Introduction
Endoscopic resection is currently accepted as an
appropriate treatment method for early-stage colorectal cancer
(eCRC). Intramucosal CRC does not metastasize to the lymph nodes
and can be curatively removed by endoscopic resection (1,2). By
contrast, 6-12% of invasive eCRC cases, in which CRC invades
through the muscularis mucosae (MM), but does not extend into the
muscularis propria (MP), are associated with lymph node metastasis,
thus requiring additional colectomy/proctectomy and lymph node
dissection for curative treatment (3-6).
eCRC is grossly classified as a superficial type
(type 0), which is further divided into protruded type (type 0-I)
and superficial type (type 0-II) according to the Japanese
Classification of Colorectal, Appendiceal, and Anal Carcinoma,
third English edition, published in 2019 by the Japanese Society
for Cancer of the Colon and Rectum (JSCCR). This is referred to as
the JSCCR rule hereafter (7). The
protruded type can be further subclassified as the pedunculated
type (type 0-Ip), subpedunculated type (type 0-Isp), or sessile
type (type 0-Is) from the presence or absence of a pedicle
(7), while the Paris classification
states that the subpedunculated type should be managed in the same
manner as the sessile type (8). The
JSCCR project study revealed that the nodal metastasis rate of eCRC
with a submucosal (SM) invasion ≥1,000 µm was 12.5% (9). Additional surgery may be warranted in
patients with a SM invasion ≥1,000 µm when other risk factors for
lymph node metastasis are present, such as lymphovascular invasion,
poorly or undifferentiated component (poorly differentiated
adenocarcinoma, mucinous adenocarcinoma, signet-ring cell
carcinoma), muconodules at the invasion front, or high grade of
tumor budding/sprouting (BD) (10-12).
The depth of SM invasion is simply determined in sessile type
cases; however, it is relatively complex in the pedunculated type
due to the presence of stalks and tangled MM. In Western countries,
the Haggitt classification is used to determine the depth of SM
invasion. This system classifies head invasion [Haggitt level (HL)
1 and HL2] and stalk invasion (HL3) as a shallow SM invasion that
is equivalent to a SM invasion <1,000 µm in sessile lesions
(13-15),
while SM invasion of the underlying bowel wall (HL4) is recognized
as a risk factor of synchronous lymph node metastasis and the need
for additional surgical resection (16-19).
By contrast, the JSCCR rule has stated that the depth of SM
invasion should be measured from the MM lower border when the MM
can be identified or estimated and from the surface of the lesion
when it cannot be identified or estimated, irrespective of
macroscopic types. In an MM-tangled pedunculated lesion, the depth
of SM invasion should be measured from the reference line (7). With the JSCCR rule, additional surgery
may be considered in some pedunculated eCRC cases with head and
stalk invasion in which additional surgery can be spared by the
Haggitt classification method.
The present study aimed to validate the JSCCR rule
of measuring the SM depth in pedunculated-type eCRC cases, and to
subsequently compare its utility in considering the need for
additional surgery with that of the Haggitt classification
method.
Materials and methods
Patients and study subjects
Consecutive patients with pedunculated-type eCRC who
underwent endoscopic cold snare polypectomy or endoscopic mucosal
resection at Shioya Hospital, International University of Health
and Welfare (IUHW; Yaita, Japan) between 2006 and 2022 and Ota
Memorial Hospital (OMH), SUBARU Health Insurance Society (Ota,
Japan) between 2014 and 2022 were included in this study. The
exclusion criteria included having a history of invasive CRC within
5 years of endoscopic resection, concurrent invasive CRCs that were
resected soon after the endoscopic resection of pedunculated
lesions, unavailable clinical information and surveillance <100
days following endoscopic resection. Any eCRC cases with HL ≥1 in
which contrast-enhanced computed tomography (CECT) was not
performed following endoscopic resection were also excluded.
Clinicopathological information was obtained through the electronic
chart systems of the respective institutions. When the
adenocarcinoma was intraepithelial carcinoma or was confined within
the lamina propria mucosae (LPM) and had negative resection
margins, the patients were recommended to undergo a colonoscopy in
12 months. When the adenocarcinoma invaded beyond the MM, but had
negative resection margins, the patients were followed-up every 6
months at the outpatient clinic for 5 years following endoscopic
resection. Serum tumor marker levels, such as carcinoembryonic
antigen and carbohydrate antigen 19-9, were measured every 6
months. CECT was performed immediately or after 6 months at the
discretion of the clinicians and at 12 months following endoscopic
resection. Colonoscopy was performed at 6 or 12 months following
endoscopic resection at the discretion of the clinicians. In cases
of carcinoma-positive resection margins, positive lymphovascular
invasion and a SM invasion of ≥1,000 µm, additional surgery was
considered. The present study was conducted in accordance with the
Declaration of Helsinki, and the study protocol was approved by the
Ethical Review Boards of the participating hospitals: IUHW,
approval number: FK-94; OMH, approval number: OR24029. Due to the
retrospective design of the study and anonymization of the data,
consent to participate was not necessary.
Pathological examination
All specimens were routinely processed for
pathological diagnosis. The tumor size (maximal diameter),
histological type and BD were evaluated using hematoxylin and eosin
(H&E)-stained sections. Desmin protein staining was examined
using immunohistochemistry (IHC) assays, as needed, to measure the
distance of SM invasion. Elastica-van Gieson (EVG) staining and
CD34 IHC assays, if necessary, were used to evaluate venous
invasion, while D2-40 IHC assays were used to determine lymphatic
invasion. Briefly, resected specimens were rapidly fixed in 10%
neutral-buffered formalin solution for 24-48 h and embedded in a
paraffin block. The blocks were then cut into 3-µm-thick sections
for H&E and EVG staining, while they were cut into 4-µm-thick
sections for IHC. Primary antibodies for IHC were FLEX monoclonal
mouse anti-human desmin antibody (clone D33, 1:1; cat. no. IR606;
Dako; Agilent Technologies, Inc.), mouse monoclonal anti-podoplanin
antibody (clone D2-40, 1:1; cat. no. 760-4395; Ventana Medical
Systems, Inc.), and CONFIRM™ anti-CD34 mouse monoclonal antibody
(clone QBEnd/10, 1:1; cat. no. 790-2927; Ventana Medical Systems,
Inc.). Antigen retrieval was performed as needed, by heating in
Cell Conditioning Solution 1 (CC1) (Roche Diagnostics) at 100˚C for
60 min. Staining was performed on the VENTANA Benchmark XT
automated slide stainer (Leica Microsystems GmbH) in combination
with the ultraView DAB universal kit (Roche Diagnostics)
according to the manufacturer's instructions. Nuclei were then
counterstained with hematoxylin (Sakura Finetek Japan Co., Ltd.).
Clinicopathological classification and staging were performed using
the World Health Organization classification of colorectal tumors
(5th edition) and the Union for International Cancer Control tumor,
node, metastasis staging system (8th edition) (20,21). In
addition, the subclassification of pathological tumor (pT)1 (the
tumor is confined within the SM) was performed using the JSCCR rule
with the following modifications: The Haggitt line, which links the
junctions between the tumor and non-tumor in the stalk, was used to
measure the depth of SM invasion instead of the reference line,
which was defined by the JSCCR rule as the boundary between the
tumor head and stalk (7) (Fig. 1A). pT1a and pT1b denote a SM invasion
of <1,000 and ≥1,000 µm, respectively (7). BD was evaluated according to the JSCCR
rule.
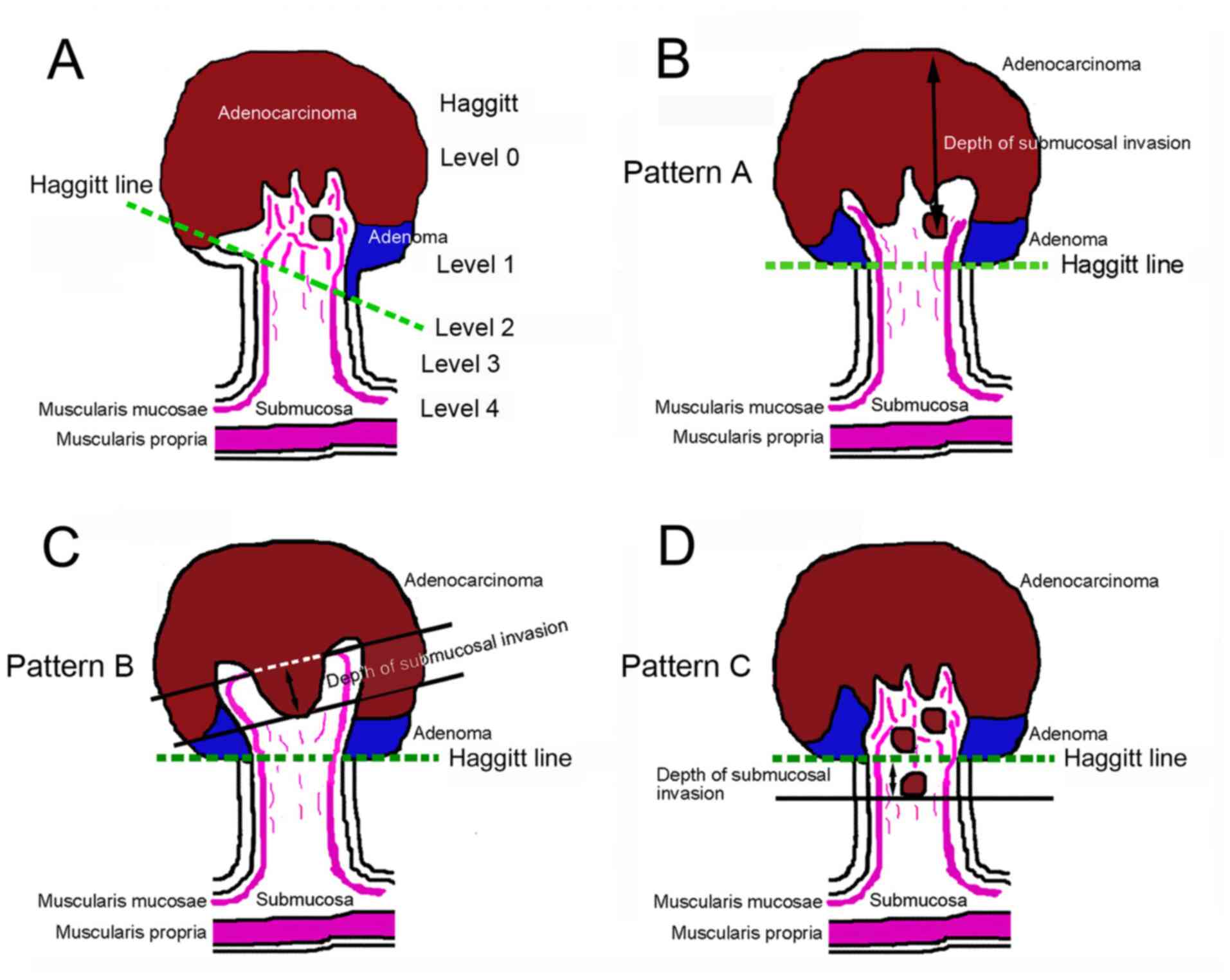 | Figure 1Method used for measuring the depth of
SM invasion in pedunculated-type eCRC. (A) The Haggitt line, which
links the junctions between the tumor and its stalk, was used to
measure the depth of SM invasion rather than the reference line,
which is defined by the JSCCR rule as the boundary between the
tumor head and stalk. (B) When the MM could not be identified or
estimated at the adenocarcinoma invasive front (designated as
pattern A), the depth of SM invasion was measured from the lesion's
surface. (C) If the MM could be identified or estimated in the
original form (designated as pattern B), then the depth of SM
invasion was measured from the lower border of MM. (D) When tangled
MM was observed (designated as pattern C), depth of SM invasion was
measured as the distance of invasion in the stalk beyond the
Haggitt line. eCRC, early colorectal cancer; JSCCR, Japanese
Society for Cancer of the Colon and Rectum; MM, muscularis mucosae;
SM, submucosal. |
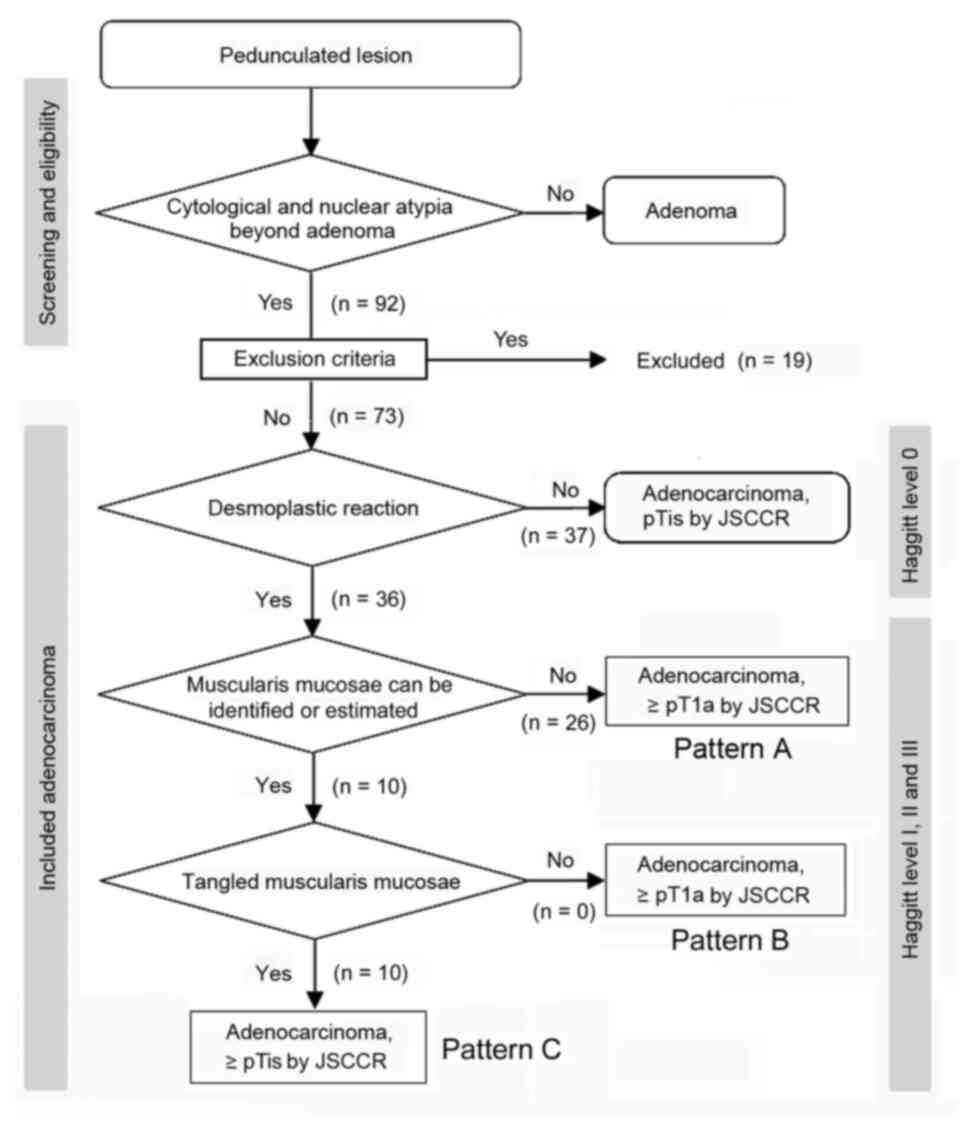
The flow diagram of the pathological staging of
pedunculated-type adenocarcinoma is presented in Fig. 2. Non-cancer glands often display SM
misplacement or pseudoinvasion in pedunculated-type tumors.
Therefore, submucosally placed glands were evaluated as
pseudoinvasion when they did not display cytological or nuclear
atypia strong enough for malignancy (Fig. 3A and B). In addition, the present study focused
on desmoplastic reaction, with adenocarcinoma cases without
desmoplastic reaction being diagnosed as intraepithelial carcinoma
or carcinoma confined within the LPM, corresponding to HL0 and pTis
by the JSCCR rule (22) (Fig. 3C and D). Adenocarcinoma cases with desmoplastic
reaction were staged as HL ≥1, which may be equivalent to pTis or
pT1 by the JSCCR rule (7).
Identification of the MM is pivotal for measuring the depth of SM
invasion and pT1 substaging by the JSCCR rule. Although the MM can
be easily identified on the H&E- and EVG-stained sections by
experienced pathologists (Fig. 3E to
H), it may be sometimes difficult to
distinguish between a tangled MM and carcinoma-associated fibrosis
(23,24). Desmin IHC assays were performed in
such cases. When the MM could not be identified or estimated at the
adenocarcinoma invasive front (pattern A) (Fig. 1B), the depth of SM invasion was
measured from the surface of the lesion (Fig. 4A and B). These cases were staged as ≥pT1a and HL
≥1. When the original MM could be identified or estimated, the
depth of SM invasion was measured from the lower border of the MM
(pattern B) (Fig. 1C). In such
cases, the lesions were staged as ≥pT1a and HL ≥1. When a tangled
MM was observed (pattern C) (Fig.
1D), the depth of SM invasion was measured as the distance of
invasion in the stalk beyond the Haggitt line (Fig. 4C and D). The SM depth of head invasion is deemed
to be <0 mm and the JSCCR rule specifies that such cases should
be described only as head invasion. Thus, cases of head invasion
with MM pattern C are practically treated as pTis. In the present
study, cases with MM pattern C are described as ≥pTis and HL ≥1.
Pathological diagnosis was independently performed by two
experienced pathologists, with diagnostic discordance resolved by
their discussion.
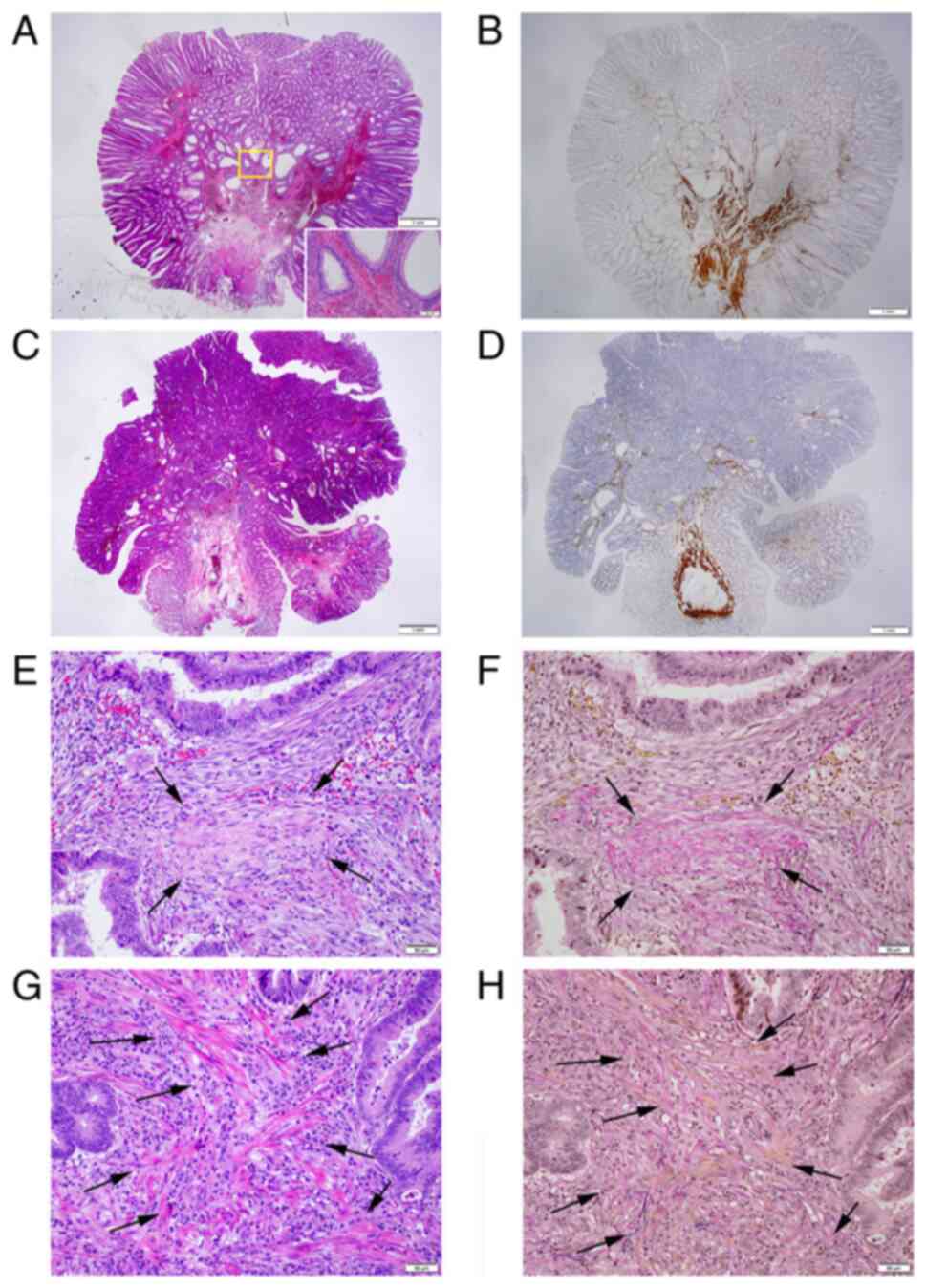 | Figure 3Pitfalls in cancer diagnosis, pT
staging and discrimination between desmoplastic reaction and a
tangled MM. (A) Representative histopathology of pedunculated-type
tubular adenoma displaying SM pseudoinvasion of adenomatous glands
(H&E; magnification, x1.25); Inset: Area within the yellow
square. Pseudoinvasive glands lack cytological atypia strong enough
for malignancy (H&E; magnification, x10). (B) Desmin IHC assays
demonstrate a tangled MM in the pedunculated-type tubular adenoma
(magnification, x1.25). (C) Pedunculated-type adenocarcinoma
initially appearing to present with head invasion with
disappearance of the MM. However, closer examination demonstrated
no desmoplastic reaction (case no. 58; H&E; magnification,
x1.25). (D) Desmin IHC assays demonstrated a tangled MM. This case
was ultimately evaluated as pTis/HL0 (magnification, x1.25). (E)
Desmoplastic reaction. Dull pink spindle cells, specifically
immature fibroblasts, irregularly proliferate with an edematous and
myxoid background and with collagen fibers deposited in the center
(arrows) (H&E; magnification, x20). (F) Desmoplastic reaction
foci with collagen fiber deposit are stained red using the EVG
staining (arrows; magnification, x20). (G) Tangled MM. Clear pink
spindle cells (arrows), specifically smooth muscle cells, run in
thin bundles (H&E; magnification, x20). (H) Smooth muscle cell
bundles (arrows) are stained yellow using the EVG staining
(magnification, x20). EVG, Elastica-van Gieson; H&E,
hematoxylin and eosin; HL, Haggitt level; IHC,
immunohistochemistry; MM, muscularis mucosae; pT, pathological
tumor; pTis, intraepithelial carcinoma or carcinoma confined within
the lamina propria mucosae; SM, submucosal. |
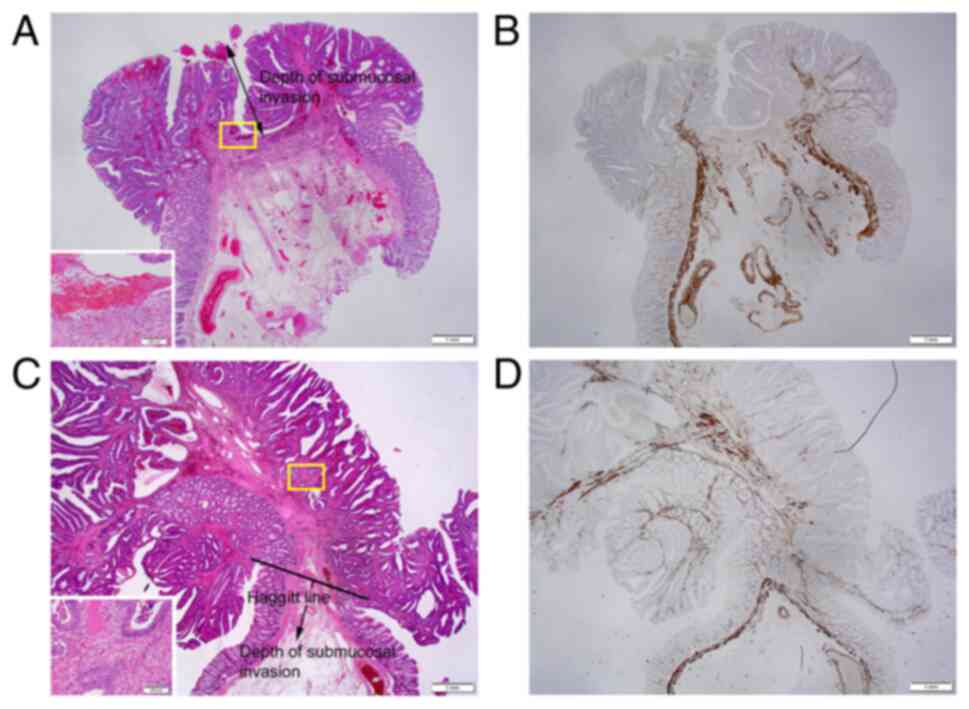 | Figure 4Representative histopathology of the
MM pattern in pedunculated-type eCRC cases. (A) When the MM could
not be identified or estimated at the adenocarcinoma invasive front
(designated as pattern A), the depth of SM invasion was measured
from the lesion's surface (H&E; magnification, x1.25); Inset:
Area within the yellow square. Desmoplastic reaction at the tumor
front, suggesting cancer invasion (H&E; magnification, x10;
case no. 74). (B) Desmin IHC assays demonstrated the disappearance
of the MM at the invasive front (magnification, x1.25). (C) When a
tangled MM was observed at the adenocarcinoma invasive front
(designated as pattern C), the depth of SM invasion was measured as
the distance of invasion in the stalk beyond the Haggitt line
(H&E; magnification, x1.25); Inset: Area within the yellow
square. Desmoplastic reaction at the tumor front, suggesting cancer
invasion (H&E; magnification, x10; case no. 41). (D) Desmin IHC
assays demonstrated a tangled MM at the invasive front
(magnification, x1.25). eCRC, early colorectal cancer; H&E,
hematoxylin and eosin; IHC, immunohistochemistry; MM, muscularis
mucosae; SM, submucosal. |
Statistical analysis
Categorical variables between two groups were
compared using the Fisher's exact test in the case of 2x2 cross
tabulations or the Chi-squared test with or without Yates'
correction as appropriate in the case of m x n cross tabulations.
Continuous variables between two groups were compared using the
Mann-Whitney U test. Continuous variables among three groups were
compared using the Kruskal-Wallis H test, with the ad-hoc test
performed by comparing two groups using the Mann-Whitney U test
with the Bonferroni correction. P-values <0.05 were considered
to indicate statistically significant differences, apart from the
Mann-Whitney U test with the Bonferroni correction, for which the
significance level was modified according to the number of groups.
Survival curves were generated using the Kaplan-Meier method and
significant differences in survival were analyzed using the
log-rank test. Statistical analyses were performed using IBM SPSS
Statistics 20 (IBM Corporation).
Results
Pathological diagnosis, evaluation of
pT stage and HL
Overall, a total of 92 cases of pedunculated-type
adenocarcinoma with/without adenoma component underwent endoscopic
resection during the study period. Among these cases, 19 cases were
excluded according to the exclusion criteria. A total of 73 cases,
of which 70 cases had success with en bloc endoscopic
resection, were subjected to further analysis. Of note, 1 of the 3
cases which failed with en bloc endoscopic resection
underwent additional surgical resection. From the absence of
desmoplastic reaction, 37 cases were diagnosed as intraepithelial
carcinoma or carcinoma confined within the LPM, corresponding to
pTis by the JSCCR rule and HL0. Cases of adenocarcinoma with
desmoplastic reaction were classified as HL ≥1, which corresponds
to pTis or pT1 by the JSCCR rule. Further pathological substaging
was performed by measuring the depth of SM invasion. When the MM
could not be identified or estimated at the adenocarcinoma invasive
front (pattern A; Fig. 1B), the
depth of SM invasion was measured from the surface of the lesion.
This pattern was observed in 26 cases, which were staged as ≥pT1a
and HL ≥1. When the MM could be identified or estimated in an
original form at the adenocarcinoma invasive front, the depth of SM
invasion was measured from the lower border of the MM (pattern B;
Fig. 1C). This pattern, which
represents ≥pT1a and HL ≥1, was not observed in any of the included
cases. When a tangled MM was observed, the depth of SM invasion was
measured as the distance of invasion in the stalk beyond the
Haggitt line (pattern C; Fig. 1D).
This pattern was observed in 10 cases, which were classified as HL
≥1, corresponding to the pTis (within the Haggitt line) or ≥pT1a
(beyond the Haggitt line) stage by the JSCCR rule.
Ultimately, 47 cases were classified as pTis and 26
cases were classified as pT1b by the JSCCR rule. The pTis cases
were significantly older than the pT1b cases (P=0.040). Of the pTis
cases, 37 cases were HL0 (surveillance, 149-6,126 days; median,
2,106 days) and 10 cases were HL1-2 (surveillance, 112-2,860 days;
median, 877 days). Of the pT1b cases, 18 cases were HL1-2
(surveillance, 104-4,079 days; median, 1,050 days) and 8 cases were
HL3 (surveillance, 501-4,140 days; median, 1,306 days) (Table I).
 | Table IClinicopathologic characteristics of
the included cases. |
Table I
Clinicopathologic characteristics of
the included cases.
| pT (JSCCR)/HL | pTis/HL0 | pTis/HL1-2 | pT1a/HL1-2 | pT1a/HL3 | pT1b/HL1-2 | pT1b/HL1-2 | pT1b/HL3 | pT1b/HL3 | P-value (P1/P2) |
|---|
| MM
patterna | NA | C | A and B | A, B, and C | A | B | A | B and C | NA |
| No. of cases | 37 | 10 | 0 | 0 | 18 | 0 | 8 | 0 | NA |
| Sex (M:F) | 24:13 | 7:3 | - | - | 16:2 | - | 5:3 | - | 0.280/0.446 |
| Age (median) | 35-88(71) | 46-77 (65.5) | - | - | 39-78(62) | - | 54-82(65) | - | 0.040/0.087 |
| Tumor site
(C:A:T:D:S:R:unknown) | 1:3:1:5:19:7:1 | 0:0:0:1:9:0:0 | - | - | 1:0:1:1:13:2:0 | - | 0:2:0:1:3:2:0 | - | 0.999/0.844 |
| Endoscopic en
bloc resection (yes:no) | 36:1 | 8:2 | - | - | 18:0 | - | 8:0 | - | 0.548/0.946 |
| ER status
(ER0:ER1:ERX) | 37:0:0 | 9:0:1 | - | - | 18:0:0 | - | 8:0:0 | - | NA |
| Additional surgery
(yes:no) | 0:37 | 1:9 | - | - | 3:15 | - | 6:2 | - |
<0.001/<0.001 |
| Surveillance after
endoscopic resection (median) (days) | 149-6,126
(2,106) | 112-2,860(877) | - | - | 104-4,079
(1,050) | - | 501-4,140
(1,306) | - | 0.363/0.063 |
The endoscopic resection margin status was unknown
in 1 case of pTis/HL1, which was excised in two sections, with the
patient later undergoing additional surgery. Additional surgical
resection was performed in 1, 3 and 6 cases of pTis/HL1, pT1b/HL1,
and pT1b/HL3, respectively. The incidence of additional surgery was
significantly more frequent in the pT1b cases than in the pTis
cases (P<0.001), and in the HL3 cases than in the HL0-2 cases
(4/65 vs. 6/8: P<0.001). The clinicopathological characteristics
of the the patients are summarized in Table I.
Pathological features, pT stage and HL
of the lesions
All but one of the cancer cases were well- or
moderately differentiated adenocarcinoma, with the other case
(pT1b/HL3) being mucinous adenocarcinoma. There was a significant
difference in tumor diameter between pTis (0.9-36 mm; median, 6.0
mm) and pT1b (2-40 mm; median, 10.5 mm) cases (P<0.001), and
among HL0 (0.9-14 mm; median, 4 mm), HL1-2 (2-36 mm; median, 10 mm)
and HL3 (6-40 mm; median, 10.5 mm) cases (P<0.001). The post hoc
analysis revealed that tumor diameter was significantly larger in
HL1-2 (P<0.001) and HL3 (P=0.001) cases compared with HL0 cases.
The absence of adenoma component was significantly more frequent in
pT1b cases than in pTis cases (P=0.002) and in HL3 cases than in
HL0 cases (P<0.001), although no significant difference was
observed between HL1-2 and HL0 cases (P=0.570). The depth of SM
invasion in pT1b cases, as defined by the JSCCR rule, was 2.0-15 mm
(median, 5.0 mm) in 18 HL1-2 cases and 2.0-18 mm (median, 4.5 mm)
in 8 HL3 cases. These results are summarized in Table II.
 | Table IIPathological features of the
lesions. |
Table II
Pathological features of the
lesions.
| pT (JSCCR)/HL | pTis/HL0 | pTis/HL1-2 | pT1b/HL1-2 | pT1b/HL3 | P-value
(P1/P2) |
|---|
| MM pattern | NA | C | A | A | NA |
| No. of cases | 37 | 10 | 18 | 8 | NA |
| Size of cancer
(median) (mm) | 0.9-14 (4.0) | 6-36 (9.5) | 2-30 (10.5) | 6-40 (10.5) |
<0.001/<0.001 |
| Histology
(W/M:P:Muc) | 37:0:0 | 10:0:0 | 18:0:0 | 7:0:1 | NA |
| Coexisting adenoma
(yes:no) | 36:1 | 10:0 | 16:2 | 3:5 |
0.002/<0.001 |
| Depth of SM
invasion (median) (mm) | - | - | 2.0-15 (5.0) | 2.0-18 (4.5) |
NA/0.955a |
| Invasion below the
Haggitt line (median) (mm) | - | - | - | 0.3-3.0 (1.3) | NA |
The lymphatic invasion rate was marginally more
frequent in pT1b cases than in pTis cases (P=0.051), while the
venous invasion rate was significantly more frequent in pT1b cases
than in pTis cases (P=0.042). There was no significant difference
in the lymphatic invasion rate and venous invasion rate among HL0,
HL1-2 and HL3 cases, and BD between HL1-2 and HL3 cases (P=0.156,
0.632 and 0.999, respectively) (Table
III).
 | Table IIIpT staging, Haggitt levels and
adverse outcomes of the lesions. |
Table III
pT staging, Haggitt levels and
adverse outcomes of the lesions.
| pT (JSCCR)/HL | pTis/HL0 | pTis/HL1-2 | pT1b/HL1-2 | pT1b/HL3 | P-value
(P1/P2) |
|---|
| MM pattern | - | C | A | A | NA |
| No. of cases | 37 | 10 | 18 | 8 | NA |
| Lymphatic invasion
(yes) | 0 | 1 | 2 | 2 | 0.051/0.156 |
| Venous invasion
(yes) | 0 | 0 | 2 | 1 | 0.042/0.632 |
| Budding
(BD1:BD2) | - | 9:1 | 15:3 | 7:1 |
0.999/0.999a |
| Positive nodes (by
pathology) | 0/37 | 0/10 (0/1) | 0/18 (0/3) | 0/8 (0/6) | 0.999 (0.999)/NA
(NA) |
| Recurrence | 0 | 1 | 0 | 0 | 0.999/0.982 |
| Recurrence-free
survival (median) (days) | 149-6,126
(2,106) | 112-2,860(748) | 104-4,079
(1,050) | 501-4,140
(1,306) | 0.338/0.059 |
pT staging, HL and adverse
outcomes
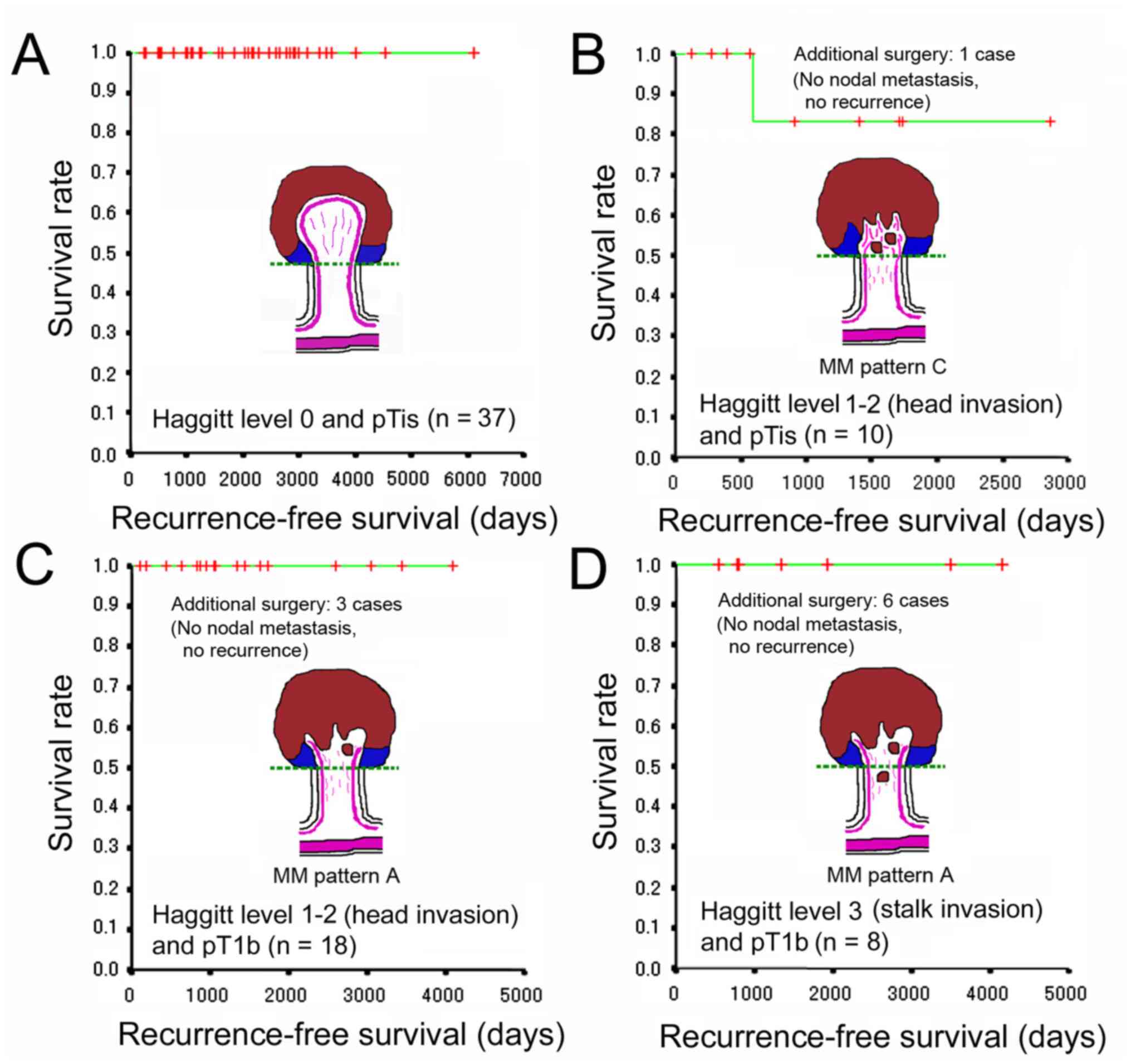
In the present study, an adverse outcome was defined
as lymph node metastasis detected by the imaging studies and/or the
pathological inspection of surgically resected specimens and
recurrence diagnosed clinically and/or pathologically. Additional
colectomy/proctectomy with lymph node dissection following
endoscopic resection was considered in the cases with
positive/unknown resection margins, lymphovascular invasion, BD2
(5-9 buddings/x200 field), and pT1b by the JSCCR rule. Due to such
risk factors and the intentions of patients, additional surgery was
performed in 1 case of pTis and 9 cases of pT1b. The inspection of
the surgical specimens from those cases revealed no nodal
metastasis. Recurrence was not observed in any of the pTis/HL0
cases. Of note, 1 case of pTis/HL1 experienced recurrence in the
peritoneum 594 days following endoscopic en bloc resection
with negative resection margins. The stalk of the lesion was 8 mm
in diameter. The total size was 36x33 mm and the size of the
adenocarcinoma was 10x10 mm. The histological type was moderately
differentiated adenocarcinoma with MM pattern C in traditional
serrated adenoma. No lymphatic or venous invasion was seen by D2-40
and EVG staining, respectively. Muconodules were observed at the
invasive front, but the case was classified as pTis (head invasion)
by the JSCCR rule. Recurrence was not observed in any of the
pT1b/HL1-2 or pT1b/HL3 cases. The adverse outcome rates were 0, 3.6
and 0% in the HL0, HL1-2 and HL3 cases, respectively. No
significant difference was observed in the recurrence rate and the
recurrence-free survival between pTis and pT1b cases and among HL0,
HL1-2, and HL3 cases. No CRC-related deaths were reported at the
time of writing the present study. These results are presented in
Fig. 5 and summarized in Table III.
Discussion
In 2004, Kitajima et al (25) reported the results of the project
study led by the JSCCR that analyzed 865 pT1 CRC cases that had
been surgically resected. Lymph node metastasis was found in 87
cases (10.1%), comprising 10 (7.1%) of the 141 pedunculated lesions
and 77 (10.6%) of the 724 non-pedunculated lesions. Lymph node
metastasis was not observed in the pedunculated lesions with head
invasion and stalk invasion presenting an SM depth <3,000 µm if
lymphatic invasion was negative. The vertical distance from the
Haggitt line to the deepest portion of invasion was designated as
the SM depth in the pedunculated lesion. The nodal metastasis rate
in the non-pedunculated lesions was 0% (0/123) when SM invasion was
<1,000 µm and 12.8% (77/601) when SM invasion was ≥1,000 µm
(25). From these results, the JSCCR
guidelines 2005 for the treatment of CRC (26) stated that additional surgery
following endoscopic resection of CRC should be considered when any
of the following conditions was noted: i) Positive SM vertical
margin; ii) SM invasion of ≥1,000 µm; iii) positive lymphatic
and/or venous invasion; and iv) poorly differentiated
adenocarcinoma and undifferentiated carcinoma. In these guidelines,
unlike in the study by Kitajima et al (25), the depth of SM invasion was
determined to be measured from the lower MM border when the MM
could be identified or estimated and from the lesion's surface when
the MM could not be identified or estimated, irrespective of the
macroscopic type. In addition, the SM depth was to be measured from
the reference line to the deepest portion of invasion in MM-tangled
pedunculated lesions (26).
Consequently, some cases with head invasion may be evaluated as
pT1b with deep SM invasion, making them candidates for additional
surgery.
In the present study, strict pathological diagnostic
criteria were applied for pT staging. In pedunculated lesions,
differentiation was required for the findings of SM localization of
normal and adenoma glands, specifically, SM misplacement and SM
pseudoinvasion. These phenomena may cause pT overstaging and result
in underestimating the prognosis of pT1 carcinoma cases. The
present study thus rigorously evaluated nuclear atypia as
malignancy and diagnosed SM invasion only when desmoplastic
reaction was observed at the invasive front, as cancer invasion in
the LPM usually does not cause desmoplastic reaction (22). Distinction between desmoplastic
reaction and a dissected or fragmented MM is usually not difficult
for experienced pathologists. However, EVG staining and desmin IHC
assays were conducted, as appropriate, in difficult cases.
Reportedly, reproducibility is not high among pathologists when
distinguishing between MM-tangled and non-tangled cases
(coincidence rate: κ value of 0.55) (27). Desmin IHC staining was used to help
distinguish between the two.
Following these pathological evaluations, the cases
included in the present study were classified as follows: 37
pTis/HL0 cases, 10 pTis/HL1-2 cases, 18 pT1b/HL1−2 cases, and eight
pT1b/HL3 cases. As the adenocarcinoma size increased, the pT stage
and HL also increased, while the rate of coexisting adenoma
decreased. As was expected, no adverse outcomes were found in the
pTis/HL0 cases. Notably, no adverse outcome was observed in any of
the 26 pT1b/HL1-3 cases, although the JSCCR guidelines assumed
lymph node metastasis in ~10% of the endoscopically resected pT1b
eCRC cases (26). Herein, the depth
of SM invasion in the pT1b/HL1−2 and pT1b/HL3 cases ranged from
2.0-15 mm (median, 5.0 mm) and 2.0-18 mm (median, 4.5 mm),
respectively, far exceeding the thickness of the normal colorectal
wall (~3.3 mm). These data suggested that pT1b substaging using the
JSCCR rule may not be useful as a tool for determining the need for
additional surgery.
The latest JSCCR guidelines 2019 for the treatment
of CRC weakly recommends additional bowel resection with lymph node
dissection for pT1b pedunculated lesions, although other factors,
such as histological subtype, lymphovascular invasion, high BD rate
(BD2/3), and the wishes of patients, need to also be taken into
consideration (9). Since the JSCCR
guidelines 2005, a limited number of studies have validated the
prognostic utility of the pT1b substaging system in the
pedunculated lesions. In 2004, Ueno et al (11) measured the SM depth in the same
manner as the JSCCR rule and investigated the association of SM
depth with lymph node metastasis. However, they did not perform pT
staging, combining the data of pedunculated and non-pedunculated
lesions and analyzing them as one dataset. In 2016, Asayama et
al (28) solely reported the
prognostic significance of pT1b substaging using the present JSCCR
rule. Adverse outcomes were also very rare in their cases. The
lymph node metastasis rate was 3.9%, while the recurrence rate was
1.3% (Table IV). Their data also
suggested that pT1b substaging using the JSCCR rule may not be
useful as a tool for determining the need for additional surgery.
Furthermore, accurate pathological pT staging is somewhat
difficult. SM misplacement and pseudoinvasion of neoplastic glands
may result in overstaging. Additionally, pathologists often
disagree on whether the MM is tangled or not. Therefore, we propose
to reconsider the definition of deep SM invasion (pT1b) in
pedunculated lesions in the JSCCR rule.
 | Table IVAdverse outcomes of JSCCR pT1
pedunculated-type lesions in the study by Asayama et al
(28) and the present study. |
Table IV
Adverse outcomes of JSCCR pT1
pedunculated-type lesions in the study by Asayama et al
(28) and the present study.
| | Rate of LN
metastasis (%) | Recurrence rate
(%) | |
|---|
| Author(s), year of
publication | pT1a | pT1b | pT1a | pT1b | (Refs.) |
|---|
| Asayama et
al, 2016 | 1/100 (1.0) | 3/76 (3.9) | 0/100 (0.0) | 1/76 (1.3) | (28) |
| Present study | - | 0/26 (0.0) | - | 0/26 (0.0) | |
| Total (%) | 1/100 (1.0) | 3/102 (2.9) | 0/100 (0.0) | 1/102 (1.0) | |
In 1985, Haggitt et al (13) reported that no nodal metastasis was
observed in 36 cases of pedunculated CRC with HL1-3, while one HL3
patient succumbed due to the disease. Several researchers have
since reported the rates of adverse outcomes, such as lymph node
metastasis, recurrence and disease-related mortality, in
pedunculated eCRC cases that underwent surgical or endoscopical
resection (11,13,25,28-30)
(Table V). According to these
previous studies and the present study, the lymph node metastasis
rates were 2.3 and 7.3% in HL1-2 and HL3 cases, respectively, while
the recurrence rates were 0.3 and 0.9% in HL1-2 and HL3,
respectively. Although Kitajima et al (25) emphasized the importance of vascular
invasion as a criterion of the need for additional surgery, Matsuda
et al (29) reported that the
incidence of nodal metastasis was 0 (0%) of 101 cases with head
invasion, in which lymphatic and/or venous invasion was observed in
29 (28.7%) cases. They thereby concluded that pedunculated invasive
eCRC can be managed by endoscopic treatment alone. These studies
suggest that additional surgery may not be necessary for
pedunculated CRC cases with HL1-2, irrespective of their vascular
invasion status. Although there are a limited number of similar
studies from Europe and the USA, the latest European (31) and American (32) guidelines suggest that only HL4 is a
risk factor of lymph node metastasis.
 | Table VAdverse outcomes of pedunculated-type
lesions according to the Haggitt level reported in the
literature. |
Table V
Adverse outcomes of pedunculated-type
lesions according to the Haggitt level reported in the
literature.
| | Rate of LN
metastasis (%) | Recurrence rate
(%) | Dead of disease
(%) | |
|---|
| Author(s), year of
publication | HL0 | HL1-2 | HL3 | HL0 | HL1-2 | HL3 | HL0 | HL1-2 | HL3 | (Refs.) |
|---|
| Haggitt et
al, 1985 | 0/18 (0.0) | 0/9 (0.0) | 0/4 (0.0) | - | - | - | 0/65 (0.0) | 0/26 (0.0) | 1/10 (10.0) | (13) |
| Ueno et al,
2004 | - | 0/42 (0.0) | 6/24 (25.0) | - | - | - | - | - | - | (11) |
| Kitajima et
al, 2004 | - | 3/53 (5.7) | 7/88 (8.0) | - | - | - | - | - | - | (25) |
| Matsuda et
al, 2011 | - | 0/101 (0.0) | 8/129 (6.2) | - | 0/219 (0.0) | 1/121 (0.8) | - | - | - | (29) |
| Asayama et
al, 2016 | - | 1/78 (1.3) | 3/98 (3.1) | - | 0/78 (0.0) | 1/98 (1.0) | - | 0/78 (0.0) | 0/98 (0.0) | (28) |
| Kimura et
al, 2016 | - | 4/30 (13.3) | 5/46 (10.9) | - | - | - | - | - | - | (30) |
| Present study | 0/37 (0.0) | 0/28 (0.0) | 0/8 (0.0) | 0/37 (0.0) | 1/28 (0.0) | 0/8 (0.0) | 0/37 (0.0) | 0/28 (0.0) | 0/8 (0.0) | |
| Total | 0/55 (0.0) | 8/341 (2.3) | 29/397 (7.3) | 0/37 (0.0) | 1/325 (0.3) | 2/227 (0.9) | 0/102 (0.0) | 0/132 (0.0) | 1/116 (0.9) | |
In the present study, notably, recurrence was
observed in 1 case of pTis/HL1 without lymphovascular invasion by
pathological inspection. Both pT staging by the JSCCR rule and the
Haggitt classification were unable to help predict recurrence in
this case, emphasizing that there is no method which can be used to
completely predict adverse outcomes. The estimated risk level by
pathological inspection can only be used as a basis for deciding
whether to perform additional resections. Despite its shortcomings,
it can be considered that the Haggitt classification may be more
useful than the JSCCR rule as it can help avoid any unnecessary
additional surgical procedures.
Finally, there are some interesting studies on the
SM depth as a prognostic factor in eCRC. According to the study
‘The stratification of risk factors for the metastasis of pT1b SM
cancer (SM invasion more than 1,000 µm)’ by JSCCR (33), the incidence of lymph node metastasis
was 1.4% in cases wherein only SM invasion depth did not satisfy
the criteria for radical cure and where no other risk factors for
metastasis were observed. On the other hand, even if surgery for T1
eCRC was carried out at first, the incidence of metastatic
recurrence was 1.5% for colon and 4.2% for rectum (34). These studies suggest that surgery
performed due to the presence of only deep SM invasion may have
only restricted merit on prognosis.
The present study has some limitations. Firstly, the
present study was retrospective and was performed at two
institutions in the northern Kanto Region in Japan. Therefore, the
numbers of events were small, with potential ethnic bias. Secondly,
cases that were endoscopically resected were included, but those
which underwent surgery without endoscopic resection were not.
Therefore, the lymph node metastasis rate may have been
underestimated.
In conclusion, using the JSCCR rule to evaluate
pedunculated eCRC may cause pT1 overstaging and unnecessary
additional surgery. Other reference lines for measuring the SM
depth, such as the Haggitt line and MM of the underlying bowel
wall, should be reconsidered. Otherwise, pT should be excluded from
the risk assessment when considering additional surgery in
pedunculated eCRC cases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YI, YO, TY and MI conceived and designed the study.
YI and SS performed the pathological diagnosis. YI analyzed the
data and wrote the manuscript. YO, TT, TY, HS and MI acquired the
clinicopathological data. YI, YO, and MI confirm the authenticity
of all the raw data. All authors critically revised the manuscript,
and have read and approved the final version to be published.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committees of Ota Memorial Hospital (Ota, Japan; approval no.
OR24029) and International University of Health and Welfare
(Nasushiobara, Japan; approval no. FK-94). Due to the retrospective
study design and anonymization of data, consent to participate was
not necessary.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morson BC, Whiteway JE, Jones EA, Macrae
FA and Williams CB: Histopathology and prognosis of malignant
colorectal polyps treated by endoscopic polypectomy. Gut.
25:437–444. 1984.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fujimori T, Kawamata H and Kashida H:
Precancerous lesion of the colorectum. J Gastroenterol. 36:587–594.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cooper HS: Surgical pathology of
endoscopically removed malignant polyps of the colon and rectum. Am
J Surg Pathol. 7:613–623. 1983.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kyzer S, Bégin LR, Gordon PH and Mitmaker
B: The care of patients with colorectal polyps that contain
invasive adenocarcinoma. Cancer. 70:2044–2050. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Minamoto T, Mai M, Ogino T, Sawaguchi K,
Ohta T, Fujimoto T and Takahashi Y: Early invasive colorectal
carcinomas metastatic to the lymph node with attention to their
nonpolypoid development. Am J Gastroenterol. 88:1035–1039.
1993.PubMed/NCBI
|
|
6
|
Tanaka S, Haruma K, Teixeira CR, Tatsuta
S, Ohtsu N, Hiraga Y, Yoshihara M, Sumii K, Kajiyama G and
Shimamoto F: Endoscopic treatment of submucosal invasive colorectal
carcinoma with special reference to risk factors for lymph node
metastasis. J Gastroenterol. 30:710–717. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Japanese Society for Cancer of the Colon
and Rectum. Japanese Classification of Colorectal, Appendiceal, and
Anal Carcinoma: The 3d English Edition [Secondary Publication]. J
Anus Rectum Colon. 3:175–195. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Participants in the Paris Workshop. The
Paris endoscopic classification of superficial neoplastic lesions:
Esophagus, stomach, and colon: November 30 to December 1, 2002.
Gastrointest Endosc. 58:S3–43. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Berman JM, Cheung RJ and Weinberg DS:
Surveillance after colorectal cancer resection. Lancet.
355:395–399. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ueno H, Mochizuki H, Hashiguchi Y,
Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H,
Ozawa K, et al: Risk factors for an adverse outcome in early
invasive colorectal carcinoma. Gastroenterology. 127:385–394.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tanaka S, Haruma K, Oh-E H, Nagata S,
Hirota Y, Furudoi A, Amioka T, Kitadai Y, Yoshihara M and Shimamoto
F: Conditions of curability after endoscopic resection for
colorectal carcinoma with submucosally massive invasion. Oncol Rep.
7:783–788. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haggitt RC, Glotzbach RE, Soffer EE and
Wruble LD: Prognostic factors in colorectal carcinomas arising in
adenomas: Implications for lesions removed by endoscopic
polypectomy. Gastroenterology. 89:328–336. 1985.PubMed/NCBI View Article : Google Scholar
|
|
14
|
ASGE Standards of Practice Committee.
Fisher DA, Shergill AK, Early DS, Acosta RD, Chandrasekhara V,
Chathadi KV, Decker GA, Evans JA, Fanelli RD, et al: Role of
endoscopy in the staging and management of colorectal cancer.
Gastrointest Endosc. 78:8–12. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon
T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P,
Bialek A, et al: Endoscopic submucosal dissection: European society
of gastrointestinal endoscopy (ESGE) guideline. Endoscopy.
7:829–854. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Resch A and Langner C: Risk assessment in
early colorectal cancer: Histological and molecular markers. Dig
Dis. 33:77–85. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Backes Y, Elias SG, Groen JN, Schwartz MP,
Wolfhagen FHJ, Geesing JMJ, Ter Borg F, van Bergeijk J, Spanier
BWM, de Vos Tot Nederveen Cappel WH, et al: Histologic factors
associated with need for surgery in patients with pedunculated T1
colorectal carcinomas. Gastroenterology. 154:1647–1659.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cubiella J, González A, Almazán R,
Rodríguez-Camacho E, Rodiles JF, Ferreiro CD, Sandoval CT, Gómez
CS, de Vicente Bielza N, Lorenzo IP, et al: pT1 colorectal cancer
detected in a colorectal cancer mass screening program: Treatment
and factors associated with residual and extraluminal disease.
Cancers (Basel). 12(2530)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Knoblauch M, Kühn F, von
Ehrlich-Treuenstätt V, Werner J and Renz BW: Diagnostic and
therapeutic management of early colorectal cancer. Visc Med.
39:10–16. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nagtegaal ID, Arends MJ and Salto-Tellez
M: Colorectal Adenocarcinoma. In: WHO classification of
tumours/Digestive system tumours. 5th edition. WHO Classification
of Tumours Editorial Board. IARC Press, Lyon, pp177-187, 2019.
|
|
21
|
Union for International Cancer Control
(UICC): Colon and Tectum. In: TNM classification of malignant
tumours. 8th edition. Brierley JD, Gospodarowicz MK and Wittekind C
(eds). Wiley Blackwell, Oxford, pp84-88, 2017.
|
|
22
|
Ohno K, Fujimori T, Okamoto Y, Ichikawa K,
Yamaguchi T, Imura J, Tomita S and Mitomi H: Diagnosis of
desmoplastic reaction by immunohistochemical analysis, in biopsy
specimens of early colorectal carcinoma, is efficacious in
estimating the depth of invasion. Int J Mol Sci. 14:13129–13136.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gascard P and Tlsty TD:
Carcinoma-associated fibroblasts: Orchestrating the composition of
malignancy. Genes Dev. 30:1002–1019. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ueno H, Kajiwara Y, Ajioka Y, Sugai T,
Sekine S, Ishiguro M, Takashima A and Kanemitsu Y:
Histopathological atlas of desmoplastic reaction characterization
in colorectal cancer. Jpn J Clin Oncol. 51:1004–1012.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kitajima K, Fujimori T, Fujii S, Takeda J,
Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et
al: Correlations between lymph node metastasis and depth of
submucosal invasion in submucosal invasive colorectal carcinoma: A
Japanese collaborative study. J Gastroenterol. 39:534–543.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR): JSCCR guidelines 2005 for the treatment of
colorectal cancer (In Japanese). https://www.jsccr.jp/guideline/2005/particular.html.
Accessed on August 16, 2024.
|
|
27
|
Fukuzawa M: Management of early
pedunculated colorectal cancer. Gastroenterol Endosc. 64:2255–2267.
2022.(In Japanese).
|
|
28
|
Asayama N, Oka S, Tanaka S, Nagata S,
Furudoi A, Kuwai T, Onogawa S, Tamura T, Kanao H, Hiraga Y, et al:
Long-term outcomes after treatment for pedunculated-type T1
colorectal carcinoma: A multicenter retrospective study. J
Gastroenterol. 51:702–710. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsuda T, Fukuzawa M, Uraoka T, Nishi M,
Yamaguchi Y, Kobayashi N, Ikematsu H, Saito Y, Nakajima T, Fujii T,
et al: Risk of lymph node metastasis in patients with pedunculated
type early invasive colorectal cancer: A retrospective multicenter
study. Cancer Sci. 102:1693–1697. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kimura YJ, Kudo S, Miyachi H, Ichimasa K,
Kouyama Y, Misawa M, Sato Y, Matsudaira S, Oikawa H, Hisayuki T, et
al: ‘Head invasion’ is not a metastasis-free condition in
pedunculated T1 colorectal carcinoma based on the precise
histopathological assessment. Digestion. 94:166–175.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hassan C, Wysocki PT, Fuccio L,
Seufferlein T, Dinis-Ribeiro M, Brandão C, Regula J, Frazzoni L,
Pellise M, Alfieri S, et al: Endoscopic surveillance after surgical
or endoscopic resection for colorectal cancer: European society of
gastrointestinal endoscopy (ESGE) and European Society of digestive
oncology (ESDO) Guideline. Endoscopy. 51:266–277. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shaukat A, Kaltenbach T, Dominitz JA,
Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman
D, Syngal S and Rex DK: Endoscopic recognition and management
strategies for malignant colorectal polyps: Recommendations of the
US multi-society task force on colorectal cancer. Gastroenterology.
159:1916–1934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tanaka S, Kashida H, Saito Y, Yahagi N,
Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, et al:
Japan gastroenterological endoscopy society guidelines for
colorectal endoscopic submucosal dissection/endoscopic mucosal
resection. Dig Endosc. 32:219–239. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kobayashi H, Mochizuki H, Morita T, Kotake
K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, et
al: Characteristics of recurrence after curative resection for T1
colorectal cancer: Japanese multicenter study. J Gastroenterol.
46:203–211. 2011.PubMed/NCBI View Article : Google Scholar
|