Introduction
Biliary tract cancer (BTC), also known as
cholangiocarcinoma, is a cancer of the digestive system that
originates in the biliary tract, which transports bile from the
liver to the digestive tract (1).
BTC is a relatively rare type of cancer worldwide. However, high
incidence rates have been reported in Asia, thus rendering it the
sixth most common cause of cancer-related mortality in Korea
(2).
BTC is also well known for its poor prognosis and
the absence of an effective therapeutic target. Despite the
benefits of targeted therapies observed in the majority of solid
cancers, including lung, breast and even colorectal cancers, since
the 2000s, well-known targeted therapies, such as anti-human
epidermal growth factor receptor 2 (HER2) or anti-EGFR agents have
only marginally benefited patients with BTC (3). While the age standardized mortality
rate for lung cancer and colorectal cancer significantly decreased
over the past decade, from 36.6 to 14.9 per 100,000 and from 10.1
to 7.2, respectively, the mortality rate of patients with BTC has
exhibited only a minimal improvement (4.5 to 4.0 per 100,000, from
2010 to 2020) (2,4).
Due to its low incidence rate and poor prognosis,
BTC has been overlooked in major clinical studies. Limited research
and a lack of the accurate understanding of its current
epidemiological characteristics are also key issues with respect to
BTC treatment. With various chemotherapy combinations and the
discovery of therapeutic targets through next-generation
sequencing, along with the use of immune checkpoint inhibitors, the
treatment landscape for biliary cancer is evolving (5). However, there is a paucity of in-depth
studies in this area. Although reports indicate that PD-L1
expression and immune infiltration influence the prognosis of
patients with liver cancer including BTC (6-8),
conclusive results require further validation through prospective
clinical trials and translational studies.
Korea, along with Thailand and Chile, has one of the
highest incidences of BTC worldwide (14.5 per 100,000) (1). These regions exhibit differences in
etiology and genomic landscape, which in turn affect the overall
prognosis of patients with BTC. Therefore, regional exploration is
essential to understand these variations (9,10). The
National Health Information Database (NHID) of the National Health
Insurance Service (NHIS) also contributes to the positioning of
Korea as a valuable cohort for BTC research. The present study
aimed to investigate the recent trends in the prognosis of patients
with BTC and explores the differences based on patient
comorbidities and tumor anatomic sites.
Patients and methods
Data source
The data used in the present study were obtained
from the NHIS of Korea. Korean citizens are obligated to enroll in
the NHIS for healthcare services, which cover ~97% of the
population of Korea. NHIS data include information from all
hospitals, including inpatient and outpatient records, using the
International Statistical Classification of Diseases and Related
Health Problems, Tenth Revision (ICD-10) codes.
The NHIS database contains various types of
information, such as diagnosis, hospitalization and outpatient
treatments, medical expenses, prescribed medications, performed
surgeries, and patient demographics such as sex and age, as well as
the date of death when applicable. This information is stored based
on the content billed by the medical institutions. The database was
provided to researchers with all personal information, such as
names, resident registration numbers, addresses and phone numbers
anonymized or removed.
The study protocol was approved by the Institutional
Review Board of Korea University Anam Hospital (Seoul, Korea; IRB
no. 2021AN0431).
Study population
The present study included patients diagnosed with
BTC between 2015 and 2020. BTC was defined as one of the following
primary diagnostic ICD-10 codes: C22.1 (intrahepatic BTC), C23.X
[gallbladder (GB) cancer], or C24.X (extrahepatic BTC). The date of
claim registration in the NHIS database was assumed to be the date
of diagnosis. Data from 2004 to 2014 were considered a washout
period to account for the absence of new drugs and to obtain
diagnostic data. Patients were included if they were aged ≥20 years
at the time of their first diagnosis of BTC and underwent surgery
within 1 year before or after the diagnosis, or were administered
gemcitabine hydrochloride or cisplatin following the diagnosis
(n=38,259). Among these, 24,659 patients who developed other types
of cancer following BTC were excluded, thus leaving a final cohort
of 13,600 patients for analysis.
Demographic variables, including age and sex were
also assessed. Previous medical history included diabetes,
hepatitis and gallstones. In the case that the corresponding
disease codes existed prior to the diagnosis of cholangiocarcinoma,
they were considered part of the medical history.
The primary outcome variables were overall and
1-year mortality rates. The analyses included comparisons between
individuals who only underwent surgery and those who only received
medication, comparisons based on the year of diagnosis, comparisons
based on diagnosis codes, and analysis of mortality rates based on
the presence of diabetes, hepatitis, and gallstones.
In the case of individuals diagnosed with BTC, those
who underwent surgical treatment were defined with the code ‘Q7380,
Q7410, Q7342, Q7221, Q7222, Q7223, Q7224 and Q7225’. Among those
diagnosed with BTC, medications were defined using the medication
codes for ‘gemcitabine (164901BIJ, 164902BIJ, 164903BIJ, 164904BIJ,
164930BIJ, 164931BIJ and 164932BIJ)’ and ‘cisplatin (134501BIJ,
134502BIJ, 134503BIJ, 134504BIJ, 134505BIJ, 134530BIJ, 134531BIJ,
134532BIJ, 134533BIJ and 134534BIJ)’. For diabetes, the codes
R81.X, E10.X and E11.X were used; chronic hepatitis B virus (HBV)
was identified using B18.X; gallstones were identified using
K80.X.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation, and categorical variables are presented as
frequencies and percentages. Cox proportional hazard regression
models were used to compare the risk of disease outcomes between
the groups, which allowed for the calculation of hazard ratios
(HRs) and 95% confidence intervals (CIs). Before analyzing the Cox
model, a log-rank test was used on the Kaplan-Meier survival curves
to confirm differences among groups, where all groups exhibited a
P-value <0.05, thus satisfying the proportional hazards
assumption. This analysis was performed as an unadjusted analysis
without any adjustments. The reason for not using an adjusted
analysis is that the present study primarily focused on identifying
raw correlations. All statistical analyses were performed using the
SAS software (version 9.4; SAS Institute). All P-values were
two-sided, and a P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Study population
Data on patients who had been diagnosed with BTC
between 2015 and 2019 were collected from the NHID. Patients
diagnosed with other types of cancer were excluded from data
collection, resulting in a final cohort of 10,222 patients for
analysis. Among these patients, 2,614 patients (25.57%) had
intrahepatic BTC, 2,757 patients (26.97%) had extrahepatic BTC and
4,851 patients (47.46%) had GB cancer. The clinical profiles of the
final cohort are summarized in Table
I.
 | Table IClinical characteristics and
comorbidities of the patients with BTC. |
Table I
Clinical characteristics and
comorbidities of the patients with BTC.
|
Characteristics | Patients
(n=10,222) |
|---|
| Age, years; mean
(SD) | 66.94 (11.58) |
| Sex | |
|
Male | 5,616 (54.94) |
|
Female | 4,606 (45.06) |
| Incidence year | |
|
2015 | 2,091 (20.46) |
|
2016 | 1,621 (15.86) |
|
2017 | 1,875 (18.34) |
|
2018 | 2,131 (20.85) |
|
2019 | 2,504 (24.50) |
| Medical
history | |
|
Diabetes
mellitus | 6,850 (67.01) |
|
Chronic
hepatitis B virus infection | 2,696 (26.38) |
|
Cholelithiasis | 5,668 (55.45) |
| Diagnosis of
BTC | |
|
Intrahepatic
cholangiocarcinoma | 2,614 (25.57) |
|
Gallbladder
cancer | 4,851 (47.46) |
|
Extrahepatic
cholangiocarcinoma | 2,757 (26.97) |
The mean age of cohort was 66.94 (SD 11.58) years,
and 54.94% were male. Prior to being diagnosed with BTC, 67.01% of
the patients had diabetes mellitus, 26.38% had hepatitis, and
55.45% had GB stones.
Survival probability according to the
year of diagnosis
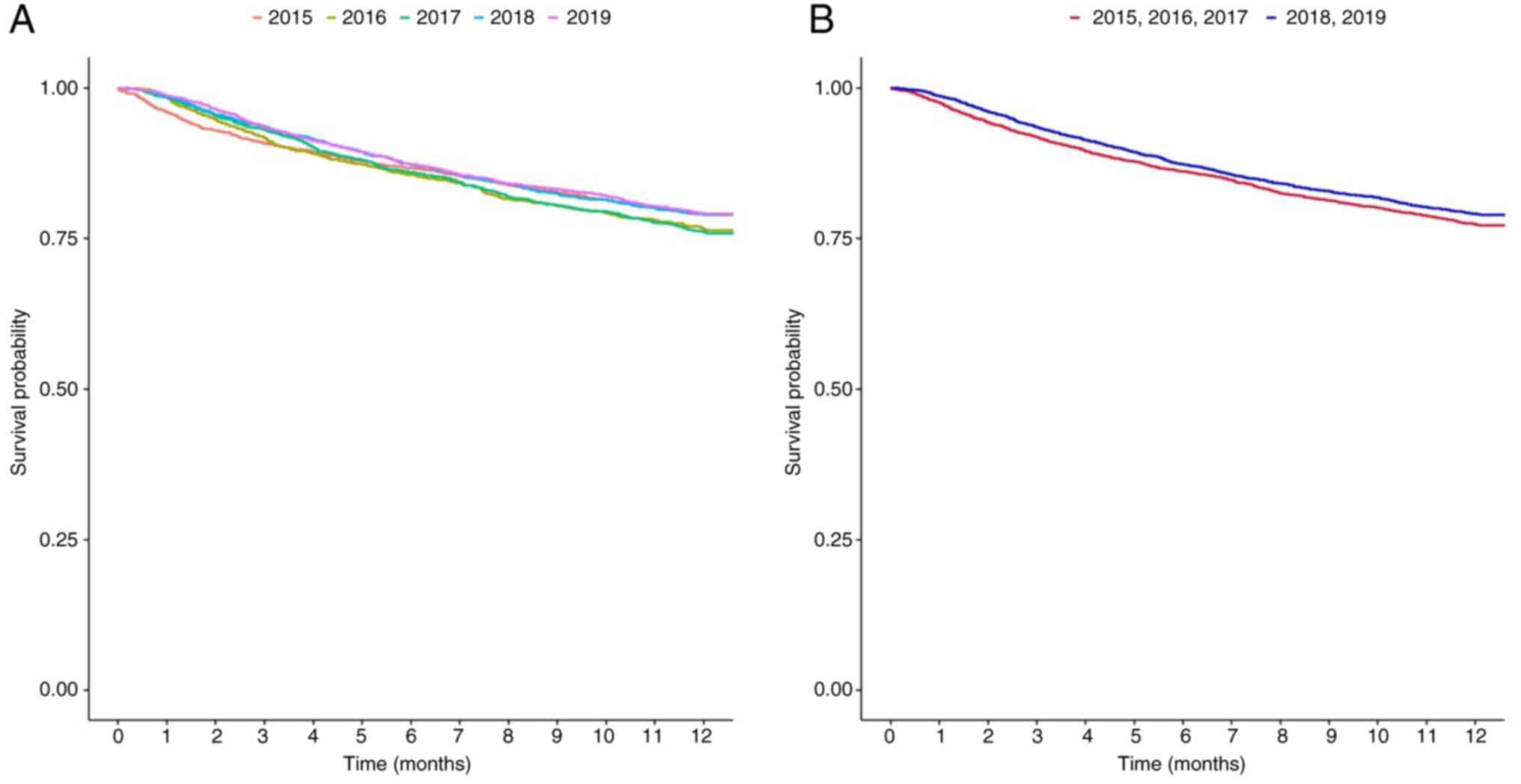
The HR of the 1-year survival rates of the patients
diagnosed each year are presented in Fig. 1 and Tables SI and SII: 1.133 (95% CI, 0.988-1.299) for 2016,
1.15 (95% CI, 1.008-1.311) for 2017, 0.992 (95% CI, 0.869-1.131)
for 2018, and 0.986 (95% CI, 0.868-1.119) for 2019, with 2015 set
as the reference year. Numerically, the 1-year survival rate of the
patients with BTC increased from 20.95% in 2015 to 24.11% in 2017,
and then decreased to 21.07% in 2018, as shown in Fig. 1A and Table SI. The HR of the 1-year survival
rate of the relatively recently diagnosed patients was lower than
that of the patients diagnosed earlier, and the difference was
statistically significant (HR, 0.908; 95% CI, 0.836-0.987;
P=0.0237) (Fig. 1B and Table SII). Table SII presents the P-value derived from
the univariate log-rank test, whereas the P-value reported in the
main text corresponds to that obtained from the Cox proportional
hazards model.
Survival probability according to
treatment setting
In the analysis of the treatment setting, to compare
surgery and chemotherapy, 491 patients who received no treatment
and 856 patients who underwent both surgery and chemotherapy were
excluded, resulting in a total of 10,222 patients being included in
the analysis. When the patients were categorized according to the
treatment they received for BTC, 9,451 patients received surgery
only, and 2,802 received chemotherapy only (Table II). The survival probability was
markedly lower in the chemotherapy-only group than in the
surgery-only group (HR, 4.857; 95% CI, 4.508-5234; P<0.001,
Fig. S1).
 | Table IIOne-year survival rates of the
patients. |
Table II
One-year survival rates of the
patients.
| Treatment | No. of events/n
(%) | Log rank z-test
statistic | Log rank test
P-value | HR (95% CI) |
|---|
| Surgery only | 1,133/9,451
(11.99%) | 45.8484 | <0.0001 | Ref. |
| Chemotherapy
only | 1,485/2,802
(53.00%) | | | 4.857
(4.508-5.234) |
Survival probability according to
anatomic site
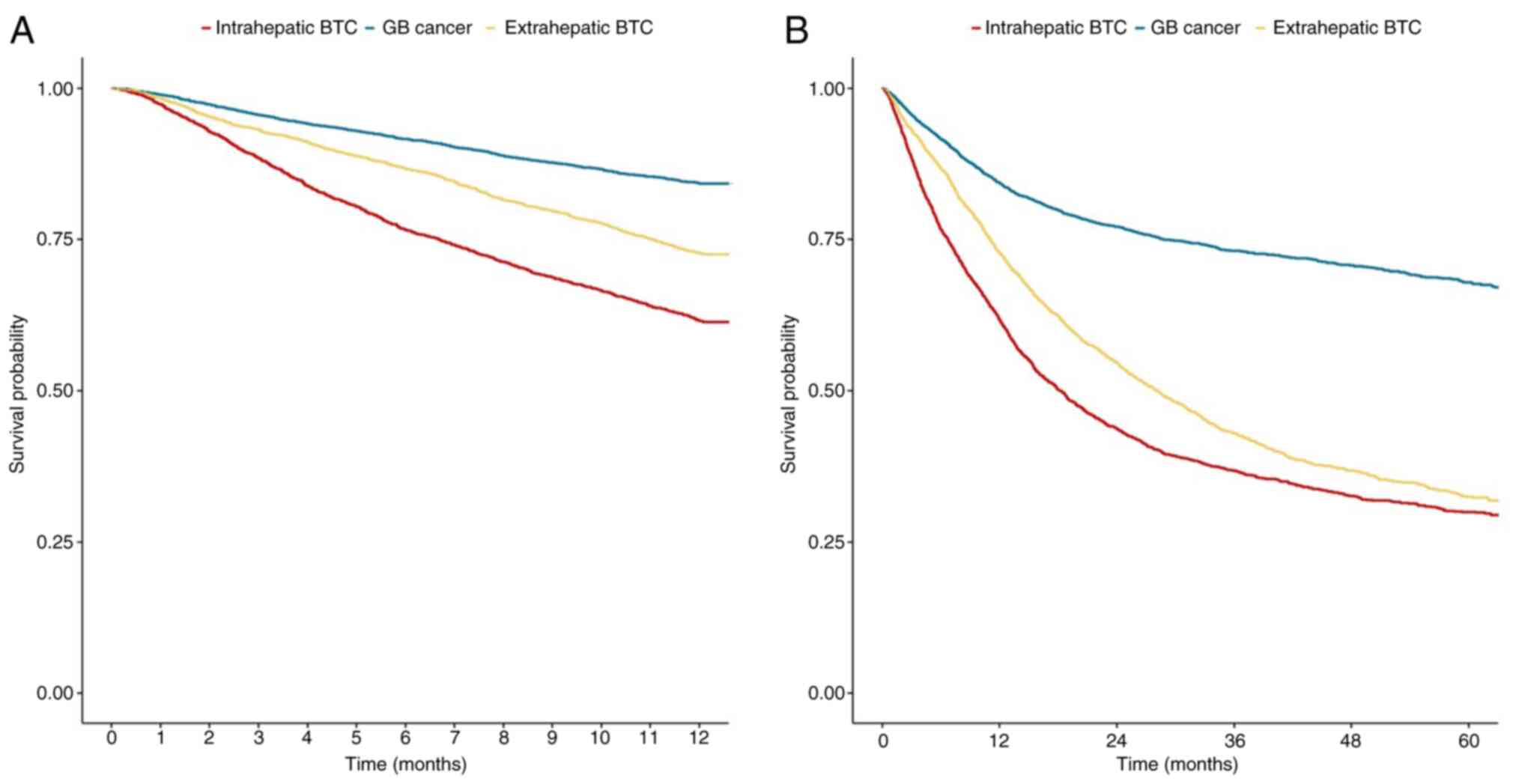
The survival rates of the patients categorized
according to anatomical tumor sites are presented in Fig. 2. The data for intrahepatic BTC,
extrahepatic BTC and GB cancer are also shown in (Tables SIII and SIV). The 1-year survival probability was
the lowest in GB cancer (HR, 0.349; 95% CI, 0.32-0.381;
P<0.001), followed by extrahepatic BTC (HR, 0.641; 95% CI,
0.589-0.698; P<0.001) when intrahepatic BTC was used as a
reference (Fig. 2A and Table SIII). The median survival
probability was not reached for intrahepatic BTC (Fig. 2B).
Survival probability according to
comorbidities
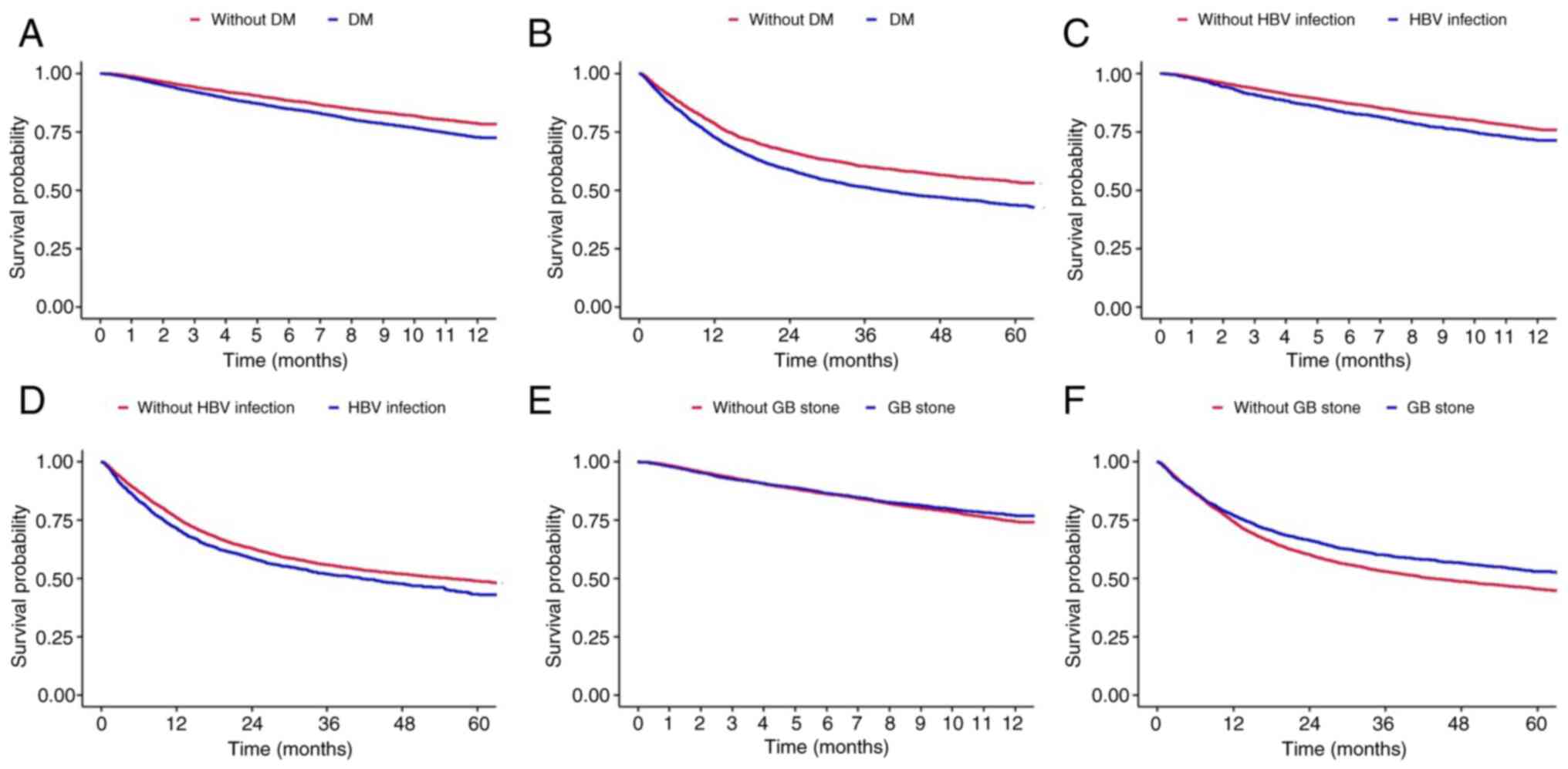
The present study conducted a subgroup analysis
according to the comorbidities of the patients with BTC (Fig. 3 and Table
SV, Table SVI, Table SVII, Table SVIII, Table SIX and Table SX). Patients who had been diagnosed
with diabetes mellitus prior to being diagnosed with BTC had a
poorer prognosis than those who had not (HR, 1.318; 95% CI,
1.225-1.418; P<0.001; Fig. 3A and
Table SV). The analysis of overall
survival revealed the same tendency (HR, 1.322; 95% CI,
1.252-1.397; P<0.001; Fig. 3B and
Table SVI). The median overall
survival of the patients without diabetes mellitus was 76.587
months (95% CI, 68.12-85.85), while that of the patients with
diabetes mellitus was 38.889 months (95% CI, 35.88-41.87) (Fig. 3B).
Patients who had chronic HBV infection also
exhibited a higher HR than patients who did not have HBV infection
(HR, 1.249; 95% CI, 1.147-1.36; P<0.001; Fig. 3C and Table SVII). The median overall survival of
the patients who had known HBV infection was 55.556 months (95% CI,
50.73-40.71; Fig. 3D). Patients with
known GB stones prior to being diagnosed with BTC had a decreased
risk of mortality (HR, 0.902; 95% CI, 0.834-0.976; P=0.01; Fig. 3E and F, and Table
SIX).
Discussion
The present study examined the 1-year survival of
patients diagnosed with BTC over a period of 5 years, beginning
from 2015. The present study investigated the impact of various
factors on the survival rates of patients with BTC. Consistent
yearly survival rates were observed with a slight decline after
2018. This decline was statistically significant when comparing the
pre- and post-2018 data. Intrahepatic BTC was associated with the
shortest survival time, whereas a history of diabetes or chronic
HBV infection negatively affected survival. However, cholelithiasis
was associated with an improved survival.
BTC is well known for its poor prognosis, with
patients with stage 4 disease having a 5-year survival rate
<10%.2 Several clinical characteristics contribute to this poor
prognosis: Diagnosis often occurs at an advanced stage due to the
absence of early symptoms, the heterogeneous nature of cancer with
a wide range of anatomies (from intrahepatic to the ampulla of
Vater) (1), and the limitation of
cytotoxic chemotherapy due to the lack of effective therapeutic
targets. While advancements in diagnostic techniques, active health
screening and the widespread adoption of genetic analyses, such as
next-generation sequencing have addressed numerous challenges
regarding BTC, the extent of the improvement in the prognosis of
patients with BTC remains elusive.
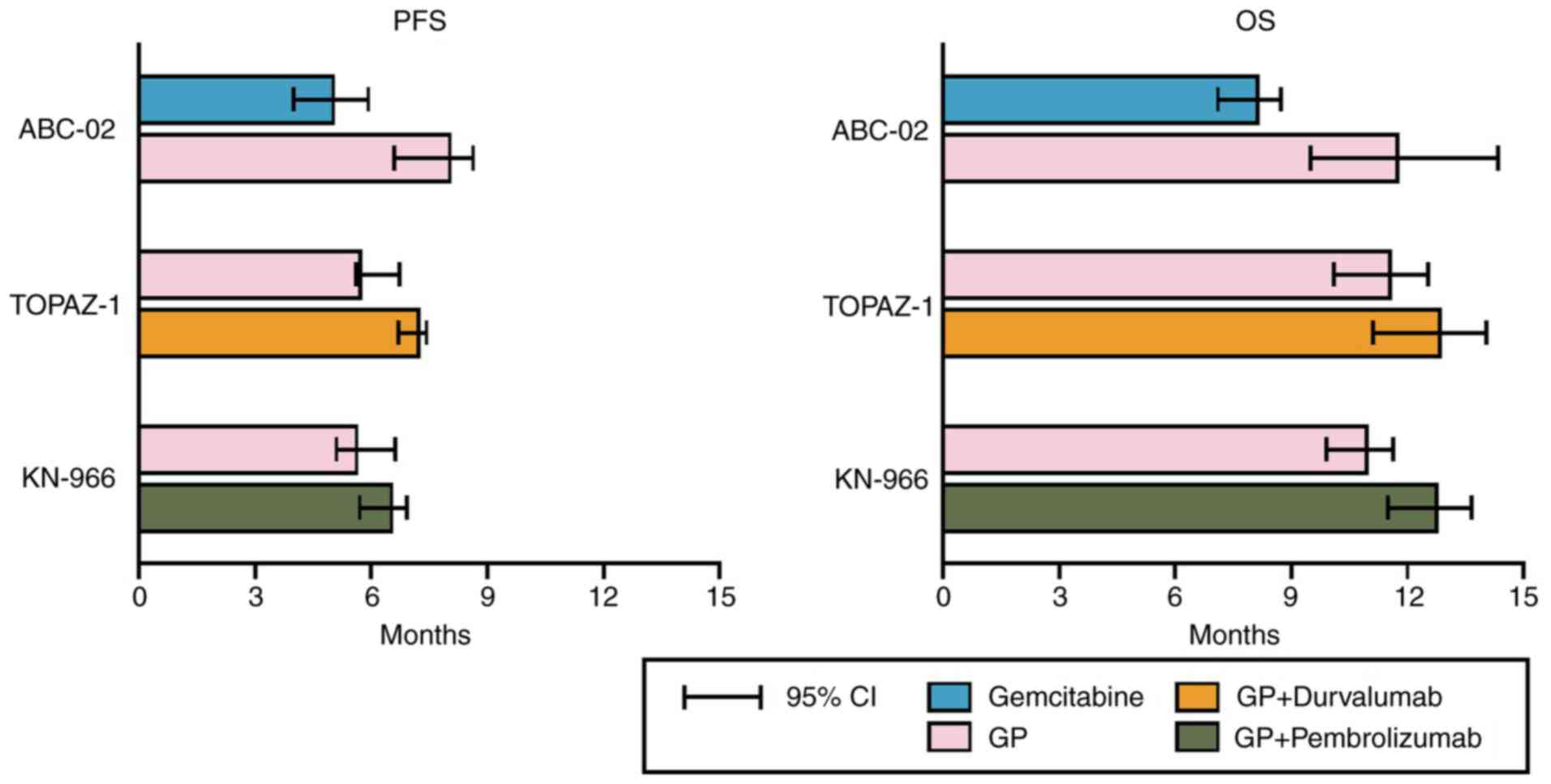
From a therapeutic perspective, combination therapy
using gemcitabine and cisplatin (GP) has been pivotal since 2010.
This combination has been shown to lead to improved response rates
and survival benefits over gemcitabine monotherapy (medial overall
survival, 11.7 vs. 8.1 months; HR, 0.64; P<0.001), establishing
a standard palliative treatment approach (11). Since then, for over a decade, no
treatment strategy has surpassed this combination therapy. A few
additional benefits of epidermal growth factor receptor and
vascular endothelial growth factor receptor inhibitors have been
observed (12). It was not until
2022 that the addition of durvalumab to GP was proven to extend
survival (medial overall survival, 12.8 months) (13); subsequently, the combination with
pembrolizumab also began to yield promising results (medial overall
survival, 12.7 months, Fig. 4)
(14).
The absence of effective subsequent therapy
following the initial treatment also contributes to the poor
prognosis of patients with BTC. Based on a phase 2 clinical trial
published in 1998, fluorouracil (5-FU)-based regimens have been
widely used empirically (15);
however, owing to the rarity of the affected population, obtaining
well-designed phase 3 data has been challenging. Since 2014, the
ABC-06 trial has tested the FOLFOX regimen (5-FU, leucovorin and
oxaliplatin), eventually yielding the first positive data for a
second line therapeutic option in 2021(16).
However, in the era of precision oncology, BTC
treatments are evolving. A number of basic studies have identified
molecular targets that are potentially useful for target-directed
therapies, such as the fibroblast growth factor receptor
(FGFR), HER2, metabolic regulators, such as isocitrate
dehydrogenase 1 and 2 (IDH1/2), and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (17-21).
Among these, FGFR2-targeting agents (e.g., pemigatinib and
futibatinib) (22-24)
and IDH1-targeting agents (e.g., ivosidenib) have been approved by
the FDA (25,26). HER2 overexpression is also a major
treatment target in GB cancer (27),
and not only existing HER2-targeted agents (e.g., trastuzumab and
pertuzumab) (28,29), but also novel agents (e.g.,
zanidatamab) are being used for treatment (30). Ethnic differences in therapeutic
targets have been consistently reported (31), and particularly in Koreans, the
prevalence of IDH1/2 and FGFR2 aberrations is lower than that
typically reported (9). However,
with the widespread use of next-generation sequencing (NGS),
efforts to identify patients who could benefit from targeted
therapy are continuing, and the impact of these therapies on
prognosis is a subject that requires thorough investigation. Owing
to these efforts, the survival rates of patients with BTC are
steadily increasing, albeit at a gradual pace. Annual cancer
statistics in Korea have also reported that the 5-year relative
survival rate of patients with BTC exhibited an upward trend until
the period of 2011-2015 (29.1%) (32); however, from to 2015-2019 (28.5%), it
exhibited a decreasing tendency (-0.6%) (33). Based on our data, it is plausible
that these changes originated in 2018. Novel drugs, such as
nanoliposomal irinotecan (NIFTY) (34) and immune checkpoint inhibitor
clinical trials (TOPAZ-1 and KEYNOTE-966) (13,14),
have recruited patients in Korea since 2018. TOPAZ-1 and
KEYNOTE-966 demonstrated that a combination of immune checkpoint
inhibitors and chemotherapy significantly extended the survival
rates of patients with advanced BTC (13,14).
Continuous real-world follow-up studies are required to determine
the changes in the survival rates of patients with BTC following
immunotherapy in clinical settings.
Anatomical heterogeneity has long been a challenging
issue in BTC, with each cancer subtype exhibiting variable survival
rates. In the present study, the patients with intrahepatic
cholangiocarcinoma had the worst prognosis. This may be attributed
to its tendency to present with fewer symptoms, such as jaundice or
abdominal pain, leading to a delayed diagnosis. This finding aligns
with the research results from the study by Tawarungruang et
al (35), that tracked prognosis
following surgical treatment (median survival time 21.8 months in
distal cholangiocarcinoma vs. 12.4 months in intrahepatic
cholangiocarcinoma). Anatomical heterogeneity is closely associated
with molecular heterogeneity (36).
However, the difference in the response to chemotherapy among
anatomical subgroups in the ABC-02 and TOPAZ-1 trials was not
distinct (11,13), indicating the need for further
research in this area.
The subgroup analysis in the present study provided
several insights. Diabetes is a well-known risk factor of BTC
(37,38). In the present study, patients with
pre-diagnosed diabetes exhibited a significantly reduced overall
survival compared to those without diabetes (38.889 vs. 76.587
months). This suggests that diabetes not only functions as a risk
factor for the development of BTC, but also significantly affects
overall survival. Additionally, it was observed that patients with
a history of cholelithiasis had an extended survival compared with
those without. Regular follow-up sessions for known GB stones and
the early detection of cancer due to symptoms, leading to prompt
treatment, are considered to have contributed to the prolonged
survival of these patients. However, the present study identified
data-driven phenomena, not biological mechanisms, necessitating
cautious interpretation. Further in vivo and prospective
studies are required to validate these findings.
The present study had several limitations, which
should be mentioned. The present study was unable to obtain
information on the clinical or pathological stages of cancer, which
significantly affect survival and prognosis. The authors attempted
to overcome this limitation through precise operational definitions
by using various treatments. NHIS research utilizes data based on
insurance claims, which means that data for items not covered by
insurance are not provided. Additionally, sensitive data that could
identify patients were not disclosed to protect personal
information, such as family history or socioeconomic status.
Detailed medical information specific to particular test results or
specific medical conditions was unavailable for research purposes.
Nevertheless, the lack of information was not concentrated in any
one subgroup, but was applied to all subjects, which the authors
believe lends value to the results as a gross outcome of
macroscopic trends from big data.
In conclusion, the present study tracked the
survival trends of patients with BTC using national healthcare big
data, suggesting an improvement in the overall prognosis of
patients with BTC since 2018. Additionally, it was revealed that
the prognosis of patients with BTC varies depending on the anatomic
site of the cancer, as well as the presence of underlying diseases,
despite the absence of detailed pathologic information.
Supplementary Material
Survival probability of patients
treated with chemotherapy only and surgery only.
One-year survival rates of the
patients (Fig. 1A).
One-year survival rates of the
patients (Fig. 1B).
One-year survival rates of the
patients (Fig. 2A).
Overall survival rates of the patients
(Fig. 2B).
One-year survival rates of the
patients (Fig. 3A).
Overall survival rates of the patients
(Fig. 3B).
One-year survival rates of the
patients (Fig. 3C).
Overall survival rates of the patients
(Fig. 3D).
One-year survival rates of the
patients (Fig. 3E).
Overall survival rates of the patients
(Fig. 3F).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant of from
Korea University (grant no. K2314161).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Both authors (JYL and JWK) contributed to the
conception and design of the study. The data were collected by JYL.
JYL and JWK performed data analysis and figure visualization. JWK
wrote the first draft of the manuscript and both authors commented
on the previous version of the manuscript. JYL and JWK authors
confirmed the authenticity of all the raw data. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All study procedures followed the ethical standards
of the responsible committee on human experimentation
(institutional and national) and the Helsinki Declaration of 1964
and later versions. The study protocol was approved by the
Institutional Review Board of Korea University Anam Hospital
(Seoul, Korea; IRB no. 2021AN0431). The data used in the present
study were obtained from the National Health Insurance Service
(NHIS) of Korea. The database was provided to researchers with all
personal information, such as names, resident registration numbers,
addresses and phone numbers anonymized or removed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valle JW, Kelley RK, Nervi B, Oh DY and
Zhu AX: Biliary tract cancer. Lancet. 397:428–444. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kang MJ, Jung KW, Bang SH, Choi SH, Park
EH, Yun EH, Kim HJ, Kong HJ, Im JS and Seo HG: Community of
population-based regional cancer registries*. Cancer statistics in
Korea: Incidence, mortality, survival, and prevalence in 2020.
Cancer Res Treat. 55:385–399. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee J, Park SH, Chang HM, Kim JS, Choi HJ,
Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW, et al: Gemcitabine and
oxaliplatin with or without erlotinib in advanced biliary-tract
cancer: A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 13:181–188. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival and prevalence in 2010. Cancer Res Treat. 45:1–14.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shi Y, Wang Y, Zhang W, Niu K, Mao X, Feng
K and Zhang Y: N6-methyladenosine with immune infiltration and
PD-L1 in hepatocellular carcinoma: novel perspective to
personalized diagnosis and treatment. Front Endocrinol (Lausanne).
14(1153802)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deng M, Li SH, Fu X, Yan XP, Chen J, Qiu
YD and Guo RP: Relationship between PD-L1 expression, CD8+ T-cell
infiltration and prognosis in intrahepatic cholangiocarcinoma
patients. Cancer Cell Int. 21(371)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xie Q, Wang L and Zheng S: Prognostic and
clinicopathological significance of PD-L1 in patients with
cholangiocarcinoma: A meta-analysis. Dis Markers.
2020(1817931)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee J and Kim DU: Recent Update of
Targeted Therapy in Cholangiocarcinoma. Korean J Pancreas Biliary
Tract. 28:59–66. 2023.(In Korean).
|
|
10
|
Dey S, Chatterjee S, Ghosh S and Sikdar N:
The geographical, ethnic variations and risk factors of gallbladder
carcinoma: A worldwide view. J Investig Genomics. 3:49–54.
2016.
|
|
11
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guion-Dusserre JF, Lorgis V, Vincent J,
Bengrine L and Ghiringhelli F: FOLFIRI plus bevacizumab as a
second-line therapy for metastatic intrahepatic cholangiocarcinoma.
World J Gastroenterol. 21(2096)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oh DY, Ruth He A, Qin S, Chen LT, Okusaka
T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, et al:
Durvalumab plus gemcitabine and cisplatin in advanced biliary tract
cancer. NEJM Evid. 1(EVIDoa2200015)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanz-Altamira PM, Ferrante K, Jenkins RL,
Lewis WD, Huberman MS and Stuart KE: A phase II trial of
5-fluorouracil, leucovorin, and carboplatin in patients with
unresectable biliary tree carcinoma. Cancer. 82:2321–2325.
1998.PubMed/NCBI
|
|
16
|
Lamarca A, Palmer DH, Wasan HS, Ross PJ,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
Second-line FOLFOX chemotherapy versus active symptom control for
advanced biliary tract cancer (ABC-06): A phase 3, open-label,
randomised, controlled trial. Lancet Oncol. 22:690–701.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
O'Rourke CJ, Munoz-Garrido P and Andersen
JB: Molecular targets in cholangiocarcinoma. Hepatology. 73 (Suppl
1):S62–S74. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goyal L, Saha SK, Liu LY, Siravegna G,
Leshchiner I, Ahronian LG, Lennerz JK, Vu P, Deshpande V,
Kambadakone A, et al: Polyclonal secondary FGFR2 mutations drive
acquired resistance to FGFR inhibition in patients with FGFR2
fusion-positive cholangiocarcinoma. Cancer Discov. 7:252–263.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Javle M, Churi C, Kang HC, Shroff R, Janku
F, Surapaneni R, Zuo M, Barrera C, Alshamsi H, Krishnan S, et al:
HER2/neu-directed therapy for biliary tract cancer. J Hematol
Oncol. 8(58)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Riener MO, Bawohl M, Clavien PA and Jochum
W: Rare PIK3CA hotspot mutations in carcinomas of the biliary
tract. Genes Chromosomes Cancer. 47:363–367. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moeini A, Sia D, Bardeesy N, Mazzaferro V
and Llovet JM: Molecular pathogenesis and targeted therapies for
intrahepatic cholangiocarcinoma. Clin Cancer Res. 22:291–300.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abou-Alfa GK, Sahai V, Hollebecque A,
Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson
D, Murphy AG, et al: Pemigatinib for previously treated, locally
advanced or metastatic cholangiocarcinoma: A multicentre,
open-label, phase 2 study. Lancet Oncol. 21:671–684.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Makawita S, K Abou-Alfa G, Roychowdhury S,
Sadeghi S, Borbath I, Goyal L, Cohn A, Lamarca A, Oh DY, Macarulla
T, et al: Infigratinib in patients with advanced cholangiocarcinoma
with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future
Oncol. 16:2375–2384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rizzo A, Ricci AD and Brandi G:
Futibatinib, an investigational agent for the treatment of
intrahepatic cholangiocarcinoma: Evidence to date and future
perspectives. Expert Opin Investig Drugs. 30:317–324.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cho SM, Esmail A, Raza A, Dacha S and
Abdelrahim M: Timeline of FDA-approved targeted therapy for
cholangiocarcinoma. Cancers (Basel). 14(2641)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roa I, de Toro G, Schalper K, de
Aretxabala X, Churi C and Javle M: Overexpression of the HER2/neu
gene: A new therapeutic possibility for patients with advanced
gallbladder cancer. Gastrointest Cancer Res. 7:42–48.
2014.PubMed/NCBI
|
|
28
|
Yarlagadda B, Kamatham V, Ritter A,
Shahjehan F and Kasi PM: Trastuzumab and pertuzumab in circulating
tumor DNA ERBB2-amplified HER2-positive refractory
cholangiocarcinoma. NPJ Precis Oncol. 3(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee CK, Chon HJ, Cheon J, Lee MA, Im HS,
Jang JS, Kim MH, Park S, Kang B, Hong M, et al: Trastuzumab plus
FOLFOX for HER2-positive biliary tract cancer refractory to
gemcitabine and cisplatin: A multi-institutional phase 2 trial of
the Korean cancer study group (KCSG-HB19-14). Lancet Gastroenterol
Hepatol. 8:56–65. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW,
Chang HM, Bao L, Sun HC, Macarulla T, Xie F, et al: Zanidatamab for
HER2-amplified, unresectable, locally advanced or metastatic
biliary tract cancer (HERIZON-BTC-01): A multicentre, single-arm,
phase 2b study. Lancet Oncol. 24:772–782. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tsilimigras DI, Stecko H,
Ntanasis-Stathopoulos I and Pawlik TM: Racial and sex differences
in genomic profiling of intrahepatic cholangiocarcinoma. Ann Surg
Oncol. 31:9071–9078. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jung KW, Won YJ, Kong HJ and Lee ES:
Community of Population-Based Regional Cancer Registries. Cancer
statistics in Korea: Incidence, mortality, survival, and prevalence
in 2015. Cancer Res Treat. 50:303–316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ,
Kong HJ, Im JS and Seo HG: Community of Population-Based Regional
Cancer Registries. Cancer statistics in Korea: Incidence,
mortality, survival, and prevalence in 2019. Cancer Res Treat.
54:330–344. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ,
Cheon J, Kang BW, Ryu H, Lee JS, Kim KW, et al: Liposomal
irinotecan plus fluorouracil and leucovorin versus fluorouracil and
leucovorin for metastatic biliary tract cancer after progression on
gemcitabine plus cisplatin (NIFTY): A multicentre, open-label,
randomised, phase 2b study. Lancet Oncol. 22:1560–1572.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tawarungruang C, Khuntikeo N, Chamadol N,
Laopaiboon V, Thuanman J, Thinkhamrop K, Kelly M and Thinkhamrop B:
Survival after surgery among patients with cholangiocarcinoma in
Northeast Thailand according to anatomical and morphological
classification. BMC Cancer. 21(497)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hang H, Jeong S, Sha M, Kong D, Xi Z, Tong
Y and Xia Q: Cholangiocarcinoma: anatomical location-dependent
clinical, prognostic, and genetic disparities. Ann Transl Med.
7(744)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ren HB, Yu T, Liu C and Li YQ: Diabetes
mellitus and increased risk of biliary tract cancer: Systematic
review and meta-analysis. Cancer Causes Control. 22:837–847.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schlesinger S, Aleksandrova K, Pischon T,
Jenab M, Fedirko V, Trepo E, Overvad K, Roswall N, Tjønneland A,
Boutron-Ruault MC, et al: Diabetes mellitus, insulin treatment,
diabetes duration, and risk of biliary tract cancer and
hepatocellular carcinoma in a European cohort. Ann Oncol.
24:2449–2455. 2013.PubMed/NCBI View Article : Google Scholar
|