revealed ptosis and a
facial droop worse on the right side compared with the left side,
4/5 strength in the majority of the proximal muscle groups, normal
reflexes, and intact all-modalities sensory examination (MGFA class
IIIb, 7 weeks from the onset of myositis/MG). The results of her
laboratory are presented in Table I.
Given her facial asymmetry, a head CT scan was obtained, which did
not reveal any notable findings. A brain MRI and c-spine with and
without contrast were also non-informative (other than moderate
cervical stenosis). A NCS and EMG revealed electrophysiologic
evidence of diffuse irritable myopathy. RNS did not reveal a
decremental response, yet concentric needle jitter analysis
revealed all six pairs with increased jitter and an elevated mean
consecutive difference of 69.2 msec, as can be seen with myasthenia
gravis.
The patient completed five sessions of plasma
exchange therapy over 10 days of hospital stay at the UAB Medical
Center. She exhibited a gradual improvement in her symptoms and
although she initially failed a swallow test, she was able to
slowly advance her diet along with mirtazapine therapy to improve
her appetite. On an outpatient follow-up at 1 week after discharge,
she exhibited an improvement in her gait and speech. The patient
continued to have difficulty with dysphagia, diplopia, and proximal
weakness and prednisone was increased to 40 mg. She was then set up
for an initiation of monthly outpatient combined plasma exchange
with IVIG 1 week later. The last time she was seen at the UAB
Outpatient Kirklin Clinic was 3 months later. She still had
bilateral mild ptosis, diplopia when looking upwards (after ~10
sec) and to the right, and 4/5 muscle strength in the bilateral
external arm rotators, hip flexor and abductor muscles (MGFA class
IIa).
Discussion
The present study describes two cases of ICI-related
myositis/MG. The first patient began to exhibit symptoms of
myositis/MG 5 weeks after receiving nivolumab and progressed to
MGFA class IV over a period of 8 weeks. The second case developed
the disease only 10 days after taking one dose of cemiplimab and
progressed to MGFA class III within 7 weeks. Both cases clearly
reflect the aggressive nature of the disease compared to what is
typically observed in autoimmune MG cases. In the first case, a
poor outcome was observed with the commencement of steroid
treatment, despite upgrading the treatment with IVIG and plasma
exchange later. In the second case, a more proactive and sustained
treatment approach, including IVIG and plasma exchange treatments
followed by maintaining the same modalities on an outpatient basis,
led to a more favorable outcome. In their study, Safa et al
(7) reported that 63% of their 65
patients had a similar aggressive course developing moderate to
severe muscle weakness (MGFA class III to V) following ICI with the
vast majority of patients (96%) requiring hospitalization. The
median time between ICI treatment initiation and the first MG
symptom was 4 weeks (range, 6 days to 16 weeks) (7). Myositis was noted in more than one
third of their patients and both of the patients in the present
study developed serological or electrophysiological evidence of
myositis. In the present study, the 1st patient developed
myocarditis and the 2nd patient developed hepatitis, which were
reported in 8 and 9% of the patients in the study by Safa et
al (7), respectively. Another
smaller cohort study demonstrated that 8 out of 10 reported
patients developed the triad of MG, myositis and myocarditis
(8). Typically, in regular cases of
MG, classes IV/V are encountered in only 2-10% of cases and the
time from onset to class IV/V status ranges between 2-3 years
(9,10). Evidence of concurrent myositis exists
in only 0.9% of typical MG cases (11).
Nivolumab and cemiplimab, the offending agents in
both cases presented, are monoclonal antibodies that bind to PD-1
receptors, found on the surfaces of activated T-cells. They prevent
the binding of tumor-secreted PD-1 ligands to the PD-1 receptor
(4,12). Blocking the PD-1 receptor can enhance
T-cell antitumor activity; however, this may also result in an
increased CD8/CD4 ratio and reduced regulatory T-cell numbers
(13). While ICIs have exhibited
notable responses in a number of malignancies, they can cause irAEs
(2). The exact mechanisms of irAEs
are not yet fully understood; however, but they are c to stem from
an imbalance between autoimmunity and immune tolerance. Immune
checkpoints, such as PD-1 and CTLA-4 play a crucial role in
maintaining self-tolerance and preventing autoimmunity. When these
natural immune ‘brakes’ are released, this can lead to unchecked
activated T-cells targeting self-antigens, the release of
inflammatory cytokines, and ultimately, in inflammation and tissue
damage, clinically presenting as autoimmune disorders (3,6). In a
previous study, peripheral blood mononuclear cellular typing was
performed before and after nivolumab treatment in a patient who
developed MG/myositis/myocarditis, and revealed an increased
expression of CD8 and cytolytic activity markers, whereas CD4
T-cell and T-regulatory cell activity were suppressed (14). An elevated CD8/CD4 ratio was reported
in another patient with nivolumab-related MG/myositis (15). Other hypotheses have been proposed,
including that the use of PD-1 inhibitors may lead to a
pro-inflammatory cascade involving T-lymphocytes, interleukins
(IL-2, IL-6, IL-17) and tumor necrosis factor-alpha, which may
contribute to the development of MG. ICIs may also increase the
availability of ligands for CD28, activating potentially
self-reactive T-cells. Nivolumab could unmask latent autoimmunity
toward the AChR that was not clinically manifest earlier (2). In the present study, case 1 also
developed concurrent myocarditis after commencing treatment with
PD-1 inhibitors. The pathophysiology of this overlap suggests that
PD-1 expression on cardiomyocytes protects the myocardium during
stress. When there is damage to the heart during ICI blockade,
T-lymphocytes are less likely to attack cardiac antigens, and PD-1
is less likely to protect the heart (6,16).
In addition to the two largest case cohorts
(7,8), herein, a review of the literature
(Table II) (2,12,14,16-29)
revealed 19 additional case reports published between 2014 and
2021. In their large cohorts, Safa et al (7) and Weaver et al (8) reported that ~1/3 of cases were
seronegative when it came to MG antibodies. An additional five
individual case reports revealed negative anti-AchR antibodies, all
consistent with both of the cases described herein (12,23,26,27,29). Of
note, 80-85% of individuals who suffer from de novo MG in
general have AchR antibodies. In generalized MG patients who are
seronegative for AChR antibodies, 50-70% test positive for MuSK
antibodies. However, these antibodies are not always found in
ICI-mediated MG, and when they are, they are much less common than
in typical MG (6). Thus far, there
are only four reported cases of anti-MuSK antibodies found in a
patient with ICI-induced MG (7,8,16). This was linked to a very poor
prognosis, resulting in severe respiratory muscle failure (7,8,16).
 | Table IIReported cases of ICI-induced
myositis/myasthenia gravis in the literature. |
Table II
Reported cases of ICI-induced
myositis/myasthenia gravis in the literature.
| Year of
publication | Authors | Age/sex | Cancer | ICI used | Onset time | AchR antibody | CK (IU/l) | Clinical
features | Electrodiagnostic
Testing | Treatment | Outcome | (Refs.) |
|---|
| 2014 | Liao et
al | 70/F | Melanoma | Ipilimumab | 2nd cycle | Positive | 1200 | Fatigue | Demyelinating
polyneuropathy | Steroids,
pyridostigmine, PLEX, and IVIG | Improved | (17) |
| 2015 | Johnson et
al | 69/F | Melanoma | Ipilimumab | 3rd cycle | Positive | No data | Diplopia, ptosis,
and dysphagia to solid foods | Decremental
response to 3Hz stimulation. | Steroids,
pyridostigmine, and PLEX | Improved | (18) |
| 2015 | Johnson et
al | 73/F | Melanoma | Ipilimumab | 2nd cycle | Positive | No data | Dyspnea, proximal
limb weakness | | Steroids and
pyridostigmine | Mortality
(cancer) | (18) |
| 2015 | Loochtan et
al | 70/M | SCLC | Ipilimumab +
nivolumab | 16 days | Positive | No data | Dyspnea and
generalized weakness | Decremental
response to 3Hz stimulation | Steroids, PLEX, and
IVIG | Death
(hospice) | (19) |
| 2016 | Kimura et
al | 80/M | Melanoma | Nivolumab | 1st cycle | Positive before and
after ICI | 7740 | Dyspnea and muscle
weakness | Data
unavailable | Steroids, PLEX, and
IVIG | Improved | (14) |
| 2016 | Maeda et
al | 79/M | Melanoma | Nivolumab | 3rd cycle | Positive | 1627 | Diplopia and facial
weakness | Data
unavailable | None | Improved | (20) |
| 2016 | Sciacca et
al | 81/M | NSCLC | Nivolumab | 3rd cycle | Positive | No data | Dyspnea | RNS negative.
Single fiber electromyography: abnormal | Steroids | Improved | (21) |
| 2016 | Shirai et
al | 81/F | Melanoma | Nivolumab | 22 days | Positive before and
after ICI | 8729 | Dyspnea | RNS negative | Denied any
treatment | Mortality (MG
crisis) | (22) |
| 2016 | Polat et
al | 65/M | NSCLC | Nivolumab | 3rd cycle | Negative | No data | Weakness | Patient declined
EMG | Pyridostigmine | Improved | (23) |
| 2017 | Chang et
al | 75/M | Bladder cancer | Nivolumab | 2nd cycle | Positive | 1587 | Dyspnea and
weakness | Decremental
response to 3Hz stimulation. | Pyridostigmine and
IVIG | Mortality
(cancer) | (24) |
| 2017 | Chen et
al | 57/M | NSCLC | Ipilimumab +
Nivolumab | 2nd cycle | Positive before and
after ICI | 2682 | Ptosis, Dyspnea,
and weakness | Active denervation
and myopathic changes. No decremental response | Steroid and
pyridostigmine | Improved | (25) |
| 2018 | Kang et
al | 75/M | Oral cavity
cancer | Nivolumab | 3 weeks | Negative | No data | Fatigue and
weakness | Patient declined
EMG | Steroids | Mortality | (12) |
| 2019 | Fazel et
al | 82/M | Melanoma | Nivolumab | 2nd cycle | Anti-MuSK positive
only | Elevated (level not
mentioned) | Ptosis and
Dysarthria | Data
unavailable | Steroid and
pyridostigmine | Mortality | (16) |
| 2020 | Veccia et
al | 65/M | NSCLC | Nivolumab | 4 weeks after 2nd
cycle | Negative | Not mentioned | Diplopia and
ptosis | Evidence of
proximal myopathy | IVIG, Steroids, and
pyridostigmine | Mortality | (26) |
| 2020 | Jeyakumar et
al | 86/M | SCC | Cemiplimab | 3 weeks after 1st
cycle | Negative | 6407 | Fatigue, decreased
vision, aches | Not performed due
to rapid decompensation | Steroids, PLEX,
IVIG | Mortality | (27) |
| 2021 | Canino et
al | 90/F | SCC | Cemiplimab | 5 weeks after 1st
cycle | Positive | 157 | Facial, weakness
bulbar symptoms, dysphagia, hypophonia | Single fiber EMG;
Nonpathological alteration | IVIG, steroids,
pyrixostigmine | Improved | (28) |
| 2021 | Canino et
al | 75/M | Melanoma | Nivolumab | Two weeks after 1st
cycle | Positive | 20 | Ptosis | Single fiber EMG;
pattern of neuromuscular junction alteration | Steroids,
pyridostigmine | Improved | (28) |
| 2021 | Bawek et
al | 68/M | Melanoma | Nivolumab | 3 weeks after 2nd
dose | Negative | Elevated | Proximal muscle
weakness, double vision, dysphagia, and ptosis | Data
unavailable | Pyridostigmine and
IVIG | Discharged on
hospice | (29) |
| 2021 | Tahir et
al | 76/M | Esophageal
cancer | Nivolumab | 6 weeks after
initial cycle | Positive | Normal | Generalized
weakness and diplopia | Patient declined
EMG | IVIG and PLEX | Hospice care | (2) |
The overlap of MG with myositis creates a diagnostic
challenge. NCS and EMG are often used for diagnosis; however,
repetitive nerve stimulation testing is generally insensitive
especially in ocular MG cases (6).
It remains unclear whether elevated CK levels reflect a concurrent
process or primary myositis predominantly involving the oculobulbar
muscles (6). In the cases described
herein, the fatigable component of weakness with the descending
distribution favored a myasthenic process. However, the negative MG
serologic and electrodiagnostic testing in case 1 in addition to
the elevated levels of CK at time of onset favored a concomitant
myopathic process.
Treatment considerations
For ICI-associated MG, treatment typically involves
discontinuing the offending ICI. Earlier reports have indicated
that patients who receive IVIG or plasma exchange as the initial
treatment have higher rates of symptom improvement compared to
those treated with only steroids (6-8).
IVIG or plasma exchange are more effective when used as the
first-line treatment option, as some patients deteriorate despite
the second-line use of IVIG or plasma exchange after initial
steroid treatment has failed (2,7). This
pattern was also noted in the cases in the present study. Fatality
due to ICI-associated MG has been reported in 20-38% of cases
(2,6,7).
Complete symptom resolution is relatively rare, and prognosis is
guarded. In the first case described herein, the patient was
discharged to hospice care with outpatient follow-up with a
neurologist. Current recommendations indicate a trial of
pyridostigmine with or without concurrent steroid therapy for
patients with MGFA class I (ocular symptoms only) or class II (mild
generalized weakness). A re-challenge with ICI can be considered in
patients with MGFA class 1 or 2, as reported in two exceptional
cases in the literature (6).
Clinical implications and
challenges
Prior to initiating ICIs, it is crucial to screen
patients for autoimmune diseases and analyze CK levels. Patients
should be informed about the risk of developing irAEs
(rheumatologic and neurologic), despite their low incidence rates.
Based on the present case reports and previous data, a closely
collaborating team comprising neurologists, rheumatologists and
oncologists is necessary to diagnose and manage life-threatening
adverse effects promptly and effectively (2,6). The
cases described herein also underscore the importance of not
relying solely on serological markers or electrodiagnostic testing,
as this can lead to diagnostic delays with a potential impact on
patient outcomes.
Limitations
The present case reports have limitations, including
a lack of comprehensive understanding of the underlying
pathophysiology of ICI-induced myositis/MG and the absence of
standardized diagnostic criteria. Single-fiber EMG was not
performed on the first patient as it was deemed to be technically
challenging as the patient spent most of his hospital course in the
ICU or the stepdown unit.
Conclusion
ICI medications have revolutionized cancer therapy.
However, they are associated with the risk of developing irAEs,
including myositis/MG, which present a unique set of diagnostic and
management challenges. The causes of ICI-induced myositis/MG remain
unclear and preclinical studies are required to elucidate the
pathophysiology of these irAEs. There are several diagnostic
challenges, including that this condition is rare and sometimes the
serological markers and electrodiagnostic testing are both
negative. The potentially associated myocarditis and hepatitis may
render the diagnosis even more challenging and require a team from
multiple disciplines to take care of such cases. Prompt recognition
and intervention are essential in preventing severe morbidity and
mortality in these patients, since the disease appears to be more
aggressive than what is observed in typical MG cases. When someone
has ICI-induced MG, they are usually treated by terminating the ICI
that is causing it, commencing treatment with corticosteroids, and
early treatment with IVIG and/or plasma exchange. Prospective
longitudinal studies enrolling larger number of patients could
define the true incidence of myositis/MG after ICI and
differentiate those who had a subclinical autoimmunity that
manifests only following exposure to ICI from new-onset disease.
They could also better answer the question whether it is safe to
reintroduce ICI treatment in a patient who was treated earlier from
ICI induced myositis/MG, since the current safety data are all
derived from case reports and small cohort studies. The two cases
described herein underscore the importance of proactive approaches
to monitoring and managing myositis/MG in ICI-treated patients,
regardless of negative serological markers and electrodiagnostic
testing. As the use of ICIs continues to expand in cancer therapy,
healthcare providers need to be vigilant and well-informed about
the potential of these multisystemic irAEs and be prepared to
provide early and specific interventions to optimize patient
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZE, VJ, MK contributed to the conception, design,
data collection and writing of the present case report. RL and AM
were responsible for the treatment and management of the patient.
ZE and MK confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Verbal consents were obtained from the patients for
their participation in the present study.
Patient consent for publication
Verbal consents were obtained from the patients for
the publication of related information in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tahir N, Mahboob A, Piao X, Ying G,
Shrestha J, Sherchan R and Zahra F: Nivolumab, a Double-Edged
Sword: A case report of nivolumab-induced myasthenia gravis. J Med
Cases. 12:424–428. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Wilgenhof S and Neyns B: Anti-CTLA-4
antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann
Oncol. 22:991–993. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kao JC, Liao B, Markovic SN, Klein CJ,
Naddaf E, Staff NP, Liewluck T, Hammack JE, Sandroni P, Finnes H
and Mauermann ML: Neurological complications associated with
anti-programmed death 1 (PD-1) antibodies. JAMA Neurol.
74:1216–1222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hottinger AF: Neurologic complications of
immune checkpoint inhibitors. Curr Opin Neurol. 29:806–812.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Haugh AM, Probasco JC and Johnson DB:
Neurologic complications of immune checkpoint inhibitors. Expert
Opin Drug Saf. 19:479–488. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Safa H, Johnson DH, Trinh VA, Rodgers TE,
Lin H, Suarez-Almazor ME, Fa'ak F, Saberian C, Yee C, Davies MA, et
al: Immune checkpoint inhibitor related myasthenia gravis: Single
center experience and systematic review of the literature. J
Immunother Cancer. 7(319)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Weaver JM, Dodd K, Knight T, Chaudhri M,
Khera R, Lilleker JB, Roberts M, Lorigan P and Cooksley T: Improved
outcomes with early immunosuppression in patients with
immune-checkpoint inhibitor induced myasthenia gravis, myocarditis
and myositis: A case series. Support Care Cancer.
31(518)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grob D, Brunner N, Namba T and Pagala M:
Lifetime course of myasthenia gravis. Muscle Nerve. 37:141–149.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gummi RR, Kukulka NA, Deroche CB and
Govindarajan R: Factors associated with acute exacerbations of
myasthenia gravis. Muscle Nerve. 60:693–699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suzuki S, Utsugisawa K, Yoshikawa H,
Motomura M, Matsubara S, Yokoyama K, Nagane Y, Maruta T, Satoh T,
Sato H, et al: Autoimmune targets of heart and skeletal muscles in
myasthenia gravis. Arch Neurol. 66:1334–1338. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kang KH, Grubb W, Sawlani K, Gibson MK,
Hoimes CJ, Rogers LR, Lavertu P and Yao M: Immune
checkpoint-mediated myositis and myasthenia gravis: A case report
and review of evaluation and management. Am J Otolaryngol.
39:642–645. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kao JC, Brickshawana A and Liewluck T:
Neuromuscular complications of programmed cell death-1 (PD-1)
Inhibitors. Curr Neurol Neurosci Rep. 18(63)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kimura T, Fukushima S, Miyashita A, Aoi J,
Jinnin M, Kosaka T, Ando Y, Matsukawa M, Inoue H, Kiyotani K, et
al: Myasthenic crisis and polymyositis induced by one dose of
nivolumab. Cancer Sci. 107:1055–1058. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen YH, Liu FC, Hsu CH and Chian CF:
Nivolumab-induced myasthenia gravis in a patient with squamous cell
lung carcinoma: Case report. Medicine (Baltimore).
96(e7350)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fazel M and Jedlowski PM: Severe myositis,
myocarditis, and myasthenia gravis with elevated anti-striated
muscle antibody following single dose of ipilimumab-nivolumab
therapy in a patient with metastatic melanoma. Case Reports
Immunol. 2019(2539493)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liao B, Shroff S, Kamiya-Matsuoka C and
Tummala S: Atypical neurological complications of ipilimumab
therapy in patients with metastatic melanoma. Neuro Oncol.
16:589–593. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson DB, Saranga-Perry V, Lavin PJ,
Burnette WB, Clark SW, Uskavitch DR, Wallace DE, Dickson MA,
Kudchadkar RR and Sosman JA: Myasthenia gravis induced by
ipilimumab in patients with metastatic melanoma. J Clin Oncol.
33:e122–e124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Loochtan AI, Nickolich MS and Hobson-Webb
LD: Myasthenia gravis associated with ipilimumab and nivolumab in
the treatment of small cell lung cancer. Muscle Nerve. 52:307–308.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maeda O, Yokota K, Atsuta N, Katsuno M,
Akiyama M and Ando Y: Nivolumab for the treatment of malignant
melanoma in a patient with pre-existing myasthenia gravis. Nagoya J
Med Sci. 78:119–122. 2016.PubMed/NCBI
|
|
21
|
Sciacca G, Nicoletti A, Rampello L, Noto
L, Parra HJ and Zappia M: Benign form of myasthenia gravis after
nivolumab treatment. Muscle Nerve. 54:507–509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shirai T, Sano T, Kamijo F, Saito N,
Miyake T, Kodaira M, Katoh N, Nishie K, Okuyama R and Uhara H:
Acetylcholine receptor binding antibody-associated myasthenia
gravis and rhabdomyolysis induced by nivolumab in a patient with
melanoma. Jpn J Clin Oncol. 46:86–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Polat P and Donofrio PD: Myasthenia gravis
induced by nivolumab therapy in a patient with non-small-cell lung
cancer. Muscle Nerve. 54(507)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chang E, Sabichi AL and Sada YH:
Myasthenia gravis after nivolumab therapy for squamous cell
carcinoma of the bladder. J Immunother. 40:114–116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen JH, Lee KY, Hu CJ and Chung CC:
Coexisting myasthenia gravis, myositis, and polyneuropathy induced
by ipilimumab and nivolumab in a patient with non-small-cell lung
cancer: A case report and literature review. Medicine (Baltimore).
96(e9262)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Veccia A, Kinspergher S, Grego E,
Peterlana D, Berti A, Tranquillini E and Caffo O: Myositis and
myasthenia during nivolumab administration for advanced lung
cancer: A case report and review of the literature. Anticancer
Drugs. 31:540–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jeyakumar N, Etchegaray M, Henry J,
Lelenwa L, Zhao B, Segura A and Buja LM: The terrible triad of
checkpoint inhibition: A case report of myasthenia gravis,
myocarditis, and myositis induced by cemiplimab in a patient with
metastatic cutaneous squamous cell carcinoma. Case Reports Immunol.
2020(5126717)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Canino F, Pugliese G, Baldessari C, Greco
S, Depenni R and Dominici M: Cemiplimab- and nivolumab-induced
myasthenia gravis: Two clinical cases. Tumori. 107:NP123–NP126.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bawek SJ, Ton R, McGovern-Poore M,
Khoncarly B and Narvel R: Nivolumab-Induced myasthenia gravis
concomitant with myocarditis, myositis, and hepatitis. Cureus.
13(e18040)2021.PubMed/NCBI View Article : Google Scholar
|