Introduction
Bladder cancer is the second most common type of
cancer in the genitourinary tract and the fourth most common cause
of cancer in males in Western industrialized countries (1). Bladder cancer leads to approximately
145,000 deaths per year worldwide (2). Approximately 80% of bladder cancers
are initially diagnosed as non-muscle-invasive tumors (3). Transurethral resection (TUR) is the
recommended treatment for non-muscle-invasive tumors. However,
there is a high incidence of recurrence and progression with TUR
treatment. To decrease the incidence of recurrence and progression
of these tumors, subsequent intravesical instillation of
chemotherapeutic agents are regularly enrolled after TUR.
Epirubicin is one of the most common drugs used in intravesical
instillation. It is important in the treatment of bladder cancer
and decreases the incidence of recurrence (4). Despite this decrease, recurrence of
this malignant tumor following TUR with subsequent intravesical
instillation remains up to 60–70% within two years. A total of
15–25% of non-muscle-invasive bladder cancers may eventually
progress to muscle invasive tumors (1). Studies have mainly attributed this
progression to the chemotherapeutic resistance of cancer cells to
epirubicin (5). Therefore, it is
significant to study the mechanism of chemotherapeutic resistance
and enhance the chemosensitivity of cancer cells to
chemotherapeutic drugs.
Our previous studies (6) determined that an oncogenic protein,
clusterin (CLU), was overexpressed in the majority of bladder
cancer cells and therefore may serve as a prognostic molecular
marker. Follow-up showed that patients with bladder cancer with a
high CLU expression had a higher incidence of recurrence than that
of the low CLU expression, even with epirubicin treatment (6). This result indicated that the
expression of CLU affected the chemosensitivity of bladder cancer
cells to epirubicin and indirectly affected the incidence of
recurrence and progression of this malignant tumor. Other studies
have also demonstrated the potential role of CLU on
chemotherapeutic resistance in several cancer cells, including lung
cancer, esophageal carcinoma and prostate tumor (5,7–9).
However, no studies have yet examined the role of CLU on
chemotherapeutic resistance of bladder cancer cells to
epirubicin.
In this study, we aimed to determine whether the
regulation of CLU expression affected the chemosensitivity of
bladder cancer cells to epirubicin. Lentivirus-mediated RNA
interference was applied to permanently silence the expression of
CLU in the EJ bladder cancer cell line which, for the first time,
had a high expression level of CLU. The silencing efficacy of the
lentivirus vector in EJ cells at the protein and mRNA levels were
examined. Cell viability, migration, invasiveness, clone formation
and cell cycle were examined to assess the role of CLU on the
chemosensitivity of EJ bladder cancer cells to epirubicin.
Materials and methods
Cell culture and reagents
Human bladder transitional cell lines T24 and 5637
were purchased from the American Type Culture Collection
(Rockville, MD, USA). The EJ and BIU-87 cells were kindly supplied
by the Institute of Urology, Beijing Medical University (Beijing,
China). Cells were maintained in RPMI-1640 (Sigma, St. Louis, MO,
USA) containing 10% fetal bovine serum (FBS) at 37°C in humidified
air containing 5% CO2 in a monolayer as previously
described (10). The 293T cell
line, which stably expressed the SV40 large T antigen and
facilitated the optimal production of viruses, was obtained from
Genechem Co., Ltd., (Shanghai, China) and was cultured in
Dulbecco’s modified Eagle’s Medium (DMEM) with 10% FBS. Epirubicin
was purchased from Zhejiang Hisun Pharmaceutical Co., Ltd
(Zhejiang, China).
Lentivirus construction and
infection
PGCSIL-GFP, a third generation self-inactivating
lentivirus vector containing a CMV-driven GFP reporter and a U6
promoter upstream of cloning restriction sites was used in this
shRNA silencing system. The synthetic oligonucleotide primers used
were: CLU; forward (5′-ccggaaccagagctcgcccttctacttcaagaga
gtagaagggcgagctctggtttttttg-3′)
and reverse (5′-aattcaaaaaaa ccagagctcgcccttctactctcttgaagtagaagggcgagctctggtt-3′).
The primers were annealed and linked into the cloning restriction
site of the vector which had been digested with the restriction
enzymes AgeI and EcoRI. The constructed vector
PGCSIL-GFP-CLU is able to produce a shRNA targeting 5′-ccaga
gctcgcccttctac-3′, which is located on the nucleotide position
740–758 of the CLU mRNA (NM_203339). It has been proven to be
efficient in CLU silencing experiments (11). The negative control sequence
(5′-ttctccgaacgtgtcacgt-3′) was used as previously described
(12). The NC-shRNA was designed
as follows: forward, 5′-ccggaattctccgaacgtgtcacgtttcaagagaacgtgacacgttcggagaatttttttg-3′
and reverse, 5′-aattcaaaaaaattctccgaacgtgtcacgttctcttgaaacgtgacacgttcggagaatt-3′.
Following PCR and sequencing of the constructed vector, it was
co-transfected with pHelper 1.0 and pHelper 2.0 into 293T cells to
package and produce the shRNA expressing lentivirus. The
supernatant was collected and concentrated 48 h after
co-transfection. The titer of lentivirus targeting CLU (LV-CLU) and
lentivirus targeting negative control (LV-NC) was examined by the
hole-by-dilution titer method. The vectors and oligonuleotide
primers were purchased from Genechem.
To knock down the CLU in the EJ bladder cancer cell
line, cells were seeded in a 6-well tissue culture plate with
2×105/well 1 day prior to infection. The complete
culture solution was replaced by infection enhancing solution with
5 μg/ml polybrene (Genechem) and the packed lentivirus was added to
the cells with multiplicity of infection (MOI) 20. Twelve hours
later, the lentivirus solution was replaced with complete culture
solution. Infected cells were subcultured every 5–7 days.
RT-PCR
To examine the effect of lentivirus-mediated shRNA
at the mRNA level, total RNA was extracted from cell lines by
TRIzol/chloroform extraction (Invitrogen Life Technologies,
Carlsbad, CA, USA). The purity and concentration of RNA was
determined using a spectrophotometer. A total of 1 μg RNA was used
for reverse transcription and the Primescript™ reverse
transcriptase (Takara Bio, Inc., Shiga, Japan) with random 6-mer
primers was used for cDNA synthesis according to the manufacturer’s
instructions. A total of 1 μl of each first-strand reaction was
subsequently used in the PCR amplifications. Oligonucleotide
primers used in the PCR were: CLU; 5′-cgcaaggcgaagaccagta-3′ and
5′-gaccctccaagcgatcagc-3′; GAPDH; 5′-gttcgacagtcagccgcatct-3′ and
5′-cctgcaaatgagccccagcct-3′. PCR conditions were as follows:
denaturation for 5 min at 94°C, then 30 sec at 94°C, annealing for
30 sec at 58°C, 31 sec at 72°C for 32 cycles and extenstion for 7
min at 72°C. The products of the PCR amplifications were separated
by 1% agarose gel electrophoresis and visualized under UV light
after staining with ethidium bromide.
Western blot analysis
For western blot analysis, cells were seeded in
6-well plates at 2×105/well. The cells were grown to
90–100% confluence, and the cells were then collected with 80
μl/well RIPA containing 1 mM phenylmethanesulfonyl fluoride (PMSF).
The protein concentration was measured using a BCA protein assay
(ThermoScientific Pierce, Rockford, IL, USA) according to the
manufacturer’s instructions. Total protein (50 μg) was used to run
electrophoresis on 10% SDS-polyacrylamide gels. After transferring
the protein to polyvinylidene fluoride membranes, the membrane was
blocked with 5% non-fat milk for 1 h and immunoblotted with primary
antibody at 4°C overnight. The monoclonal CLU antibody was
purchased from Millipore (Billerica, MA, USA) and diluted to 1:100
in 5% non-fat milk containing 0.05% Tween-20. α-tubulin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was diluted to 1:1000 and used
as the internal control. After washing the membrane three times
with Tris-buffered saline Tween-20, it was blotted with a secondary
antibody for 2 h at room temperature. An ECL kit (ThermoScientific
Pierce) was used to detect the bands. The quantification of protein
was performed by band analysis using Gel-Pro Analyzer 4.0 software
(Media Cybernetics, Bethesda, MD, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT
assay
The cells were prepared at a concentration of
3×104 cells/ml and dispensed into 96-well plates at 100
μl/well, then incubated for 0, 1, 3 and 5 days. The MTT assay was
performed by adding 20 μl of MTT (5 mg/ml) and incubating for 4 h.
When the MTT incubation was complete, the supernatants were
removed. A total of 150 μl dimethyl sulfoxide (DMSO) was added to
each well and after 15 min the absorbance value (OD) of each well
was measured using a microplate reader set at 490 nm. Experiments
were performed in triplicate.
Matrigel invasion assay
Cells were trypsinized, washed and resuspended in
RPMI-1640 without FBS. Samples of 5×104 cells were
placed in the upper chamber of each Transwell device (Falcon, BD
Labware, Bedford, MA, USA) with an 8-μm Matrigel-coated
polycarbonate membrane filter inserted in 24-well plates. RPMI-1640
with 10% FBS was placed in the lower chamber. After 24 h of
incubation, the non-invading cells were removed by wiping the upper
surface of the filter with a cotton swab. The remaining cells were
fixed in 100% methanol for 20 min, stained with Giemsa (Sigma), and
rinsed with distilled water several times. The degree of invasion
was quantified by selecting five different predetermined views
(original magnification, ×200) and counting the cells on the
underside of the filters under a microscope.
Wound healing assay
Cells were seeded at 5×105 cells/well in
6-well plates and cultured for ~24 h until almost confluent. The
cell monolayer was manually scratched with a pipette tip, washed
twice with PBS and allowed to migrate in RPMI-1640 without FBS for
24 h. Phase contrast micrograph images were captured immediately
and 22 h after the wound was made. The relative distance traveled
by the leading edge from 0 to 22 h was assessed using Adobe
Photoshop CS3 software (Adobe Inc.) (n=6).
Plate clone formation assay
The cells were seeded at 1×102 cells/well
in 6-well plates and incubated at 37°C for 14 days. The cells were
then washed twice with PBS and fixed in 100% methanol for 20 min,
prior to staining with Giemsa solution for 30 min. The number of
colonies containing ≥50 cells were counted under a microscope and
calculated using the equation: [Plate clone formation efficiency =
(number of colonies/number of cells inoculated) × 100%].
Cell cycle analysis
To study the cell cycle progression following RNA
interference, the monoparametric FACS analysis method following PI
(propidium iodide) staining for total DNA content was conducted as
previously described (13).
Briefly, after treatment with epirubicin for 24 h, cells from each
experimental group were collected and fixed in 70% ethanol at −20°C
overnight. After washing twice with PBS, cells were incubated in
PBS containing 100 μg/ml RNase at 37°C for 30 min. The cells were
then stained with 0.5 mg/ml PI for 30 min in the dark at 37°C. Cell
cycle progression was analyzed using a flow cytometer (Beckman
Coulter, Miami, FL, USA). During cell cycle analysis, gating and
voltage were carefully set to exclude clumped cells and cell
debris. The data were analyzed using CXP Analysis 2.0 (Beckman
Coulter).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD). The statistical correlations in the data between the groups
were analyzed by the Student’s t-test. P<0.05 (two-sided)
was considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 16.0 software (SPSS
Inc.).
Results
RT-PCR and western blot analysis of CLU
expression in bladder cancer cell lines
To select a cell line with a high level of CLU
expression, we used RT-PCR and western blot analysis to analyze the
CLU expression at the mRNA and protein levels in BIU-87, 5637, EJ
and T24 bladder cancer cell lines. The results suggest that the EJ
cell line had the highest expression level of CLU (Fig. 1A).
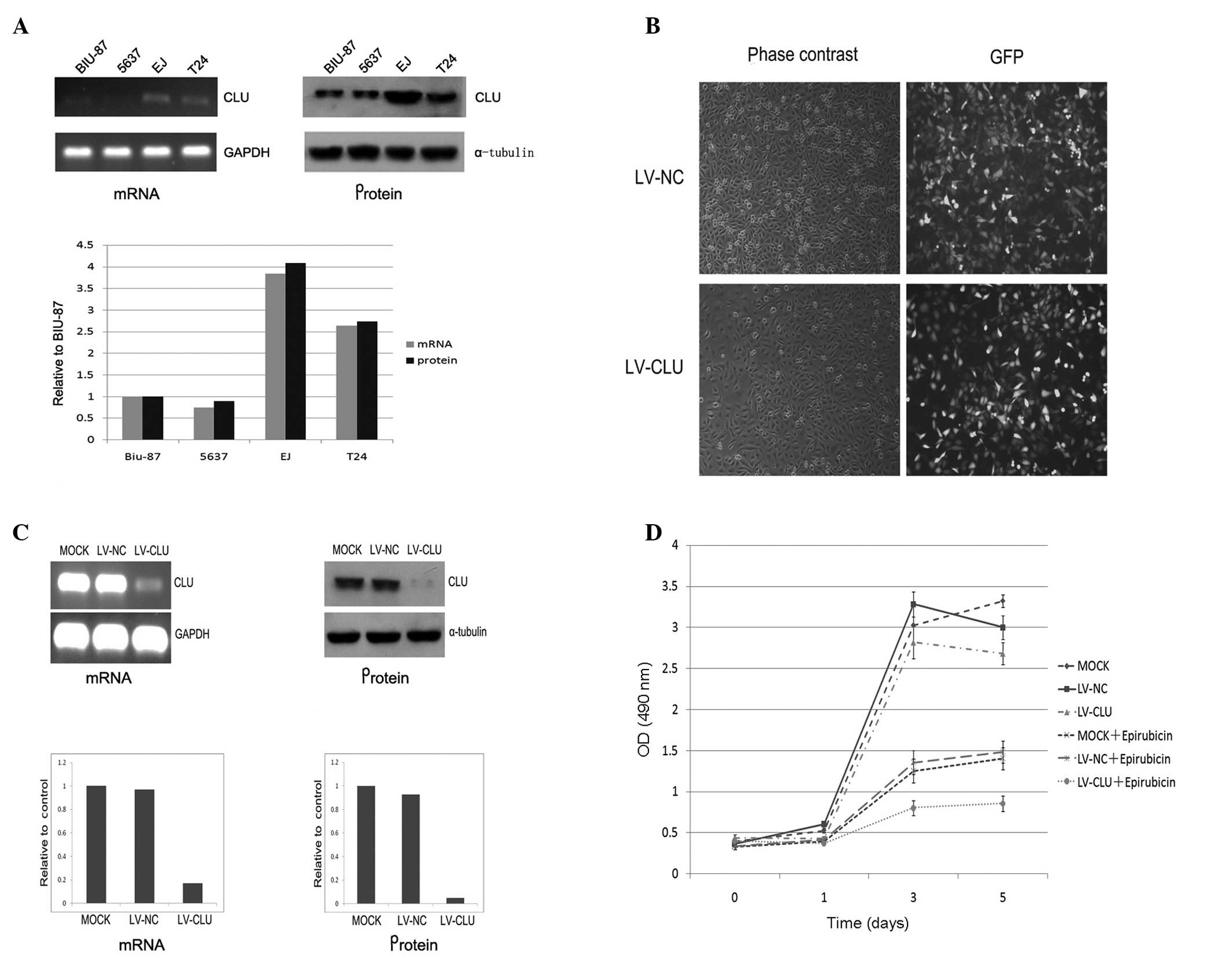 | Figure 1Establishment of stable CLU silencing
EJ cell line and detection of the growth velocity. (A) Analysis of
levels of CLU expression in mRNA and protein in 5637, BIU-87, EJ
and T24 bladder cancer cell lines. Results indicate that the EJ
cell line had the highest level of CLU expression. (B) The
infection efficiency of EJ cells (MOI 20) is shown. The cells were
infected with LV-CLU or LV-NC. Four days later, the infection
efficiency achieved was >90% (magnification, ×100). (C) Analysis
of CLU mRNA expression in the EJ cell line after lentivirus
infection. RT-PCR analysis demonstrated that LV-CLU reduced CLU
mRNA by 83.3% in EJ cells, while CLU mRNA expression was almost
unaffected by the LV-NC. Western blot analysis of the CLU protein
expression demonstrated a proximal result. LV-CLU reduced the CLU
protein expression by 94.8%. (D) An MTT assay demonstrated the
effect of LV-CLU on cell susceptibility to epirubicin. Epirubicin
untreated groups have a higher cell viability and proliferation
compared to the epirubicin-treated groups. Moreover, in the
epirubicin-treated groups, LV-CLU-infected EJ cells had a lower
cell viability and proliferation than the mock EJ cells and
CLU-NC-infected EJ cells. Therefore, inhibition of the CLU
expression increased the cytotoxicity induced by epirubicin (50
ng/ml). CLU, clusterin; LV-CLU lentivirus targeting CLU; LV-NC,
lentivirus targeting negative control; MOI, multiplicity of
infection. |
Establishment of stable CLU-silenced EJ
cell line
After the PGCSIL-GFP-CLU vector was constructed, PCR
and sequencing analysis were performed. The results obtained proved
satisfactory (data not shown). Results of the titer analysis
revealed an expression of 3×108 transducing (T) U/ml of
LV-CLU versus 5×109TU/ml of LV-NC (13). The EJ cell line, which had the
highest level of CLU expression of the four bladder cancer cell
lines, was used to study the role of CLU in chemotherapeutic
resistance. Four days after infection, the stably infected EJ cells
were observed under a fluorescence microscope. The infection
efficiency achieved was >90% (Fig.
1B).
Inhibition of CLU expression using
lentivirus-mediated shRNA
To identify the specificity and potency of the
lentivirus-mediated shRNA inhibition of the CLU expression, the
effect of lentivirus on the CLU mRNA and protein levels was
determined using RT-PCR and western blot analysis, respectively. As
shown in Fig. 1C, five days after
lentivirus infection, lentivirus-CLU reduced the CLU mRNA level by
83.3%, while the CLU mRNA expression was almost unaffected by the
lentivirus-NC. Inhibition of the CLU protein expression in EJ cells
was also observed 5 days after infection (Fig. 1C).
Inhibition of CLU expression enhances the
efficiency of epirubicin on cell viability
To determine whether inhibition of the CLU
expression by LV-CLU enhances the cytotoxity induced by epirubicin,
a cell viability assay was performed after treatment with
epirubicin (50 ng/ml) using an MTT assay. The growth curve
demonstrated that the LV-CLU-infected EJ cells had a significantly
higher susceptibility to epirubicin. CLU knockdown combined with
epirubicin treatment had a maximum cytotoxic effect on EJ cells
(Fig. 1D).
Inhibition of CLU expression enhances the
efficiency of epirubicin on cell invasiveness and migration
Migration and invasion present two important aspects
that lead to the ability of cancer cells to metastasize. In this
study, a Matrigel invasion assay was used to determine whether CLU
knockdown would affect cell invasion. Cells from each experimental
group were seeded in transwell chambers and cultured for 24 h. Our
results demonstrated that LV-CLU-infected cells combined with
epirubicin treatment had minimum invasiveness (Fig. 2A). Statistical analysis revealed a
significant difference between the LV-NC and LV-CLU groups with
epirubicin treatment (P<0.01).
The role of CLU on cell migration was assessed by a
classic wound healing assay. LV-NC- and LV-CLU-infected cells were
treated with or without epirubicin (50 ng/ml), respectively, for 22
h. The relative cell migrating distance was calculated using Adobe
Photoshop CS3 software (Adobe). The data demonstrated a
statistically significant difference between the LV-CLU- and the
LV-NC-infected cells. The LV-CLU-infected cells had a lower
migration. Thus, CLU silencing enhances the toxicity of epirubicin
on cell migration (Fig. 2B).
CLU knockdown increases the toxicity of
epirubicin on clone formation
A plate clone formation assay was used to assess the
role of CLU on clone formation in bladder cancer cells. Mock EJ
cells, LV-NC- and LV-CLU-infected EJ cells were seeded in 6-well
plates, respectively, and cultured with or without epirubicin for
14 days. The number of colonies containing ≥50 cells were counted
under a microscope. Our results demonstrated that the CLU knockdown
EJ cells combined with epirubicin treatment had a clone formation
rate of 21±5%, which was lower than that of the other groups
(Fig. 2C). Therefore, the CLU
knockdown combined with epirubicin treatment effectively decreased
the clone formation of EJ bladder cancer cells.
CLU knockdown increases the potency of
epirubicin on cell cycle arrest
Following treatment with or without epirubicin (50
ng/ml) in each experimental group, a flow cytometer was used to
quantify changes in the cell cycle. In our study, CLU silenced
cells demonstrated G0/G1 phase arrest and G2/M and S-phase
reduction. A total of 98.3% of cells were blocked in the G0/G1
phase in the CLU knockdown EJ cells, while only 64.9% of cells were
blocked in the G0/G1 phase in LV-NC-infected cells after treatment
with epirubicin (Fig. 2D). The
difference was statistically significant.
Discussion
CLU, also known as SP-40, sulfated glycoprotein 2,
testosterone-repressed prostate message-2 and apolipoprotein J was
first isolated from ram rete testis fluid by Blaschuk et
al(14) in 1983. This single
copy gene locates on chromosome 8p12-p21 and encodes an mRNA of
approximately 2 kb which produces a glycoprotein of 449 amino acids
which is cleaved into an α and β subunit. These appear as a smear
of approximately 40 kDa on an SDS polyacrylamide gel (14). CLU is generally expressed in almost
all fluids and tissues in humans and is overexpressed in a number
of cancers, including breast, colon, bladder and melanoma (15–18).
Previous studies have found that the majority of bladder cancers
were accompanied with CLU overexpression, and the recurrence-free
survival rate of patients with a strong CLU expression was
significantly lower than that of patients with a weak expression
(16,19). Recent studies have considered CLU
as a key contributor to chemoresistance to anticancer agents
(5). CLU knockdown was found to
significantly chemosensitize cancer cells, including prostate, and
breast and lung cancer (9,20–23).
However, no study has examined whether CLU silencing is able to
chemosensitize bladder cancer cells to epirubicin, one of the most
common drugs, after TUR, used for intravesical instillation in
patients with non-muscle-invasive bladder cancer. In this study, we
aimed to determine whether CLU knockdown enhances the
chemosensitivitiy of bladder cancer cells to epirubicin. Our
results demonstrated that a combined treatment of CLU silencing and
epirubicin had a maximum cytotoxic effect on bladder cancer cells,
with regards to cell proliferation, migration, invasion, clone
formation or cell cycle arrest. This finding indicated that
silencing of CLU may be an alternative method to treating the
chemoresistance of bladder cancer cells to epirubicin.
In this study, lentivirus-mediated shRNA was used,
for the first time, to knock down CLU. Despite OGX-11, a second
generation of antisense oligonucletide targeting CLU currently
being widely used in numerous experiments (24,25),
it has certain disadvantages, including transient CLU silencing and
low transfection efficiency, particularly in non-dividing primary
cells. To overcome these limitations, we designed and constructed
lentivirus-mediated shRNA to obtain a stable CLU knockdown effect.
Lentivirus-mediated shRNA provides specific, long-lasting silencing
and maximal inhibition of gene expression at lower concentrations
in a variety of human cells, including primary non-dividing cells
and also in the transgenic mouse (26–28).
CLU has been proven to be safe for humans and has been used in
several clinical trials with no evident side effects (29,30).
In our study, we revealed that CLU is a powerful tool which
decreased mRNA expression by over 80% and protein expression by
over 90%. Thus, we recommend lentivirus-mediated shRNA as an
improved method to knock down CLU.
Our study indicates that lentivirus-mediated shRNA
targeting CLU effectively sustains the knockdown of CLU gene
expression in EJ bladder cancer cells. This study describes the
successful construction of a lentivirus RNAi vector targeting CLU
that may become a useful tool for the study of the function of the
CLU gene in bladder cancer cells. Our findings markedly suggest
that CLU is important in the chemoresistance of bladder cancer
cells to epirubicin. CLU knockdown combined with epirubicin
treatment may prove to be a more effective approach for controlling
recurrence and progression than epirubicin treatment alone in
intravesical instillation. It represents a novel approach to
regulating bladder cancer recurrence and progression.
Acknowledgments
This study was supported by Grants from the Natural
Science Foundation of Guangdong Province (8151008901000149;
06300794), the National Nature Science Foundation of China (Nos.
30900539 and 81172429), the Guangdong Provincial Science and
Technology Foundation (2008B030301112 and 2010B031600036), the
Major State Basic Research Program of China (2006CB910104), the
Fundamental Research Funds for the Central Universities (09ykpy35)
and the Jiangmen Science and Technology Project
(20120020078044).
References
|
1
|
Amling CL: Diagnosis and management of
superficial bladder cancer. Curr Probl Cancer. 25:219–278. 2001.
View Article : Google Scholar
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Richie JP: Intravesical chemotherapy.
Treatment selection, techniques, and results. Urol Clin North Am.
19:521–527. 1992.PubMed/NCBI
|
|
4
|
Kuroda M, Niijima T, Kotake T, Akaza H and
Hinotsu S: Effect of prophylactic treatment with intravesical
epirubicin on recurrence of superficial bladder cancer - The 6th
Trial of the Japanese Urological Cancer Research Group (JUCRG): a
randomized trial of intravesical epirubicin at dose of 20mg/40ml,
30mg/40ml, 40mg/40ml. Eur Urol. 45:600–605. 2004.
|
|
5
|
Djeu JY and Wei S: Clusterin and
chemoresistance. Adv Cancer Res. 105:77–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo JH, Xie D, Chen W, et al: Correlation
of clusterin expression to prognosis of bladder carcinoma. Ai
Zheng. 24:743–747. 2005.PubMed/NCBI
|
|
7
|
He LR, Liu MZ, Li BK, et al: Clusterin as
a predictor for chemoradiotherapy sensitivity and patient survival
in esophageal squamous cell carcinoma. Cancer Sci. 1000:2354–2360.
2009.PubMed/NCBI
|
|
8
|
Cheng CY, Cherng SH, Wu WJ, et al:
Regulation of chemosensitivity and migration by clusterin in
non-small cell lung cancer cells. Cancer Chemother Pharmacol.
69:145–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narita S, So A, Ettinger S, et al: GLI2
knockdown using an antisense oligonucleotide induces apoptosis and
chemosensitizes cells to paclitaxel in androgen-independent
prostate cancer. Clin Cancer Res. 14:5769–5777. 2008. View Article : Google Scholar
|
|
10
|
Li M, Gu FL, Li WB, Song YS, Zhou AR and
Guo YL: Introduction of wild-type p53 gene downregulates the
expression of H-ras gene and suppresses the growth of bladder
cancer cells. Urol Res. 23:311–314. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trougakos IP, So A, Jansen B, Gleave ME
and Gonos ES: Silencing expression of the clusterin/apolipoprotein
j gene in human cancer cells using small interfering RNA induces
spontaneous apoptosis, reduced growth ability, and cell
sensitization to genotoxic and oxidative stress. Cancer Res.
64:1834–1842. 2004. View Article : Google Scholar
|
|
12
|
Lin X, Yu Y, Zhao H, Zhang Y, Manela J and
Tonetti DA: Overexpression of PKCalpha is required to impart
estradiol inhibition and tamoxifen-resistance in a T47D human
breast cancer tumor model. Carcinogenesis. 27:1538–1546. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bettuzzi S, Scorcioni F, Astancolle S,
Davalli P, Scaltriti M and Corti A: Clusterin (SGP-2) transient
overexpression decreases proliferation rate of SV40-immortalized
human prostate epithelial cells by slowing down cell cycle
progression. Oncogene. 21:4328–4334. 2002. View Article : Google Scholar
|
|
14
|
Blaschuk O, Burdzy K and Fritz IB:
Purification and characterization of a cell-aggregating factor
(clusterin), the major glycoprotein in ram rete testis fluid. J
Biol Chem. 258:7714–7720. 1983.PubMed/NCBI
|
|
15
|
Redondo M, Villar E, Torres-Munoz J,
Tellez T, Morell M and Petito CK: Overexpression of clusterin in
human breast carcinoma. Am J Pathol. 157:393–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyake H, Gleave M, Kamidono S and Hara I:
Overexpression of clusterin in transitional cell carcinoma of the
bladder is related to disease progression and recurrence. Urology.
59:150–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie D, Sham JS, Zeng WF, et al: Oncogenic
role of clusterin overexpression in multistage colorectal
tumorigenesis and progression. World J Gastroenterol. 11:3285–3289.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shannan B, Seifert M, Leskov K, et al:
Clusterin (CLU) and melanoma growth: CLU is expressed in malignant
melanoma and 1,25-dihydroxyvitamin D3 modulates expression of CLU
in melanoma cell lines in vitro. Anticancer Res. 26:2707–2716.
2006.PubMed/NCBI
|
|
19
|
Kruger S, Mahnken A, Kausch I and Feller
AC: Value of clusterin immunoreactivity as a predictive factor in
muscle-invasive urothelial bladder carcinoma. Urology. 67:105–109.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyake H, Chi KN and Gleave ME: Antisense
TRPM-2 oligodeoxynucleotides chemosensitize human
androgen-independent PC-3 prostate cancer cells both in vitro and
in vivo. Clin Cancer Res. 6:1655–1663. 2000.PubMed/NCBI
|
|
21
|
July LV, Beraldi E, So A, et al:
Nucleotide-based therapies targeting clusterin chemosensitize human
lung adenocarcinoma cells both in vitro and in vivo. Mol Cancer
Ther. 3:223–232. 2004.PubMed/NCBI
|
|
22
|
So A, Sinnemann S, Huntsman D, Fazli L and
Gleave M: Knockdown of the cytoprotective chaperone, clusterin,
chemosensitizes human breast cancer cells both in vitro and in
vivo. Mol Cancer Ther. 4:1837–1849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yom CK, Woo HY, Min SY, Kang SY and Kim
HS: Clusterin overexpression and relapse-free survival in breast
cancer. Anticancer Res. 29:3909–3912. 2009.PubMed/NCBI
|
|
24
|
Lamoureux F, Thomas C, Yin MJ, et al:
Clusterin inhibition using OGX-011 synergistically enhances Hsp90
inhibitor activity by suppressing the heat shock response in
castrate-resistant prostate cancer. Cancer Res. 71:5838–5849. 2011.
View Article : Google Scholar
|
|
25
|
Baylot V, Andrieu C, Katsogiannou M, et
al: OGX-427 inhibits tumor progression and enhances gemcitabine
chemotherapy in pancreatic cancer. Cell Death Dis. 2:Oct
20–2011.(Epub ahead of print).
|
|
26
|
Nishitsuji H, Ikeda T, Miyoshi H, Ohashi
T, Kannagi M and Masuda T: Expression of small hairpin RNA by
lentivirus-based vector confers efficient and stable
gene-suppression of HIV-1 on human cells including primary
non-dividing cells. Microbes Infect. 6:76–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubinson DA, Dillon CP, Kwiatkowski AV, et
al: A lentivirus-based system to functionally silence genes in
primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Lin MC, Yao H, et al:
Lentivirus-mediated RNA interference targeting enhancer of zeste
homolog 2 inhibits hepatocellular carcinoma growth through
down-regulation of stathmin. Hepatology. 46:200–208. 2007.
View Article : Google Scholar
|
|
29
|
Manilla P, Rebello T, Afable C, et al:
Regulatory considerations for novel gene therapy products: a review
of the process leading to the first clinical lentiviral vector. Hum
Gene Ther. 16:17–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bank A, Dorazio R and Leboulch P: A phase
I/II clinical trial of beta-globin gene therapy for
beta-thalassemia. Ann NY Acad Sci. 1054:308–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
















