Introduction
Streptococcus thoraltensis (S.
thoraltensis) is an alpha-hemolytic nonmotile, anaerobic,
non-sporulating, Gram-negative Streptococcus. It was
initially discovered in 1997(1). It
commonly inhabits the urogenital and gastrointestinal tracts of
pigs and rabbits (2) and constitutes
a component of the normal gut microbiota of quadruped mammals
(3). The overuse of antibiotics in
livestock may cause mutations in this microorganism, thus aiding
its spread to other species (4,5).
Currently, S. thoraltensis is not considered pathogenic to
humans. However, it has been found to colonize the oral mucosa of
diesel industry workers (6).
Furthermore, it has been identified as an etiological agent in
cases of chorioamnionitis (7),
postpartum pneumonia (8) and fever
of unknown origin secondary to bacteremia (9). Recently, S. thoraltensis was
isolated from the blood samples of an elderly individual with a
prosthetic heart valve diagnosed with endocarditis (10). In 2020, the first case of bacterial
endocarditis attributable to S. thoraltensis emerged in
Mexico, affecting an immunocompetent patient with no prior history
of heart valve replacement, confirmed via histopathological
analysis (11).
Therefore, in line with this emerging pattern, the
present study describes the case of a 38-year-old healthy female
patient diagnosed with an abscess in the posterior uterine region
due to S. thoraltensis.
Case report
A 38-year-old female patient with no prior medical
relevant history was admitted to the Emergency Department of the
Hospital Regional Universitario of Colima (Colima, Mexico). The
individual reported an acute episode of abdominal pain localized in
the left iliac fossa, which radiated to the hypogastrium and right
iliac fossa over the past 10 days; the pain was intermittent and
accompanied by dysuria, vesical tenesmus and constipation
persisting for 4 days. Following hospital admission, the patient
was transferred to the Gynecology and Obstetrics Department.
Following the diagnosis, the patient underwent biochemical and
hematological testing. The initial complete blood count revealed
low levels of erythrocytes (3.71 million/µl) and hemoglobin (10.30
g/dl). Additionally, leukocytosis was observed (26,500/mm³),
predominantly due to neutrophils (86%, with a total of 22,800
neutrophils/mm³). An increase in acute-phase reactants was also
noted, with an erythrocyte sedimentation rate of 16 mm/h and
C-reactive protein levels of 25 mg/dl. Subsequently, an abdominal
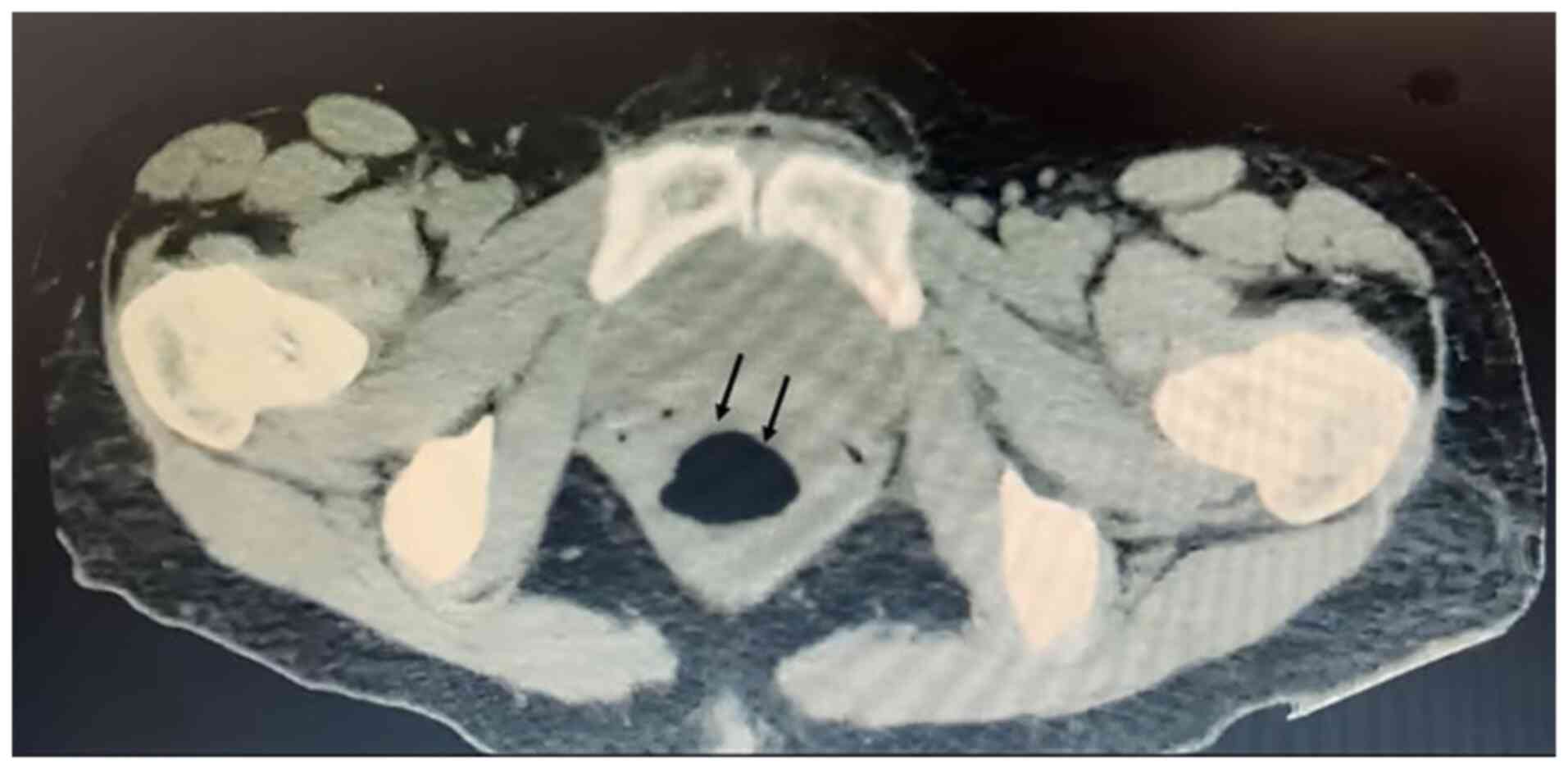
tomography scan was requested which revealed a cystic lesion
measuring 81x72x67 mm, anteriorly displaced from the uterus,
probably due to adnexal origin, with attenuation coefficients of 15
Hounsfield units (HU) and posterior to the rectum (Fig. 1). Evidence of mechanical subocclusion
was observed. Thus, surgical management was decided.
An exploratory laparotomy was performed, in which it
is not possible to identify the ovarium due to the presence of
multiples adherences, whereby adhesiolysis through the blunt
technique was executed. In the posterior region of the uterus and
sigmoid, an abscess was identified. Therefore, the cavity was
drained and 150 cc of purulent material were obtained, which were
sent for microbiological culture. Bacteriological analysis using
the VITEK 2.0 system® (supplied by BioMérieux Mexico)
revealed the presence of S. thoraltensis with 97% diagnostic
certainty. Treatment was initiated with a triple antibiotic
regimen, including doxycycline (100 mg orally every 12 h),
metronidazole (500 mg orally every 12 h) and ceftriaxone (1 g
daily) for 7 days. At 24 h following the initiation of
antimicrobial therapy, follow-up tests were conducted, which
revealed a decrease in the erythrocyte sedimentation rate and
C-reactive protein levels (5 mm/h and 10 mg/dl, respectively), as
well as a reduction in the leukocyte count (14,000/mm³), with 82.8%
neutrophils (11,600 neutrophils/mm³). Due to infrastructural
limitations, a follow-up computed tomography scan was not
performed. However, based on the clinical and paraclinical
improvement observed during the follow-up of the patient, the
resolution of the clinical condition was established. The symptoms
of the patient continued to improve, and following 72 h of
treatment, all biochemical and hematological test results were
normalized.
Discussion
The present study describes a concise case report
detailing the clinical presentation of a 38-year-old female patient
without any notable medical or surgical history, who was diagnosed
with a bacterial abscess in the posterior uterine region secondary
to S. thoraltensis infection. To the best of our knowledge,
information about S. thoraltensis involvement in human
infection is recent and limited.
Recent reports published in 2024 have indicated an
increase in the incidence of endocarditis attributed to this
etiological agent. In fact, there is a growing number of reports
linking endocarditis to the presence of this pathogen. The first
case, published by Abid et al (12), described a middle-aged male patient
with no prior medical history, who presented with acute infectious
endocarditis. Upon analysis, the presence of S. thoraltensis
was confirmed as the causative agent. Notably, the patient had a
history of recreational drug use and occupational exposure to
livestock (rabbits), which are known reservoirs of S.
thoraltensis (12). The latest
report published in 2024 describes the case of a 65-year-old male
patient with multiple comorbidities, including congestive heart
failure due to dilated cardiomyopathy, chronic obstructive
pulmonary disease, and alcoholic cirrhosis resulting from 20 years
of chronic alcoholism, presented with an acute clinical onset.
Endocarditis was confirmed both clinically and via ultrasound. The
isolated etiological agent was S. thoraltensis, after which
antibiotic treatment was initiated. However, the clinical condition
of the patient deteriorated, ultimately leading to his death due to
septic shock (13).
Fortunately, in the case described herein, the
patient responded adequality to treatment with doxycycline and
metronidazole, similar to a previous report in which the double
antibiotic regimen exhibited efficacy (14). It should be mentioned that mammals,
particularly quadrupeds, serve as the natural reservoir for this
bacterium. Consequently S. thoraltensis infection can be
classified as a zoonotic disease, with its transmission mechanism
plausible associated to food industry (15). Notably, in recent times the number of
zoonotic diseases has been increased, encompassing around 75% of
all emergent diseases (16). A
common strategy employed to diminish the risk of zoonotic diseases
is the routine administration of broad-spectrum antibiotics in
farms and the food industry. It is estimated that these sectors
annually administer thousands of tons of antibiotics (17). On this point, a previous report noted
that the excessive use of antibiotics may facilitate the
development of pathogenic characteristics in previously innocuous
microorganisms (18).
This phenomenon reveals the increasingly evident
impact of climate change, which modifies the biochemical
interactions between microorganisms and their environment. These
changes can disrupt the normal colonization patterns of biological
niches and negatively affect the genetic and molecular barriers
between host and vectors. It has been theorized that in response to
these changes, microorganisms can modify their gene expression to
produce unpredictable interactions (19,20).
Moreover, the rise in global temperatures accelerates the
metabolism of bacteria, although this effect can be mitigated with
antibiotics. The selective elimination of pathogenic bacteria could
indirectly favor colonization by atypical species, as has been
reported for some species of streptococcus (21). Subsequently, if this trend continues,
an artificial regulatory effect will be exerted that favors the
appearance of emerging pathogens that were previously considered
benign.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IJGA and GARR participated equally in the
preparation of the present manuscript. Both authors participated in
the medical care process and during data collection, literature
search, information synthesis, and writing of this manuscript. IJGA
and GARR confirm the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the ethical standards of the Declaration of Helsinki, 1964.
Informed consent was obtained from the patient for inclusion in the
study. Ethics approval was waived by the local committee as no
personal data were used.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
related images.
Competing interests:
The authors declare that they have no competing
interests.
References
|
1
|
Devriese LA, Pot B, Vandamme P, Kersters
K, Collins MD, Alvarez N, Haesebrouck F and Hommez J:
Streptococcus hyovaginalis sp. nov. and Streptococcus
thoraltensis sp. nov., from the genital tract of sows. Int J
Syst Bacteriol. 47:1073–1077. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Borø S, McCartney CA, Snelling TJ, Worgan
HJ and McEwan NR: Isolation of Streptococcus thoraltensis
from rabbit faeces. Curr Microbiol. 61:357–360. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abed SM, Jasim HJ, Alomari MM and Hussein
GH: Comparison between antimicrobial and antibiofilm activity of
exopolysaccharides (EPS) extracted from lactobacillus reuteri and
streptococcus mitis against oral bacteria. Arch Razi Inst.
77:2215–2221. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Araby E, Nada HG, El-Nour SA and Hammad A:
Detection of tetracycline and streptomycin in beef tissues using
Charm II, isolation of relevant resistant bacteria and control
their resistance by gamma radiation. BMC Microbiol.
20(186)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
León-Sampedro R, Novais C, Peixe L,
Baquero F and Coque TM: Diversity and evolution of the
Tn5801-tet(M)-like integrative and conjugative elements among
enterococcus, streptococcus, and staphylococcus. Antimicrob Agents
Chemother. 60:1736–1746. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
AlWakeel SS: Microbiological and molecular
identification of bacterial species isolated from nasal and
oropharyngeal mucosa of fuel workers in Riyadh, Saudi Arabia. Saudi
J Biol Sci. 24:1281–1287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vukonich M, Moline H, Chaussee M, Pepito B
and Huntington MK: Case report: Chorioamnionitis attributed to
Streptococcus thoraltensis. S D Med. 68:298–299.
2015.PubMed/NCBI
|
|
8
|
Wazir M, Grewal M, Jain AG and Everett G:
Streptococcus thoraltensis Bacteremia: A case of pneumonia
in a postpartum patient. Cureus. 11(e5659)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Petridis N, Apsemidou A, Kalopitas G,
Pilianidis G and Avramidis I: Streptococcus thoraltensis
bacteremia: First described case as a fever of unknown origin in
human. Case Rep Infect Dis. 2018(7956890)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hai PD, Son PN, Huong NT, Binh NT, Hoa LT
and Dung NM: A case of Streptococcus thoraltensis bacteremia
and prosthetic valve endocarditis in a 68-year-old Vietnamese man.
Am J Case Rep. 21(e925752)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Flores-Morales A, Jacobo-Ruvalcaba A,
Acevedo-Meléndez AC, Fernández-Muñoz MJ, Carmona-Ruiz HA,
Borrayo-Sánchez G and Orihuela-Rodríguez O: Infectious endocarditis
without intracardiac devices or underlying structural heart
disease. Cir Cir. 91:535–541. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abid S, Stolear A, Dulgher M, Sethi S and
Zarich S: A rare cause of acute bacterial native valve endocarditis
caused by Streptococcus thoraltensis. Cureus.
16(e70934)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chiorescu RM, Buksa SB, Botan A, Mocan M,
Costache C and Toc DA: Vancomycin-Resistant Streptococcus
thoraltensis: A case report of bacterial endocarditis and
review of literature on infections caused by this pathogen.
Microorganisms. 12:1–12. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gomez-Torres J, Nimir A, Cluett J,
Aggarwal A, Elsayed S, Soares D, Teatero S, Chen Y, Gottschalk M
and Fittipaldi N: Human case of streptococcus suis disease,
Ontario, Canada. Emerg Infect Dis. 23:2107–2109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duchenne-Moutien RA and Neetoo H: Climate
change and emerging food safety issues: A review. J Food Prot.
84:1884–1897. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Żbikowska K, Michalczuk M and Dolka B: The
use of bacteriophages in the poultry industry. Animals (Basel).
10(872)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Filho WL, Ternova L, Parasnis SA, Kovaleva
M and Nagy GJ: Climate change and Zoonoses: A review of concepts,
definitions, and bibliometrics. Int J Environ Res Public Health.
19(893)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang S, Zeng X, Yang Q and Qiao S:
Antimicrobial peptides as potential alternatives to antibiotics in
food animal industry. Int J Mol Sci. 17(603)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Friedman DZP and Schwartz IS: Emerging
fungal infections: New patients, new patterns, and new pathogens. J
Fungi (Basel). 5(67)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tiedje JM, Bruns MA, Casadevall A, Criddle
CS, Eloe-Fadrosh E, Karl DM, Nguyen NK and Zhou J: Microbes and
climate change: A research prospectus for the future. mBio.
13(e0080022)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Numberger D, Siebert U and Weigand PV:
Survival and adaptation of Streptococcus phocae in host
environments. PLoS One. 19(e0296368)2024.PubMed/NCBI View Article : Google Scholar
|