Introduction
Although blood donation is generally a safe
procedure, undesirable reactions can occur during or after the
collection of whole blood or blood components. The most common
adverse event is a vasovagal reaction (VVR; or vasovagal syncope).
VVRs are considered to be triggered by various physical (e.g.,
standing up after losing 500 ml blood) and psychological stimuli
(e.g., pain, stress and fear) (1).
During a VVR, there is a decrease in the arterial blood pressure
and cerebral perfusion of the donor, which reduces the blood flow
to the brain (2). The highest
frequency of VVRs in whole blood donors occurs during needle
removal and when leaving the donation chair (3). A small proportion (9-12%) of reported
VVRs occurs after the donor leaves the center. However, the number
of off-site reactions is often underestimated due to underreporting
by donors (4). The symptoms of VVRs
include general weakness, dizziness, pallor, sweating, anxiety and
nausea; additionally, some donors may experience more severe
symptoms, such as a transient loss of consciousness (syncope),
convulsions, or incontinence (5).
Fainting and falling can result in accidental injuries. VVRs also
prevent individuals from donating again, decreasing the likelihood
of repeat donations by >50% (6,7).
VVRs affect donor safety and future donation
behavior. They also negatively affect donor waiting times,
appointment management and the number of completed blood
collections (7). Therefore, risk
factors for VVR need to be identified, and preventive policies need
to be developed. The present study aimed to investigate the
frequency and causes of VVR in whole blood donors at the
Transfusion Center, Faculty of Medicine, Necmettin Erbakan
University.
Subjects and methods
Donor information
A total of 742 donors who applied for whole blood
donation at the Necmettin Erbakan University Transfusion Center
(Konya, Turkey) were included in the study. The present study was
conducted between September and October, 2024. Donor eligibility
was evaluated according to the National Blood and Blood Component
Preparation, Use, and Quality Assurance Guide (8). All donors were provided with
information about the donation process prior to the donation.
Potential complications were explained. At this stage, those who
decided to withdraw from blood donation were not pressured to
continue. Following the donation, donors were taken to the rest
area where they were served fruit juice and encouraged to sit and
rest for 15 min. In cases of VVR, necessary interventions were
performed by the doctor. To assess the health status of the donors
and determine their eligibility for blood donation, blood pressure,
body temperature, and pulse rate were measured prior to the
donation.
An average of 450±45 cc of whole blood was collected
from the donors. Donor age, sex, comorbidities, fasting status,
time since last meal, blood donation history, leukocyte count,
hemoglobin level and platelet count were recorded. The frequency
and timing of VVRs were determined, and the association between
VVRs and the donor characteristics was analyzed. The present study
was approved by the Necmettin Erbakan University, Ethics Committee
for Non-Pharmaceutical and Non-Medical Device Research (2024-5381).
Written consent was obtained from the participants.
Statistical analysis
Statistical analysis was performed using the SPSS
IBM program, version 23.0 (IBM Corp.). The distribution
characteristics of continuous variables were evaluated using the
Kolmogorov-Smirnov test. Descriptive statistics for normally
distributed data are expressed as the mean ± standard deviation,
while non-normally distributed data are presented as the median
(range). Group comparisons for continuous variables were conducted
using the Mann-Whitney U test. Categorical variables are expressed
as percentages (%) and compared using the Fisher's test. Values of
P<0.05 were considered to indicate a statistically significant
difference.
Results
A total of 742 blood donors were evaluated. The
median age of the donors was 36 years (range, 18-67 years), with 69
(9.8%) female and 673 (90.2%) male donors. The median age of the
donors was similar between the male and female donors (35 years vs.
36 years).
VVR was detected in 37 (4.9%) donors. A total of 7
out of 62 females (10.1%) and 30 out of 643 males (4.45%) developed
VVR, with no significant difference. The median age of the donors
who experienced VVR was significantly lower than that of those who
did not (P=0.04). Of note, 16% of the donors (119 individuals) were
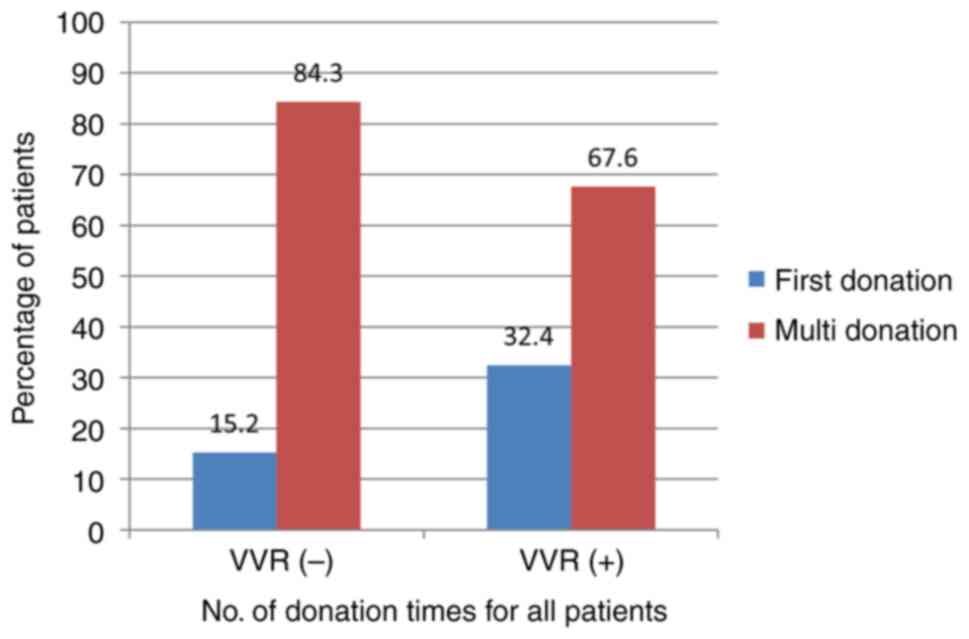
first-time donors. VVRs developed in 10% (12 individuals) of the
first-time donors. First-time blood donors had a higher incidence
of VVR (32.4% vs. 15.2%, P=0.010) (Fig.
1). The median blood donation rate for donors who experienced
VVRs was 2, compared to 4 for those who did not, and this
difference was statistically significant (P<0.001) (Table I). No significant effects of fasting
status, sex, comorbidities, leukocyte count, hemoglobin,
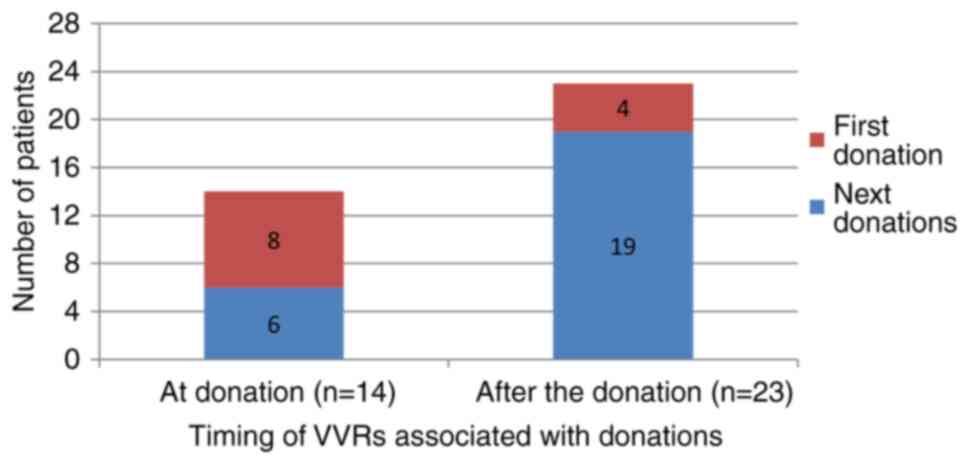
hematocrit, or platelet count on VVRs were found (Table I). As regards the timing of VVRs,
among the 37 donors who experienced VVRs, 14 (37.8%) had it during
the donation, and 23 (62.2%) after the donation. First-time donors
had a higher rate of VVR during the donation (57.1% vs. 17.4%,
P=0.027) (Table I and Fig. 2).
 | Table IEpidemiological and laboratory
comparison of the donors with and without VVRs. |
Table I
Epidemiological and laboratory
comparison of the donors with and without VVRs.
| Parameter | VVR (-) (n:705) | VVR (+) (n:37) | P-value |
|---|
| Sex, n (%) | | | 0.072a |
|
Female | 62 (8.8) | 7 (18.9) | |
|
Male | 643 (91.2) | 30 (81.1) | |
| Additional disease,
n (%) | | | NSa |
|
Yes | 18 (2.6) | 0 | |
|
No | 687 (97.4) | 37(100) | |
| Fasting status, n
(%) | | | NSa |
|
Yes | 11 (1,6) | 0 | |
|
No | 694 (98.4) | 37 | |
| Donation
history | | |
0.010a |
|
First-time | 107 (15.2) | 12 (32.4) | |
|
Repeat | 598 (84.8) | 25 (67.6) | |
| Age (median,
range) | 36 (18-67) | 30 (18-56) |
0.04b |
| Time since last
meal (h) | 2 (0.5-12) | 1 (0.5-4) | 1.29b |
| No. of blood
donations | 4 (0-80) | 2 (0-20) |
<0.001b |
| Leukocyte count
(mm3) | 7,745
(4,100-11,800) | 7,550
(5,400-12,500) | 0.611b |
| Hemoglobin
(g/dl) | 15.3 (12,4-18) | 15 (12.5-17.5) | 0.072b |
| Hematocrit (%) | 44.6
(36.1-52.1) | 43.6
(28.2-50.9) | 0.064b |
| Platelet count
(x103/µl) | 277.5
(112-475) | 294 (122-393) | 0.79b |
| No. of donation
times, n (%) | | | 0.010a |
|
First
donation | 107 (15.2) | 12 (32.4) | |
|
Multiple
donations | 598 (84.3) | 25 (67.6) | |
| The timing of
VVRsc, n (%) | | | 0.027a |
|
During the
donation (n=14) | | | |
|
First
donation | | | |
|
Multiple
donations | | 8 (57.1) | |
|
After the
donation (n=23) | | 6 (42.9) | |
|
First
donation | | | |
|
Multiple
donations | | 4 (17.4) | |
| | | 19 (82.6) | |
Discussion
In blood donation, moderate VVRs occur in 1.4 to 7%
of individuals, and severe VVRs occur in 0.1 to 0.5% of cases
(4,9-18).
The present study found a VVR rate of 4.9%, which is similar to
that observed in the literature. The hospitalization rate due to
blood donation complications has been reported as 1/198,000, with
two-thirds of these events associated with VVRs. Falls leading to
injuries have been found to be the most significant cause in female
donors (4). None of the donors in
the present study required hospitalization.
The following risk factors have been shown to be
associated with an increased risk of VVRs: The female sex (7,19,20), a
low body weight (21,22), a low estimated blood volume (9,20,23), a
younger age (7,9,19,23),
first-time donation (7,19,22,23), a
low resting blood pressure (21),
insufficient sleep prior to donation (24,25),
donation site (23) and a history of
symptoms during previous donations (26,27). The
frequency of VVRs has been found to be higher in first-time, young
and female donors (21,28,29).
Additionally, donors who experienced VVRs had a significantly lower
total blood donation count. This may be due to regular donations
from those who did not experience VVRs in previous donations, while
those who experienced VVRs tend to avoid further donations. Unlike
other studies, the female sex was not found to be a risk factor for
VVRs in the present study, possibly due to the smaller number of
female donors. The primary reason for the low number of female
donors is iron deficiency anemia, which develops in menstruating
women. Iron deficiency affects 29 to 58% of healthy females of
reproductive age, while the prevalence of iron deficiency anemia
ranges from 27 to 47% (30-32).
Another reason is the reluctance to select women who have had
pregnancies as donors in Turkey. The female donor rate and the
frequency of VVR occurrence among female donors in different
countries is presented in Table II.
The frequency of female donors varies greatly across countries.
Upon reviewing these studies, it appears that factors, such as the
level of development of the countries and religious beliefs play a
prominent role in influencing the rate of female donors. Similar to
these countries, in the authors' country, Turkey, the
socio-cultural situation may be another main reason for women
donating less in certain segments of society.
 | Table IIIncidence of vasovagal reactions in
blood donation in different countries worldwide. |
Table II
Incidence of vasovagal reactions in
blood donation in different countries worldwide.
| Country (year of
publication) | Sample size | % of bood donation
in females | % of females
developing VVR | % of blood donation
in first donors | % of VVR improving
in first-time donors | Incidence of VVR
(%) | (Refs.) |
|---|
| USA (2008) | 422,231 | 57.9 | 1.86 | 24.6 | 2.75 | 1.43 | (9) |
| USA (2010) | 793,293 | 48 | 0.65 | 21 | 0.89 | 0.41 | (4) |
| Saudi Arabia
(2017) | 18,936 | 1.4 | 1.2 | 47 | 1.6 | 1.1 | (12) |
| Italy (2009) | 183,855 | 8.6 | | 19.5 | | 0.19 | (13) |
| India (2014) | 88,201 | 3.9 | | 35.8 | | 1.23 | (14) |
| Pakistan
(2016) | 41,579 | 0.2 | 0 | | | 1.03 | (15) |
| Germany (2015) | 928,411 | 47.8 | | 8.2 | 2.78 | 0.76 | (16) |
| Iran (2011) | 5,285 | 4.4 | 8.6 | 18 | 5.6 | 2 | (17) |
| Brazil (2012) | 724,861 | 30 | 3.39 | 31.5 | 4.28 | 2.2 | (18) |
| Turkey (2025) | 742 | 9.8 | 10.1 | 16 | 10 | 4.9 | Present study |
Setting weight limits for blood donation is critical
for protecting donors from adverse effects, particularly vasovagal
episodes and anemia. It has been shown that a low body weight and
low blood volume are independent predictors of VVRs (10,21).
Trouern-Trend et al (21)
demonstrated that a low body weight increases the risk of
developing VVRs. They demonstrated that VVRs developed in 4.6% of
individuals with a body weight <120 lb and in 0.4% of those with
a body weight >210 lb (21).
Another study found that body weight was a significant determinant
of the rate of vasovagal reactions in first-time blood donors
(22). In general, it is accepted
that the total volume of donated blood should not exceed 13% of the
blood volume of the donor. For example, a donor must weigh at least
45 kg to donate 350 ml (±10%) of blood, and at least 50 kg to
donate 450 ml (±10%) of blood (38).
There is no specific upper weight limit for blood donation. In
Turkey, the donor selection criteria require that the donor weighs
>50 kg (8).
Psychological stresses, such as pre-donation sleep
duration, a history of fainting epidodes, anxiety traits, a fear of
blood and injury, and a fear of needles have been associated with
VVRs (24,34-37).
In their study, Takanashi et al (24) compared the records of 4,924 Japanese
donors who experienced VVR with a control group of 43,948 donors
who did not experience any such complications which were related to
donation. As observed in their study, for both male and female
donors, factors such as being a first-time donor, having a
pre-donation pulse rate of ≥90 beats per minute, a diastolic blood
pressure ≤70 mmHg, <6 h of sleep (compared to >8 h of sleep),
not eating within the previous 4 h, and factors such as age, sex,
body mass index, pulse rate and systolic blood pressure were all
significantly associated with an increased risk of having VVRs
(24).
The underlying mechanisms of VVRs include the
orthostatic effects of hypovolemic static status (i.e., a decrease
in blood pressure when standing up following the donation) and
psychological stress related to the procedure (e.g., pain
associated with needle insertion or phlebotomy) (38). Due to the complexity of the
mechanisms involved, current prevention strategies target both the
physiological and psychological aspects of the reaction (39).
Various strategies have been proposed to prevent
VVRs. For example, the World Health Organization (WHO) recommends
pre-donation hydration and the application of active muscle tension
(AMT) to increase blood flow and blood pressure. The EU Domaine
Project supports pre-donation hydration and AMT, as well as
caffeine loading, distraction techniques, supportive care, and
educational materials (40-42).
Blood services worldwide have adopted various strategies to prevent
VVRs, with water loading and AMT being the most common (43,44).
Compared to the empirical literature focusing on
pre-donation hydration and AMT, only a limited number of studies
have addressed the psychological aspects of the donation and their
association with vasovagal symptoms. Studies evaluating
psychological methods primarily focus on reducing stress or anxiety
by providing distraction or social support during the procedure
(39). In a previous study, the
effectiveness of audiovisual distraction for first-time donors was
investigated by classifying individuals based on their coping
styles as monitoring (attending to the situation) or blunting
(distracting, denying, or reinterpreting the situation) (45). Donors with blunting coping styles who
were provided with a distracting environment reported significantly
lower self-reported vasovagal symptoms compared to participants in
the untreated control condition (45). Hanson and France (46) demonstrated that donors who were
accompanied by a trained research assistant for support had lower
VVR levels and were more willing to donate again compared to donors
who did not receive support.
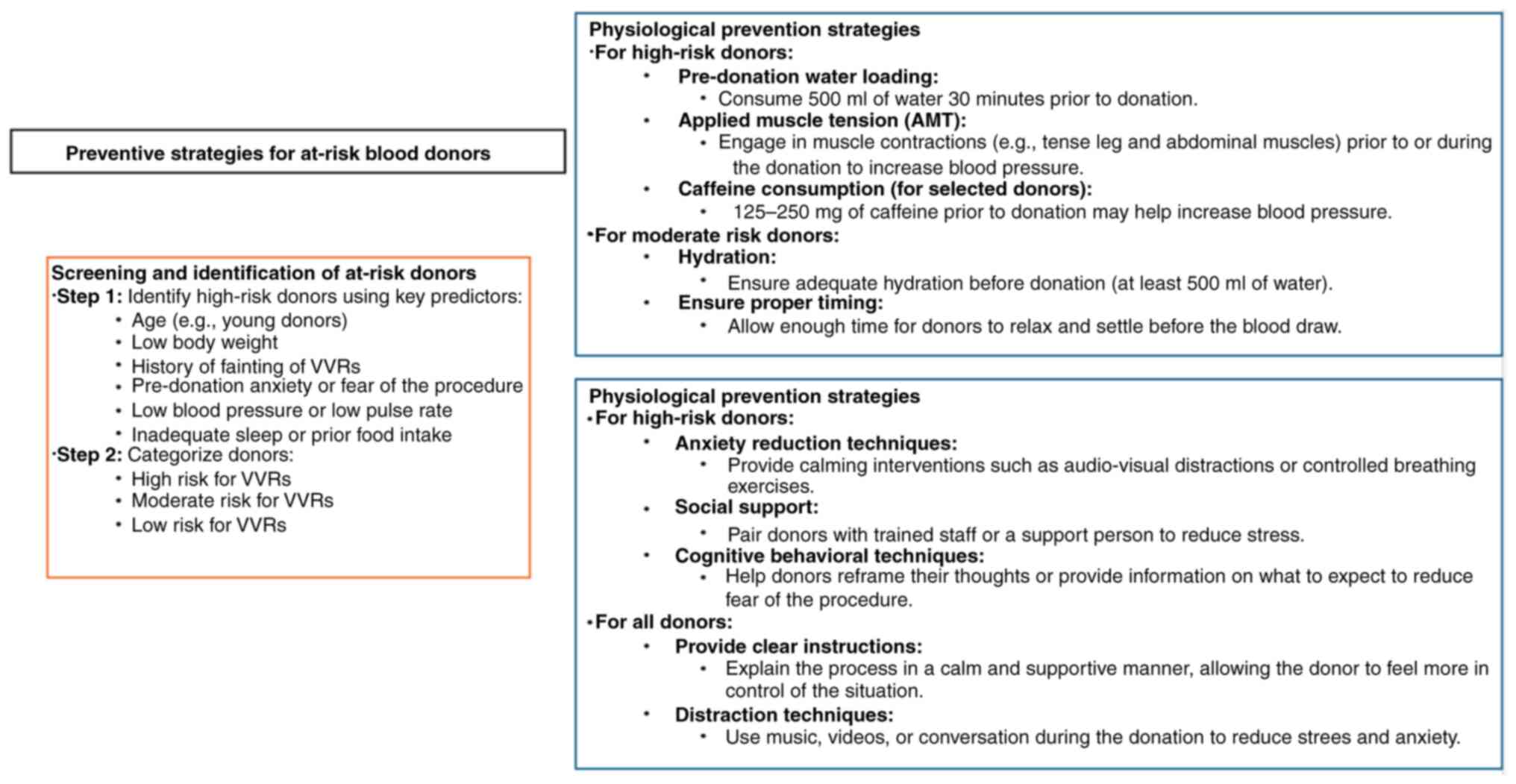
VVRs can be minimized with simple precautions. First
and foremost, donor education is essential. Donors should be
advised to avoid coming on an empty stomach, refrain from smoking
before and after donation, avoid standing up immediately after
donation (and instead sit up first), and ensure adequate fluid
intake before and/or after donation. These measures will prevent
the majority of cases of VVRs. However, despite these precautions,
VVRs may still occur, particularly in first-time blood donors, due
to psychological stress. Donors who exhibit signs of psychological
stress should receive psychological support before proceeding with
blood donation. Therefore, blood donation centers should have
trained personnel capable of providing psychological support.
Another potential factor in reducing VVR is ensuring that the blood
donation environment is spacious, clean, well-ventilated, and
comfortable. Additionally, a friendly attitude from the staff,
effective communication, reassuring the donor, and good technical
skills are important factors in reducing VVR (Fig. 3).
A limitation of the present study is the lack of the
evaluation of body weight, blood pressure, body temperature, oxygen
saturation and psychological stress levels prior to donation. These
factors could provide valuable insight into the overall health and
well-being of donors, potentially affecting the donation process
and outcomes. In future studies on this topic, the inclusion of
these parameters would enhance the value of the research and offer
a more comprehensive understanding of the factors influencing
donation. Another limitation of the present study is the
significantly lower number of females compared to males. However,
this is not a choice of the authors, but rather a result of the
general tendency of females in Turkey to donate blood.
As a result, in the present study, the frequency of
VVRs was found to be 4.9%, and it was observed that VVRs developed
more frequently in younger individuals and first-time donors.
Although numerous intervention studies have evaluated the
effectiveness of physiological and psychological methods in
preventing VVRs and/or vasovagal symptoms, questions regarding the
efficacy of these techniques remain. A reasonable approach may be
to apply both physiological and psychological preventive methods to
young first-time donors to prevent them from experiencing VVRs.
This is due to the fact that those who experience VVRs during their
first blood donation tend to avoid donating again, which leads to a
decrease in the number of blood donors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD was a main contributor to the conception of the
study, as well as to the literature search for related studies. SD,
HD and ATe were involved in the literature review, in the writing
of the manuscript, and in the analysis and interpretation of the
patient data. ATü and ATo collected data. HD and Ate confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Necmettin
Erbakan University, Ethics Committee for Non-Pharmaceutical and
Non-Medical Device Research (2024-5381). Written consent was
obtained from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilchrist PT and Ditto B: Sense of
impending doom: Inhibitory activity in waiting blood donors who
subsequently experience vasovagal symptoms. Biol Psychol.
104:28–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wieling W, Thijs RD, van Dijk N, Wilde
AAM, Benditt DG and van Dijk JG: Symptoms and signs of syncope: A
review of the link between physiology and clinical clues. Brain.
132:2630–2642. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tomasulo P, Bravo M and Kamel H: Time
course of vasovagal syncope with whole blood donation. ISBT Sci
Ser. 5:52–58. 2010.
|
|
4
|
Kamel H, Tomasulo P, Bravo M, Wiltbank T,
Cusick R, James RC and Custer B: Delayed adverse reactions to blood
donation. Transfusion (Paris). 50:556–565. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wiersum-Osselton JC, Marijt-van der Kreek
T, Brand A, Veldhuizen I, van der Bom JG and de Kort W: Risk
factors for complications in donors at first and repeat whole blood
donation: A cohort study with assessment of the impact on donor
return. Blood Transfus. 12 (Suppl 1):S28–S36. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rader AW, France CR and Carlson B: Donor
retention as a function of donor reactions to whole-blood and
automated double red cell collections. Transfusion (Paris).
47:995–1001. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
France CR, Rader A and Carlson B: Donors
who react may not come back: Analysis of repeat donation as a
function of phlebotomist ratings of vasovagal reactions. Transfus
Apher Sci. 33:99–106. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
National Guide for Blood and Blood
Components Preparation, Usage and Quality Assurance. Republic of
Turkey, Ministry of Health, 2016. Available from: https://shgmkanhizmetleridb.saglik.gov.tr/TR,71523/ulusal-kan-ve-kan-bilesenleri-hazirlama-kullanim-ve-kalite-guvencesi-rehberi-2016.html.
|
|
9
|
Wiltbank TB, Giordano GF, Kamel H,
Tomasulo P and Custer B: Faint and prefaint reactions in
whole-blood donors: An analysis of predonation measurements and
their predictive value. Transfusion. 48:1799–1808. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin JT, Ziegler DK, Lai C and Bayer W:
Convulsive syncope in blood donors. Ann Neurol. 11:525–528.
1982.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Newman BH: Blood donor complications after
whole-blood donation. Curr Opin Hematol. 11:339–345.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Almutairi H, Salam M, Alajlan A, Wani F,
Al-Shammari B and Al-Surimi K: Incidence, predictors and severity
of adverse events among whole blood donors. PLoS One.
12(e0179831)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crocco I, Franchini M, Garozzo G, Gandini
AR, Gandini G, Bonomo P and Aprili G: Adverse reactions in blood
and apheresis donors: Experience from two Italian transfusion
centres. Blood Transfus. 7:35–38. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Philip J, Sarkar R and Jain N: A
single-centre study of vasovagal reaction in blood donors:
Influence of age, sex, donation status, weight, total blood volume
and volume of blood collected. Asian J Transfus Sci. 8:43–46.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sultan S, Baig MA, Irfan SM, Ahmed SI and
Hasan S: Adverse reactions in allogeneic blood donors: A tertiary
care experience from a developing country. Oman Med J. 31:124–128.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Burkhardt T, Dimanski B, Karl R, Sievert
U, Karl A, Hübler C, Tonn T, Sopvinik I, Ertl H and Moog R: Donor
vigilance data of a blood transfusion service: A multicenter
analysis. Transfus Apher Sci. 53:180–184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Assarian Z, Haghighi BA, Javadi I, Fotouhi
A, Seighali F and Akbari N: Risk factors for vasovagal reactions
during blood donation. Sci J Iran Blood Transfus Organ. 7:221–226.
2011.
|
|
18
|
Gonçalez TT, Sabino EC, Schlumpf KS,
Wright DJ, Leao S, Sampaio D, Takecian PL, Proietti AB, Murphy E,
Busch M, et al: Vasovagal reactions in whole blood donors at three
REDS-II blood centers in Brazil. Transfusion. 52:1070–1078.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eder AF, Hillyer CD, Dy BA, Notari EP IV
and Benjamin RJ: Adverse reactions to allogeneic whole blood
donation by 16- and 17-year-olds. JAMA. 299:2279–2286.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goldman M, Osmond L, Yi QL, Cameron-Choi K
and O'Brien SF: Frequency and risk factors for donor reactions in
an anonymous blood donor survey. Transfusion. 53:1979–1984.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trouern-Trend JJ, Cable RG, Badon SJ,
Newman BH and Popovsky MA: A case-controlled multicenter study of
vasovagal reactions in blood donors: Influence of sex, age,
donation status, weight, blood pressure, and pulse. Transfusion.
39:316–320. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Newman BH: Vasovagal reaction rates and
body weight: Findings in high- and low-risk populations.
Transfusion. 43:1084–1088. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bravo M, Kamel H, Custer B and Tomasulo P:
Factors associated with fainting-before, during and after whole
blood donation. Vox Sang. 101:303–312. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takanashi M, Odajima T, Aota S, Sudoh M,
Yamaga Y, Ono Y, Yoshinaga K, Motoji T, Matsuzaki K, Satake M, et
al: Risk factor analysis of vasovagal reaction from blood donation.
Transfus Aphere Sci. 47:319–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ke S, Xu P, Xiong J, Xu L, Ma M, Du X and
Yang R: Long-term poor sleep quality is associated with adverse
donor reactions in college students in Central China: A
population-based cross-sectional study. Vox Sang. 118:455–462.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eder AF, Notari EP IV and Dodd RY: Do
reactions after whole blood donation predict syncope on return
donation? Transfusion. 52:2570–2576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rudokaite J, Ong LLS, Ertugrul IO, Janssen
MP and Veld EMJ: Predicting vasovagal reactions to needles with
anticipatory facial temperature profiles. Sci Rep.
13(9667)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Newman BH, Pichette S, Pichette D and
Dzaka E: Adverse effects in blood donors after whole-blood
donation: A study of 1000 blood donors interviewed 3 weeks after
whole-blood donation. Transfusion. 43:598–603. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Newman BH, Satz SL, Janowicz NM and
Siegfried BA: Donor reactions in high-school donors: The effects of
sex, weight, and collection volume. Transfusion. 46:284–288.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Young I, Parker HM, Rangan A, Prvan T,
Cook RL, Donges CE, Steinbeck KS, O'Dwyer NJ, Cheng HL, Franklin JL
and O'Connor HT: Association between haem and non-haem iron intake
and serum ferritin in healthy young women. Nutrients.
10(81)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hwalla N, Al Dhaheri AS, Radwan H, Alfawaz
HA, Fouda MA, Al-Daghri NM, Zaghloul S and Blumberg JB: The
prevalence of micronutrient deficiencies and inadequacies in the
Middle East and approaches to interventions. Nutrients.
9(229)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Di Santolo M, Stel G, Banfi G, Gonano F
and Cauci S: Anemia and iron status in young fertile
non-professional female athletes. Eur J Appl Physiol. 102:703–709.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Blood Donor Selection: Guidelines on
assessing donor suitability for blood donation. World Health
Organization, p118, 2013.
|
|
34
|
Meade MA, France CR and Peterson LM:
Predicting vasovagal reactions in volunteer blood donors. J
Psychosom Res. 40:495–501. 1996.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ditto B and France CR: Vasovagal symptoms
mediate the relationship between predonation anxiety and subsequent
blood donation in female volunteers. Transfusion. 46:1006–1010.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ditto B, Gilchrist PT and Holly CD:
Fear-related predictors of vasovagal symptoms during blood
donation: It's in the blood. J Behav Med. 35:393–399.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
France CR, France JL, Himawan LK, Stephens
KY, Frame-Brown TA, Venable GA and Menitove JE: How afraid are you
of having blood drawn from your arm? A simple fear question
predicts vasovagal reactions without causing them among high school
donors. Transfusion. 53:315–321. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wieling W, France CR, van Dijk N, Kamel H,
Thijs RD and Tomasulo P: Physiologic strategies to prevent fainting
responses during or after whole blood donation. Transfusion.
51:2727–2738. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thijsen A and Masser B: Vasovagal
reactions in blood donors: Risks, prevention and management.
Transfus Med. 29 (Suppl 1):S13–S22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
World Health Organization. (2012). Blood
Donor Selection: Guidelines on Assessing Donor Suitability for
Blood Donation. World Health Organization. Available from:
https://iris.who.int/handle/10665/76724.
|
|
41
|
De Kort WVI: Donor management manual.
DOMAINE project c/o Sanquin Blood Supply Foundation. 2010.
Available from: https://www.sanquin.org/binaries/content/assets/en/research/donor-studies/en-donor-mange
ment-manual-part-1.pdf. Accessed February 6, 2023.
|
|
42
|
Joint United Kingdom (UK) Blood
Transfusion and Tissue Transplantation Services Professional
Advisory Committee. Guidelines for the Blood Transfusion Services.
2013. Available from: https://www.transfusionguid elines.org/dsg. Accessed
February 6, 2023.
|
|
43
|
Tips For A Successful Blood Donation | Red
Cross Blood Services. Available from: https://www.redcrossblood.org/donate-blood/blood-donat
ion-process/before-during-after.html. Accessed February 6,
2023.
|
|
44
|
Blood donation process at Canadian Blood
Services. Available from: https://www.blood.ca/en/blood/donating-blood/donation-process.
Accessed February 6, 2023.
|
|
45
|
Bonk VA, France CR and Taylor BK:
Distraction reduces self-reported physiological reactions to blood
donation in novice donors with a blunting coping style. Psychosom
Med. 63:447–452. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hanson SA and France CR: Social support
attenuates presyncopal reactions to blood donation. Transfusion.
49:843–850. 2009.PubMed/NCBI View Article : Google Scholar
|