Introduction
In 1992, Hashimoto et al (1) coined the term transient myoclonic state
with asterixis (TMA) in a group of older Japanese individuals
presenting with myoclonus and asterixis occurring at the same time.
In this context, Mizutani et al (2) were the first to describe in the
literature a similar stereotyped myoclonus. Notably, the patient
reported by Mizutani et al (2) had positive serum Epstein-Barr virus
antibodies, which was likely a coincidental finding. Following the
reports by Hashimoto et al (1) and Mizutani et al (2), other patients with transient myoclonic
state (TMS) and no history of asterixis were reported (3). In this context, TMA and TMS represent
different conditions within the same spectrum, which are hereby
collectively referred to as TMS.
Myoclonus has a hyperkinetic phenomenology defined
by jerk-like movements that occur secondary to neuronal discharges
(4). Myoclonus can either present as
physiological jerks or may be associated with some underlying
neurodegenerative disorder (5). It
can originate in the cortex, brainstem, spinal cord or peripheral
nerves. Myoclonus usually persists for a number of years without
any identifiable physiological cause (6). Positive myoclonus are jerks caused by
the activation of a determined group of muscles, whereas negative
myoclonus, also known as asterixis, are jerks that occur due to the
cessation of ongoing muscular activity (4). In addition, positive myoclonus occurs
more commonly, and negative myoclonus occurs mainly in hospital
settings (6).
A small number of patients have presented with TMS,
considered a unique type of myoclonic jerk, as it primarily
involves faciobrachial structures and is characterized by
repetitive and irregular movements. However, lower extremity
involvement has been reported in ~50% of cases (7). A single jerk consisted of a burst of
myoclonic muscle contractions and lasted for a few seconds. These
bursts of myoclonic contractions can be combined with
asterixis-like movements in some patients. TMS is most commonly
reported amongst older patients concomitant with comorbidities,
such as chronic kidney injury, diabetes mellitus and hypertension.
These patients, however, are independently carrying out their
activities of daily living.
A key concern regarding TMS is the limited
understanding of its condition definition (7). TMS presents with classic clinical
symptoms of repetitive bilateral myoclonus lasting for a few
seconds with consciousness fully intact. The syndrome has a benign
prognosis and is usually self-limiting. Despite such characteristic
presentation, the limited knowledge of TMS can lead to the
misdiagnosis of the condition as metabolic encephalopathy or
epilepsy (3). Furthermore, frequent
recurrence of the syndrome has been reported. Therefore, the timely
diagnosis of the condition is of utmost importance so that proper
management can be provided to the patients (8). The present systematic review aimed to
provide critical insight and up-to-date information on the latest
information about the pathophysiology and therapy of TMS.
Data and methods
Study selection
A comprehensive search was conducted across six
major databases to identify all available reports on TMS, published
in electronic format up to November, 2024. The databases, Latin
American and Caribbean Health Sciences Literature (Lilacs)
(https://lilacs.bvsalud.org/en/),
Excerpta Medica (Embase) (https://www.embase.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com/), Scientific
Electronic Library Online (Scielo) (https://www.scielo.org/), ScienceDirect (https://www.sciencedirect.com/) and CiNii Japan
Science and Technology Agency (https://cir.nii.ac.jp/) were searched. The search
terms used were ‘transient myoclonus’, ‘transient myoclonus state’,
‘transient myoclonus state with asterixis’, ‘positive and negative
myoclonus’ (Table I).
 | Table IFree text and medical subject heading
search terms used in the US National Library of Medicine. |
Table I
Free text and medical subject heading
search terms used in the US National Library of Medicine.
| Query | Search term | Results |
|---|
| Transient
myoclonus | (‘transiently’[All
Fields] OR ‘transients and migrants’[MeSH Terms] OR
(‘transients’[All Fields] AND ‘migrants’[All Fields]) OR
‘transients and migrants’[All Fields] OR ‘transient’[All Fields] OR
‘transients’[All Fields]) AND (‘myoclonus’[MeSH Terms] OR
‘myoclonus’[All Fields]) | 273 |
| Transient myoclonus
state | (‘transiently’[All
Fields] OR ‘transients and migrants’[MeSH Terms] OR
(‘transients’[All Fields] AND ‘migrants’[All Fields]) OR
‘transients and migrants’[All Fields] OR ‘transient’[All Fields] OR
‘transients’[All Fields]) AND (‘myoclonus’[MeSH Terms] OR
‘myoclonus’[All Fields]) AND (‘state’[All Fields] OR ‘states’[All
Fields] OR ‘stated’[All Fields] OR ‘states’[All Fields] OR
‘stating’[All Fields]) | 40 |
| Transient myoclonus
state with asterixis | (‘transiently’[All
Fields] OR ‘transients and migrants’[MeSH Terms] OR
(‘transients’[All Fields] AND ‘migrants’[All Fields]) OR
‘transients and migrants’[All Fields] OR ‘transient’[All Fields] OR
‘transients’[All Fields]) AND (‘myoclonus’[MeSH Terms] OR
‘myoclonus’[All Fields]) AND (‘state’[All Fields] OR ‘states’[All
Fields] OR ‘stated’[All Fields] OR ‘states’[All Fields] OR
‘stating’[All Fields]) AND (‘dyskinesias’[MeSH Terms] OR
‘dyskinesias’[All Fields] OR ‘asterixis’[All Fields]) | 16 |
| Positive and
negative myoclonus | (‘positive’[All
Fields] OR ‘positively’[All Fields] OR ‘positiveness’[All Fields]
OR ‘positives’[All Fields] OR ‘positivities’[All Fields] OR
‘positivity’[All Fields]) AND (‘negative’[All Fields] OR
‘negatively’[All Fields] OR ‘negatives’[All Fields] OR
‘negativities’[All Fields] OR ‘negativity’[All Fields]) AND
(‘myoclonus’[MeSH Terms] OR ‘myoclonus’[All Fields]) | 202 |
Inclusion and exclusion criteria
All types of articles, including reports on TMS,
were included. No language restrictions were applied; for
manuscripts that did not provide sufficient information in English,
Google Translate was used (9).
Study selection
The present study was conducted in accordance with
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) 2020 checklist, which provides a structured
framework for systematically identifying, selecting, and
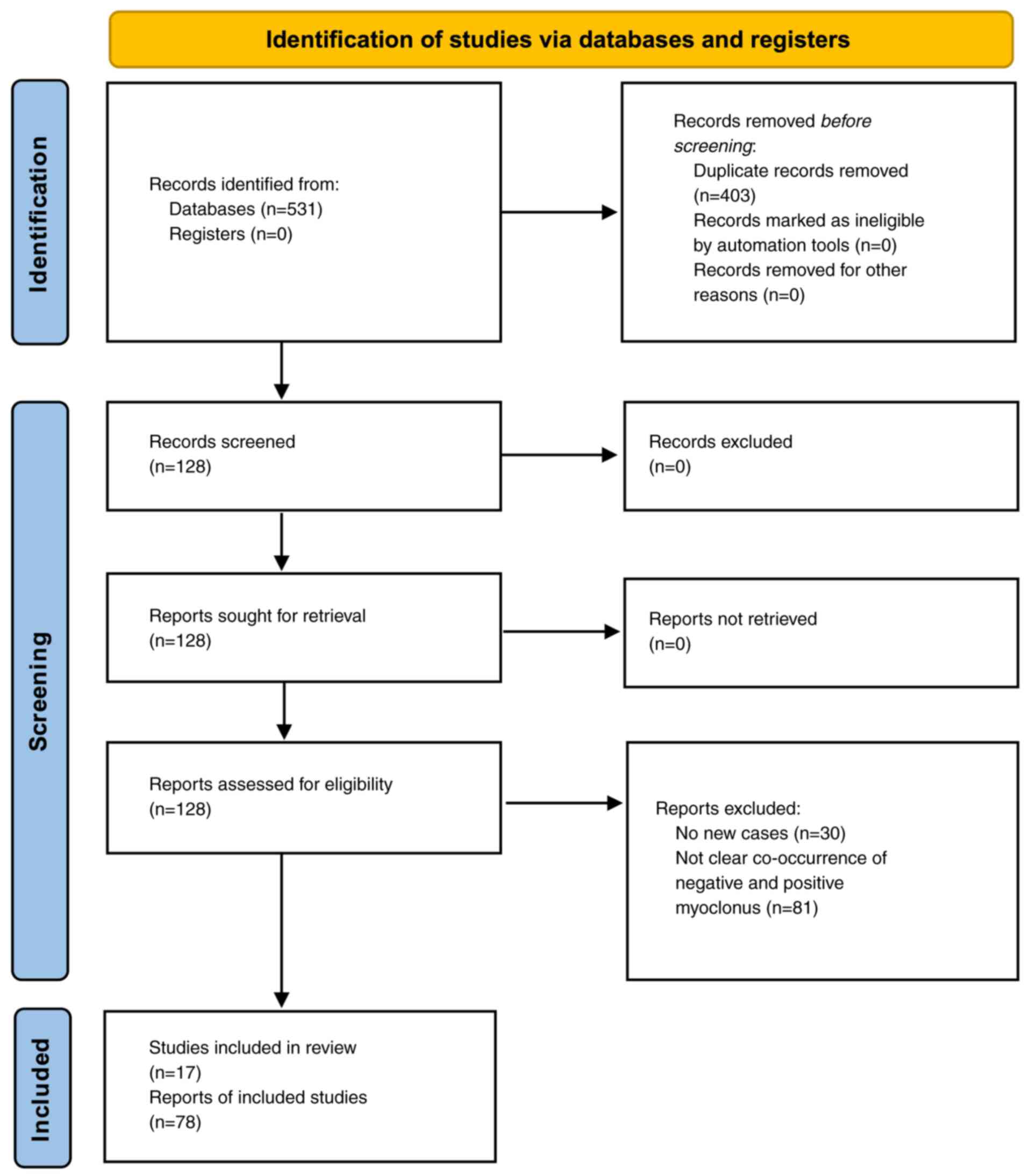
synthesizing relevant studies (Fig.
1) (10). This approach ensures
transparency, reproducibility and a comprehensive assessment of the
available evidence. The assessment of the risk of bias in the
included studies was evaluated with Joanna Briggs Institute (JBI)
Critical Appraisal Checklist for Case Reports (11) (Table
II) (1-3,7,8,12-22).
 | Table IIQuality evaluation of case reports by
the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for
Case Reports. |
Table II
Quality evaluation of case reports by
the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for
Case Reports.
| Author(s), year of
publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | (Refs.) |
|---|
| Mizutani et
al, 1986 | Y | Y | Y | Y | Y | U | Y | N | (2) |
| Hashimoto et
al, 1992 | Y | Y | Y | Y | Y | Y | Y | N | (1) |
| Negoro et
al, 1994 | Y | Y | Y | Y | Y | Y | Y | N | (12) |
| Fujihara et
al, 1995 | Y | Y | Y | Y | Y | Y | Y | N | (13) |
| Tsuchiyama et
al, 1995 | U | U | U | U | U | U | U | N | (23) |
| Hirata et
al, 1997 | Y | Y | Y | Y | Y | Y | Y | N | (14) |
| Iijima et
al, 1997 | Y | Y | Y | Y | Y | U | Y | N | (15) |
| Yamamoto, 1997 | Y | Y | Y | Y | Y | U | Y | N | (16) |
| Kohono et
al, 1998 | Y | Y | Y | Y | Y | Y | Y | N | (17) |
| Okuma et al,
1999 | Y | Y | Y | Y | Y | Y | Y | N | (18) |
| Kawakami, 2007 | N | N | N | N | Y | Y | U | N | (19) |
| Hitomi et
al, 2011 | Y | Y | Y | Y | Y | Y | Y | N | (20) |
| Hiraga et
al, 2014 | Y | Y | Y | Y | Y | Y | Y | N | (7) |
| Sethi, 2014 | N | N | N | N | Y | U | Y | N | (21) |
| Doden et al,
2015 | Y | Y | Y | Y | Y | Y | Y | Y | (3) |
| Umemura et
al, 2015 | Y | Y | N | U | Y | Y | Y | N | (22) |
| Harada et
al, 2024 | Y | N | N | N | Y | Y | Y | N | (8) |
Results
The present study found 17 reports containing 78
cases in the literature about TMS (Table III). Almost all the reports were
from Japan, apart from the one by Sethi (21). The mean age of the patients was 75.67
years (standard deviation, 5.8 years), with a median of 75 years
(range, 54-84 years). Sex was reported for 74 cases, and 60.8% of
the individuals were male. A precipitating factor, such as an
infectious disease or the introduction of a new medication, was
observed in 24 cases (30.7%). All individuals achieved full
recovery; however, 53 patients (67.9%) required benzodiazepine
therapy, while the remaining individuals improved
spontaneously.
 | Table IIILiterature review of reports of
transient myoclonic state. |
Table III
Literature review of reports of
transient myoclonic state.
| Author(s), year of
publication | Prefecture,
country | No. of cases | Age
(years)/sex | Clinical
manifestations | Precipitator | Recovery | Management | (Refs.) |
|---|
| Mizutani et
al, 1986 | Tokyo, Japan | 1 | 84/M | Lasted 2-7 days.
Recurred five times in 2 years. Viral capsid antigen IgG antibodies
for Epstein-Barr virus were positive. | 1 IF | 1 SP | None | (2) |
| Hashimoto et
al, 1992 | Kyoto, Japan | 7 | 72.3 (mean)/ 3 F
and 4 M | Recurrent. | 1 IF, 1 drug | 7 BZD | IM/IV DZP | (1) |
| Negoro et
al, 1994 | Yamaguchi,
Japan | 9 | 70.5 (mean)/ 4 F
and 5 M | Lasted 0.5-4 days.
Recurrent. | 3 IF | 3 SP and 6 BZD | PO CZP | (12) |
| Fujihara et
al, 1995 | Hiroshima,
Japan | 1 | 76/M | Recurrent. | 0 IF | 1 BZD | PO CZP | (13) |
| Tsuchiyama et
al, 1995 | Japan | 1 | NA | NA | NA | 1 BZD | NA | (23) |
| Hirata et
al, 1997 | Hyogo, Japan | 1 | 54/F | No recurrence. | 0 IF | 1 BZD | PO CZP and IM/IV
DZP | (14) |
| Iijima et
al, 1997 | Tokyo, Japan | 1 | 84/F | No recurrence. | 0 IF | 1 SP | None | (15) |
| Yamamoto, 1997 | Saga, Japan | 2 | 67.5 (mean)/ 1 F
and 1 M | Elevated serum
amyloid A protein. Recurrent. | 1 IF | 2 SP | None | (16) |
| Kohono et
al, 1998 | Ibaraki, Japan | 2 | 60.5 (mean)/ 2
M | Recurrent. | 1 IF | 1 BNZ and 1 SP | PO CZP | (17) |
| Okuma et al,
1999 | Tokyo, Japan | 5 | 71.5 (mean)/ 1 F
and 4 M | No recurrence. | 1 IF | 3 SP and 2 BZD | PO CZP, IV/IM
DZP | (18) |
| Kawakami, 2007 | Tochigi, Japan | NA | NA | NA | NA | NA | NA | (19) |
| Hitomi et
al, 2011 | Kyoto, Japan | 6 | 84 (mean)/ 2 F and
4 M | There was no
increase in SEP amplitudes during symptoms, and JLA showed a
positive spike occurring before the myoclonic jerks. | 1 IF | 6 BZD | PO CZP, IM/IV
DZP | (20) |
| Hiraga et
al, 2014 | Chiba, Japan | 11 | 75 (mean)/ 5 F and
6 M | Lasted 1-4
days. | 5 IF | 7 SP and 4 BZD
DZP | PO CZP, IM/IV | (7) |
| Sethi, 2014 | USA | 3 | NA | NA | NA | 3 SP | None | (21) |
| Doden et al,
2015 | Nagano, Japan | 26 | 79.7 (mean)/ 10 F
and 16 M | Lasted days. | 9 IF | 22 BZD and 4
SP | PO CZP, IV/IM
DZP | (3) |
| Umemura et
al, 2015 | Kyoto, Japan | 1 | 79/ M | Iodine-123
iodoamphetamine revealed increased perfusion in the bilateral
primary motor cortex. | NA | 1 BZD | PO CZP | (22) |
| Harada et
al, 2024 | Tokyo, Japan | 1 | 68/ F | NA | NA | 1 BZD | PO CZP and IV/IM
DZP | (8) |
Notably, chronic kidney disease was present in some
cases of TMS. Reported creatinine levels ranged from 2 to 4 mg/dl
(23). Notably, 29% (2 out of 7) of
individuals described by Hashimoto et al (1) had stage 2-3 chronic kidney disease, and
1 patient in another study required dialysis (12).
The patient described by Kawakami (19) had a notable medical history of
cardiac arrest. Electroencephalographic findings were non-specific,
but may suggest hypoxic-ischemic brain injury, including cortical
irritation, neuronal dysfunction, or epileptiform activity
resulting from global hypoxia or ischemia.
A precipitating factor was identified in several
cases of TMS. Cisplatin was reported in 1 case as a precipitating
factor for the occurrence of the myoclonic jerks (1). Another patient received β-blocker and
calcium channel blocker in therapeutic doses 24 h prior to the
occurrence of myoclonic jerks. Initially, the authors considered
drug-induced myoclonus as a potential differential diagnosis for
TMS (14). Nevertheless, there was
no occurrence of involuntary jerks after the reintroduction of
these anti-hypertensive medications in the patients; thus, the
drugs were unlikely to be related to the occurrence of TMS.
Furthermore, the probability of TMS occurrence in drug-induced
cases was also evaluated using the Naranjo algorithm (24), and these cases were categorized as
doubtful.
Sethi (21) described
the first case of TMS outside Japan, in a letter to the editor
referencing the manuscript of Hiraga et al (7). Sethi (21) reported three cases with similar
features that subsided spontaneously. However, Sethi (21) did not describe the phenomenology of
the cases occurring with associated asterixis or the body
localization. In addition, no details about ethnicity were
provided. Based on the data from prior studies, the cases described
by Sethi (21) lacked sufficient
evidence to confirm a diagnosis of TMS. Moreover, the slowing shown
in electroencephalograms was not related with encephalopathy in the
cases described from Japan (25).
Discussion
Definition
TMS is characterized by an unusual type of
involuntary jerk, which is recurring, involving mainly in the
faciobrachial region. In addition, asterixis-like movement can
occasionally be observed during these abnormal movements in 30-50%
of the individuals with TMS (3).
This abnormal involuntary movement was already described in the
literature as ‘transient myoclonic state with asterixis (1)’, ‘benign transient shuddering-like
involuntary movement (12)’ or
‘isolated transient myoclonus (7)’.
Doden et al (3) defined that
a single myoclonic burst of TMS has a duration of 44 msec (standard
deviation, 12 msec) with a frequency of 9 hertz (standard
deviation, 2 hertz). Ictal impairment and loss of consciousness are
not observed following an episode of TMS; thus, the individual
remains fully alert and is aware without experiencing any cognitive
dysfunction or altered state of awareness during or after the
episode.
Older populations with mild chronic systemic
conditions are usually susceptible to TMS for unknown reasons.
These patients are fully conscious, no disturbance of amnesia or
paralysis is observed, and they responded to simple and complex
commands. Movement or posturing leads to the aggravation of
myoclonus, whereas sleep causes the jerk to settle and improve the
myoclonus of the patient. The myoclonus exhibits no exacerbation by
sensory stimuli. These jerks present acutely within a day and
resolve spontaneously within a few days or respond well to
treatment with benzodiazepines. Patients with TMS have exhibited a
frequent recurrence of myoclonus and almost half the patients have
experienced recurrence within a year and a half.
Periods of silence have been observed in
electromyography (EMG) in patients with TMS. In fact, in the
majority of cases, the brief period of silence has been observed
during the refractory phases following positive myoclonic
discharges, indicating a temporary cessation of neural activity
between the bursts of involuntary muscle contractions (3). Ugawa et al (26) categorized the interruption of
muscular activities into two types. The first type is the
classically described form of asterixis, which is the silent period
that follows an interval with no change in the background EMG
activity, suggesting that muscle activity remained stable while the
myoclonic discharges subsided. The second type of asterixis
typically occurs after a sudden, involuntary muscle contraction
triggered by voluntary movement or external stimuli. The technique
known as ‘silent period locking’ involves precisely recording EMG
during the silent period, allowing for the analysis of its
association with preceding EEG activity. This technique has
revealed that the EEG patterns prior to the silent period were
specifically associated with the second type of asterixis silent
periods, indicating a distinct neurophysiological connection
between the brain’s electrical activity and the subsequent muscle
inactivity (26). Some movement
disorders specialists believe that asterixis associated with TMS
should be classified as type II asterixis.
Etiology
The etiology of TMS can vary widely, with no
evidence of a definitive cause. Studies have shown a consistent
occurrence of TMS in the older population. The majority of cases
reported in previous studies are older males and older females,
suggesting that aging can precipitate TMS (3). In addition, sex can likely be a
predisposing factor, as it was observed that TMS occurs more
frequently in males than females. The majority of these patients
were found to have mild chronic comorbidities. Significant fluid
status changes in patients with renal and cardiovascular disorder
were likely associated with the occurrence of TMS (8).
TMS is considered a spontaneous cortical myoclonus,
with cortical hyperexcitability potentially indicating a genetic
cause. However, the argument for a genetic basis is weak, as
geographic clustering does not necessarily imply a genetic factor.
This clustering could be attributed to environmental factors,
underreporting in other regions, or publication bias. Notably, TMS
cases have been reported exclusively in Japan, though there is no
clear, specific geographical distribution within the country
(Fig. 2).
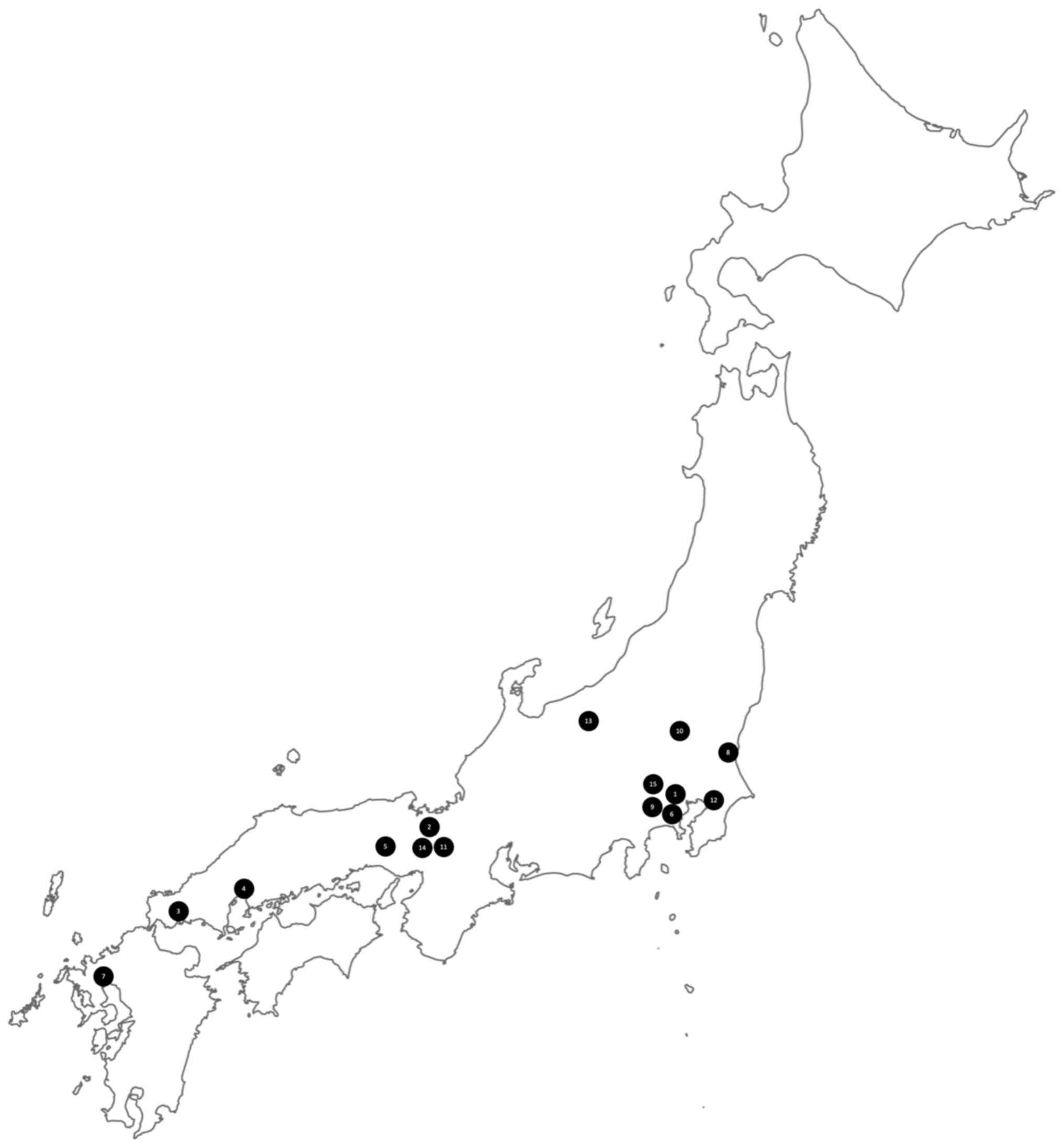 | Figure 2Geographical distribution in Japan of
the cases reported in the literature of transient myoclonic state.
The case described in the study by Sethi (21) was not included as it was outside
Japan. In addition, there were insufficient data from the study by
Tsuchiyama et al (23) to
provide the geographical localization. The locations are indicated
by numbers on the map and refer to the following studies: 1,
Mizutani et al (2); 2,
Hashimoto et al (1); 3,
Negoro et al (12); 4,
Fujihara et al (13); 5,
Hirata et al (14); 6, Iijima
et al (15); 7, Yamamoto
(16); 8, Kohono et al
(17); 9, Okuma et al
(18); 10, Kawakami (19); 11, Hitomi et al (20); 12, Hiraga et al (7); 13, Doden et al (3); 14, Umemura et al (22); 15, Harada et al (8). |
A small number of TMS cases with increased levels of
pyruvate and lactate were also reported; however, there is no
definite evidence to prove this change of biochemistry in their
body as a likely cause of TMS. This could have been a mere
coincidence, and can be explained by continual muscle contractions
(1). Another precipitating factor
reported by patients in some studies is infection. Myoclonic jerks
can occur in the initial, recovery, or the transition between these
two phases, and this was observed in almost half of the patients
with TMS (7). Elevated titer of
Epstein-Barr virus IgG antibodies was also reported in some
patients (2). Drugs such as
cisplatin, risperidone and levofloxacin are among the other
etiological factors causing myoclonic jerks. According to some
studies, the initiation of the above-mentioned drugs has led to the
onset of TMS due to its role in temporary metabolic changes and
increased sympathetic nervous system activity (1,3). All the
external etiological factors are likely to have caused TMS;
however, a number of patients have no history of any event
occurring prior to the onset of TMS. Thus, precipitating factors
are not mandatory for TMS occurrence (20).
Pathophysiology
TMS is characterized as a cortical myoclonus that is
spontaneous and non-reflex (1).
Variations in the physiological parameters with fluctuations in
both brain electrical activity and muscle response have been
observed. In electrophysiological studies, jerk-locked back
averaging (JLA) revealed cortical activity before the occurrence of
the involuntary muscle activity (18). Hitomi et al (20) reported that fluctuations in EMG
signal variability were associated with the severity of myoclonus.
In addition, increased amplitude or heightened response in the
cortical area when sensory stimuli are applied [giant somatosensory
evoked potentials (SEPs)] have been observed, although this is a
rare phenomenon. This enlargement suggests heightened neural
excitability or enhanced processing of sensory information within
the brain’s sensory pathways.
Cortical spikes preceding myoclonic jerk, shown by
JLA, suggests hyperexcitability present in primary motor cortex
(PMC) during the myoclonic occurrence. The decrease on the
amplitude of these positive spikes by the administration of
benzodiazepines, resulted in the clinical improvement of the
patient. This further suggests that selective activation at the PMC
is likely the cause of involuntary jerks in TMS. Furthermore, the
administration of benzodiazepines simultaneously suppresses the
spikes in the PMC and myoclonus. In JLA, the spike that precedes
the myoclonus has a positive polarity in the reflex myoclonus of
cortical origin and a negative polarity in the spontaneous
myoclonus (27). The negative
polarity in JLA may result from a pathologically excitable cortex,
similar to an epileptic state. Thus, a positive polarity suggests
that TMS is a cortical myoclonus.
Motor-evoked potentials have been reported to be
normal during the asymptomatic period, suggesting no
hyperexcitability at the PMC. This suggests that cortical
hyperexcitability was recorded only at PMC during the symptomatic
phase, as shown by JLA, and it is associated with myoclonus in
patients with TMS (3).
Other electrophysiological studies have demonstrated
no giant SEPs during symptomatic or asymptomatic periods. SEP
amplitudes tend to increase as a person ages; hence, the SEP
amplitudes in TMS do not markedly increase (28,29). In
addition, an overall amplitude increase does not necessarily
indicate a giant SEP. EEG is usually normal, and there are no
epileptiform discharges during the myoclonus, suggesting that an
epileptic factor is unlikely to cause TMS (28).
The laboratory tests of patients with TMS were found
to be within normal limits. Neuroimaging was unrevealing (7). Notably, areas of hyperperfusion in the
bilateral precentral gyri during the myoclonic jerks were observed.
This focal hyperperfusion persisted for three months in the
precentral gyri even after the disappearance of the myoclonus. The
hyperperfusion supports the hyperactivity in the PMC during the
symptomatic period. It also indicates that hyperactivity can occur
without presenting symptoms and contribute for TMS or its
recurrence (22).
Subcortical structures may play a role in TMS. The
brainstem and motor systems play a crucial role in the development
of axial and bilateral myoclonus. The brainstem, which is
responsible for basic autonomic and motor functions, coordinates
movement and muscle control, while the motor systems involve
pathways that regulate muscle tone and voluntary movements. When
either of these systems is disrupted, it can lead to involuntary
muscle jerks or twitches, particularly affecting the axial muscles
(trunk and neck) and bilaterally (on both sides of the body)
(1). Some authors have also reported
the resemblance of TMS and shivering; thus, the hypothalamic region
that is related to the thermal regulation may play a role in the
development of this pathology (30).
Of note, subcortical myoclonus cannot be excluded, as the cortical
and subcortical regions can originate asterixis and positive
myoclonus. In this manner, the cortical than the subcortical source
is more likely to be related to the occurrence of TMS, although
there is no clear definition and multiple sources from different
locations in the brain may lead to this disorder (20).
Another hypothesis suggests that the excessive
activation of specific areas of the brain may lead to TMS, while
enhanced inhibitory effects could contribute to the development of
negative myoclonus. Of note, a combination of negative and positive
myoclonus has already been observed in multiple myoclonic syndromes
such as post hypoxic myoclonus and myoclonic epilepsy syndromes
(31).
Diagnostic approach to transient
myoclonic state. Initial assessment of patients with TMS
In terms of medical history, it is important to
obtain the specific timeframe of the myoclonus onset, the
progression of symptoms over time (acute vs. subacute/chronic), the
body parts most commonly affected, alleviating and worsening
factors, neurological family history and comorbidities (32). It is also important to determine
whether symptoms are stable or progressive (32).
The categorization of the type of myoclonus largely
depends on the age of onset. The onset of myoclonus in young
adulthood, alongside with generalized epileptic seizures and
neurocognitive impairment are suggestive of progressive myoclonus
epilepsy (PME) (32). Moreover, in
individuals who are > 65 years of age, myoclonus associated with
cognitive impairment is frequently observed Lewy body dementia,
corticobasal degeneration, in prion diseases and in advanced stages
of Alzheimer’s disease (33). On the
other hand, opsoclonus-myoclonus syndrome in childhood is most
often associated with paraneoplastic disorders underlying
malignancies, such as medulloblastoma and neuroblastoma (34). When manifesting in middle-aged
adults, myoclonus can be part of paraneoplastic syndromes
associated with skin and lung cancers. Myoclonus could also be
secondary to drugs and other systemic conditions (35).
In terms of different timeframes of clinical
manifestations, the acute onset of myoclonus is generally observed
in hepatic and renal failure, as well as in other toxic-metabolic
conditions, thyrotoxicosis, electrolyte abnormalities, hypoglycemia
nonketotic hyperglycemia, and in central nervous system infections,
such as herpes simplex encephalitis and neuroborreliosis (32). Myoclonus can also occur following
hypoxic brain injury. The recent initiation of new medications or
an increase in dosage should always be considered a potential cause
of abnormal involuntary movements (35). Additionally, neurodegenerative
diseases and PME are generally associated with myoclonus with a
slow and progressive onset, worsening overtime (33).
It is worth mentioning that the presence of
neurological findings apart from myoclonus may lead to algorithms
assisting with the etiological diagnosis of involuntary movements.
Metabolic disorders and even PME have a pattern of inheritance for
genetic traits where a child inherits a mutated gene from each
parent (32). On the other hand,
dominant causes of myoclonic jerks involve non-neurodegenerative
conditions like myoclonus-dystonia syndrome (33).
In the context of the neurological exam, it is
essential to evaluate for the presence of postural myoclonus while
the patient is keeping arms outstretched, at rest, and during
movement (36). Spinal or brainstem
sources are generally observed with myoclonus at rest, whereas
myoclonus from cortical source is usually triggered by and
generally manifests as focal distribution (37). Spinal segmental myoclonus is also
considered focal, as it typically affects specific regions of the
spinal cord and associated muscles, often localized to a particular
segment or area (36).
Stimulus sensitivity analysis is another part of the
neurological examination of myoclonus. Myoclonus can be triggered
by gently touching the outstretched fingers, with the response
being particularly sensitive to auditory stimuli (38). In this case, even subtle sounds may
provoke involuntary muscle jerks, demonstrating the heightened
responsiveness of the condition to auditory input. Hand-clapping
may also induce myoclonus (38).
Neurophysiology of cortical vs.
subcortical myoclonus
The neurophysiology of myoclonus can be categorized
based on whether it originates from cortical or subcortical
regions, each with distinct mechanisms. Cortical myoclonus is
typically associated with the motor cortex, which controls
voluntary movement. The abnormal electrical activity arises from
the cortex, often due to an issue in the pyramidal tract or
cortical circuitry. It may occur in response to voluntary movement,
sensory stimuli, or even spontaneously. Cortical myoclonus can be
triggered by external stimuli (e.g., tactile, auditory) and is
usually characterized by rapid, jerky muscle contractions. It is
often seen in conditions such as epilepsy (e.g., juvenile myoclonic
epilepsy) and neurodegenerative diseases (e.g., corticobasal
degeneration). Subcortical myoclonus arises from deeper structures,
such as the brainstem, basal ganglia, or spinal cord. It is
typically less responsive to cortical stimuli and may result from
dysfunction in the brainstem motor control centers or altered
processing within the basal ganglia circuits. Subcortical myoclonus
often involves more generalized or bilateral muscle jerks, which
are not typically triggered by external stimuli. It is observed in
conditions such as brainstem lesions, neurodegenerative diseases,
or metabolic disorders. Both types of myoclonus result from
abnormal neuronal firing but differ in their origin and the
specific pathways involved (32).
Differential diagnosis
Notably, non-epileptic myoclonic movements are more
prevalent than their epileptic counterparts, particularly during in
younger patients, such as infancy and childhood. Parents often
report myoclonic movements, whether confirmed or not, making the
differential diagnosis challenging. Various factors, including
intellectual disabilities and EEG irregularities, can further
complicate the diagnosis. Non-epileptic myoclonic movements can
occur during the neonatal stage. One condition to consider in
infancy is Fejerman myoclonus, which is considered to arise from a
subcortical source. Myoclonus in this condition may be triggered by
certain stimuli, such as touch or auditory cues, and can be part of
a broader clinical presentation that includes other neurological
signs (32).
It is important to differentiate Fejerman myoclonus
from other similar conditions, such as benign neonatal myoclonus or
early-onset myoclonus, before proceeding to genetic testing
(39). Careful clinical evaluation
is key to ruling out other forms of myoclonus. Research has
indicated that conditions resembling myoclonus, such as head atonic
episodes, often begin in the first months or years of life, with
normal psychomotor development and a neurologically normal
assessment (40). These conditions
tend to spontaneously regress after a few months without long-term
consequences. Misdiagnosis can have significant implications for
both individuals and their families, as Fejerman myoclonus and head
atonic episodes are frequently mistaken for epileptic seizures,
leading to unnecessary treatments and emotional stress for parents
and caregivers. Accurate diagnosis is critical to avoid
mismanagement and ensure appropriate care (41).
Non-epileptic symptoms usually do not react to
antiseizure medications (ASMs), leading to some children being
wrongly diagnosed with epilepsy or even drug-resistant epilepsy.
When the start of an ASM coincides with a natural reduction in
episodes, this reaction can be inaccurately attributed to the ASM,
resulting in the continuation of an unnecessary treatment for a
number of years (42).
Differentiating between epilepsy and other conditions can be
effectively done through a more detailed history and by using
videos taken by parents or recordings from video-polygraphy.
Although neurophysiology can help determine the classification, it
lacks specificity; therefore, it cannot provide the causes of the
myoclonus (43).
There are some similarities between TMS and benign
adult familial myoclonus epilepsy (BAFME) and familial cortical
myoclonic tremor with epilepsy (FCMTE) (42). These conditions are relevant to
discuss due to their overlap in clinical presentation, particularly
in terms of myoclonus and tremor. However, TMS can be
differentiated from BAFME and FCMTE by several key factors. These
include the family history of the patient, which may reveal genetic
predispositions; the age at which symptoms first appear, helping to
understand the progression of the condition; the specific patterns
of myoclonus experienced, which can vary in their distribution
across the body; and the presence or absence of seizures, which may
indicate different underlying mechanisms of the disorder. Each of
these elements provides valuable insight into the nature of TMS and
aids in its diagnosis and management (20).
Future studies and specialist
recommendations
Future studies on TMS are required to address
several critical areas to enhance our understanding and treatment
of this condition. First, research should focus on elucidating the
neurobiological and genetic mechanisms underlying transient
myoclonus, employing advanced neuroimaging techniques and molecular
studies. Comprehensive clinical characterization through
longitudinal studies is necessary to distinguish transient
myoclonus from other movement disorders, identify subtypes, and
develop standardized diagnostic criteria.
Epidemiological studies are vital for understanding
the prevalence, incidence and risk factors associated with
transient myoclonus. Population-based research and multicenter
collaborations could provide insight into demographic and
environmental factors, aiding in developing preventive strategies.
Investigating potential genetic predispositions to TMS is critical,
particularly in cases where there is a familial pattern. Further
studies are warranted to identify genes linked to myoclonus and
other movement disorders, which could provide insight into the
etiology and lead to targeted therapies.
Further research is also required to investigate the
impact of transient myoclonus on the quality of life and long-term
outcomes of patients, using patient-reported outcome measures to
assess treatment effectiveness. Research into the long-term
prognosis of individuals with TMS is crucial. Further studies are
required assess the likelihood of recurrence, the potential for
neurodevelopmental delays, or any lasting neurological impairments.
Finally, advances in genomics and biomarker discovery should be
prioritized to identify diagnostic and prognostic markers, enabling
early diagnosis and personalized treatment approaches.
Collaborative, multidisciplinary efforts are crucial to advancing
our understanding and improving care for transient myoclonus
patients.
The present study reviewed all the particular
features associated with TMS. An overview of the characteristics of
TMS is presented in Table IV.
 | Table IVTransient myoclonic state features
(as indicated in the manuscript). |
Table IV
Transient myoclonic state features
(as indicated in the manuscript).
| 1. Older
individuals and those with chronic illnesses are vulnerable. |
| 2. The state of
consciousness is almost normal or just slightly compromised. |
| 3. Myoclonic jerks
happen unexpectedly and are somewhat intensified during movement,
primarily observed in the fasciobrachial region. |
| 4. Voice can be
tremulous. However, no opsoclonus or palatal myoclonus are
observed. |
| 5. No noticeable
myoclonic jerks are triggered by sensory stimuli. |
| 6. Negative
myoclonus is present in the muscles affected by myoclonic jerks,
and similar movements resembling negative myoclonus can also be
observed when the tongue is extended. |
| 7. Neurological
examination apart from myoclonus is within normal limits. |
| 8. The
electroencephalogram reveals nonspecific slowing or irregular
patterns, with no evidence of epileptiform abnormalities.
Photoparoxysmal response is not observed. |
| 9. The beginning of
this condition is relatively sudden, but it slowly became more
noticeable over the course of several hours. |
| 10. Myoclonus and
asterixis disappear altogether within three days of onset with
benzodiazepine therapy. |
| 11. No lasting
effects were observed, although the transient myoclonic state has a
tendency to recur. |
| 12.
Electrophysiological results indicate the absence of giant
somatosensory evoked potentials during both symptomatic and
asymptomatic phases, as well as a lack of cortical reflex. A
positive spike in jerk-locked back averaging is observed prior to
the myoclonus. Motor evoked potential findings are normal during an
asymptomatic phase. |
In conclusion, the present systematic review
emphasizes the intricate nature of TMS or TMA. Future research
endeavors are required to prioritize uncovering the biological and
genetic mechanisms that contribute to this condition, as well as
refining the criteria used for its diagnosis. Notably, TMS appears
to have a distinct geographical preference, suggesting at a
potential genetic distribution among affected populations.
Understanding the pathophysiological mechanisms associated with
this specific form of stereotyped myoclonus could provide valuable
insight into other types of myoclonus as well. It is essential to
engage in open discussions with patients and their caregivers about
the typically benign progression of this disorder and to clarify
that, in certain cases, the use of benzodiazepines may be necessary
to manage symptoms effectively.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors’ contributions
JPR, NMV, NS, SS and ALFC conceived and designed the
methodology of the systematic review. JPR, NMV, NS, SS and ALFC
extracted and collected the relevant information from the
literature and drafted the manuscript. JPR and ALFC supervised the
article selection, and reviewed and edited the manuscript. JPR and
ALFC reviewed and edited the manuscript. NMV, NS and SS confirm the
authenticity of all the raw data. All authors have read and agreed
to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hashimoto S, Kawamura J, Yamamoto T,
Kinoshita A, Segawa Y, Harada Y and Suenaga T: Transient myoclonic
state with asterixis in elderly patients: A new syndrome? J Neurol
Sci. 109:132–139. 1992.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mizutani T, Ida M, Egarashi O, Daida H and
Shiozawa R: Recurrent myoclonus associated with Epstein-Barr virus
infection in an elderly patient. Rinsho Shinkeigaku. 26:1042–1046.
1986.PubMed/NCBI(In Japanese).
|
|
3
|
Doden T, Sato H and Hashimoto T: Clinical
characteristics and etiology of transient myoclonic state in the
elderly. Clin Neurol Neurosurg. 139:192–198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shibasaki H and Hallett M:
Electrophysiological studies of myoclonus. Muscle Nerve.
31:157–174. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thomas B and Frucht SJ: Myoclonus: An
update. Curr Opin Neurol. 37:421–425. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kojovic M, Cordivari C and Bhatia K:
Myoclonic disorders: A practical approach for diagnosis and
treatment. Ther Adv Neurol Disord. 4:47–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hiraga A, Kamitsukasa I and Kuwabara S:
Isolated transient myoclonus in the elderly: An under-recognized
condition? Clin Neurol Neurosurg. 117:51–54. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harada T, Yamasato K, Nakanishi T and
Nakai M: In-hospital onset of transient myoclonic state in older
adults: A case report. Eur J Case Rep Intern Med.
11(004254)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Vries E, Schoonvelde M and Schumacher
G: No longer lost in translation: Evidence that google translate
works for comparative bag-of-words text applications. Polit Anal.
26:417–430. 2018.
|
|
10
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ma LL, Wang YY, Yang ZH, Huang D, Weng H
and Zeng XT: Methodological quality (risk of bias) assessment tools
for primary and secondary medical studies: What are they and which
is better? Mil Med Res. 7(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Negoro K, Fukusako T, Tsuda N, Nogaki H
and Morimatsu M: Clinical analysis of benign transient
shuddering-like involuntary movement in elderly people. Rinsho
Shinkeigaku. 34:1153–1156. 1994.PubMed/NCBI(In Japanese).
|
|
13
|
Fujihara K, Iwato K and Ohta M: Transient
involuntary shuddering movement in the aged. Neurol Med (Tokyo).
43(90)1995.
|
|
14
|
Hirata K, Kawamoto M, Takatsuka K,
Yoshikawa N and Kawamura J: Transient myoclonic state with
asterixis: A case report. Neurol Med (Tokyo). 46:53–56. 1997.
|
|
15
|
Iijima M, Osawa M and Iwata M: Transient
myoclonus-like involuntary movements in an elderly patient: A case
report. Neurol Med (Tokyo). 46:413–416. 1997.
|
|
16
|
Yamamoto A: Elevated serum amyloid A
protein in senile transient myoclonus. Neurol Med (Tokyo).
47(546)1997.
|
|
17
|
Kohono Y, Hayashi A, Ishii A, Hoshino S
and Shoji S: Two cases of benign transient shivering-like
involuntary movements. JMDD. 8:109–113. 1998.
|
|
18
|
Okuma Y, Komine M, Shimo Y, Miwa H and
Mizuno Y: Transient myoclonic state in elderly patients; clinical
and physiological study. Rinsho Nouha. 41:780–785. 1999.(In
Japanese).
|
|
19
|
Kawakami T: Transient myoclonic state with
asterixis. Neurol Med (Tokyo). 66:117–121. 2007.
|
|
20
|
Hitomi T, Ikeda A, Inouchi M, Imamura H,
Nakagawa T, Fumuro T, Matsumoto R and Takahashi R: Transient
myoclonic state with asterixis: Primary motor cortex
hyperexcitability is correlated with myoclonus. Intern Med.
50:2303–2309. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sethi NK: Isolated transient myoclonus in
the elderly-cortical vs subcortical. Clin Neurol Neurosurg.
122(137)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Umemura A, Oeda T and Sawada H: Transient
myoclonic state with asterixis presenting as persistent
hyperperfusion on single-photon emission computed tomography: A
case report. Neurol Clin Neurosci. 3:101–102. 2015.
|
|
23
|
Tsuchiyama M, Yuasa R, Matsuura M and
Tomoda K: Transient myoclonic state with asterixis in elderly
patients. 2 cases of (Hashimoto). Rōka to shikkan (Ageing and
diseases). 8:1644–1646. 1995.https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200902147153567053.
|
|
24
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hiraga A and Kuwabara S: Reply to letter
to the Editor: Isolated transient myoclonus in the elderly. Clin
Neurol Neurosurg. 120(142)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ugawa Y, Shimpo T and Mannen T:
Physiological analysis of asterixis: Silent period locked
averaging. J Neurol Neurosurg Psychiatry. 52:89–93. 1989.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mima T, Nagamine T, Ikeda A, Yazawa S,
Kimura J and Shibasaki H: Pathogenesis of cortical myoclonus
studied by magnetoencephalography. Ann Neurol. 43:598–607.
1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lüders H: The effect of aging on the wave
form of the somatosensory cortical evoked potential.
Electroencephalogr Clin Neurophysiol. 29:450–460. 1970.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tanosaki M, Ozaki I, Shimamura H, Baba M
and Matsunaga M: Effects of aging on central conduction in
somatosensory evoked potentials: Evaluation of onset versus peak
methods. Clin Neurophysiol. 110:2094–2103. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kanosue K, Zhang YH, Yanase-Fujiwara M and
Hosono T: Hypothalamic network for thermoregulatory shivering. Am J
Physiol. 267:R275–R282. 1994.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rissanen SM, Hyppönen J, Silvennoinen K,
Säisänen L, Karjalainen PA, Mervaala E and Kälviäinen R: Wearable
monitoring of positive and negative myoclonus in progressive
myoclonic epilepsy type 1. Clin Neurophysiol. 132:2464–2472.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chandarana M, Saraf U, Divya KP, Krishnan
S and Kishore A: Myoclonus-a review. Ann Indian Acad Neurol.
24:327–338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Riva A, D’Onofrio G, Ferlazzo E,
Pascarella A, Pasini E, Franceschetti S, Panzica F, Canafoglia L,
Vignoli A, Coppola A, et al: Myoclonus: Differential diagnosis and
current management. Epilepsia Open. 9:486–500. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cantarín-Extremera V, Jiménez-Legido M,
Aguilera-Albesa S, Hedrera-Fernández A, Arrabal-Fernández L,
Gorría-Redondo N, Martí-Carrera I, Yoldi-Pedtri ME, Sagaseta-De
Ilúrdoz M and González-Gutiérrez-Solana L: Opsoclonus-myoclonus
syndrome: Clinical characteristics, therapeutic considerations, and
prognostic factors in a Spanish paediatric cohort. Neurologia (Engl
Ed): July 8, 2020 (Epub ahead of print).
|
|
35
|
Rissardo JP, Fornari Caprara AL, Bhal N,
Repudi R, Zlatin L and Walker IM: Drug-induced myoclonus: A
systematic review. Medicina (Kaunas). 61(131)2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shibasaki H: Neurophysiological
classification of myoclonus. Neurophysiol Clin. 36:267–269.
2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kakigi R and Shibasaki H: Generator
mechanisms of giant somatosensory evoked potentials in cortical
reflex myoclonus. Brain. 110:1359–1373. 1987.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hallett M, Chadwick D and Marsden CD:
Cortical reflex myoclonus. Neurology. 29:1107–1125. 1979.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Caraballo RH, Capovilla G, Vigevano F,
Beccaria F, Specchio N and Fejerman N: The spectrum of benign
myoclonus of early infancy: Clinical and neurophysiologic features
in 102 patients. Epilepsia. 50:1176–1183. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Capovilla G: Shaking body attacks: A new
type of benign non-epileptic attack in infancy. Epileptic Disord.
13:140–144. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Capovilla G, Montagnini A, Peruzzi C and
Beccaria F: Head atonic attacks: A new type of benign non-epileptic
attack in infancy strongly mimicking epilepsy. Epileptic Disord.
15:44–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Menozzi E, Balint B, Latorre A, Valente
EM, Rothwell JC and Bhatia KP: Twenty years on: Myoclonus-dystonia
and ε-sarcoglycan-neurodevelopment, channel, and signaling
dysfunction. Mov Disord. 34:1588–1601. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shibasaki H: Electrophysiological studies
of myoclonus. Muscle Nerve. 23:321–335. 2000.PubMed/NCBI View Article : Google Scholar
|