Introduction
Mesenchymal stem cells (MSCs), which originate from
the mesodermal germ layer, are a subset of non-hematopoietic stem
cells that exist in the bone marrow (1,2).
MSCs have been described as an adherent, fibroblast-like population
and have the ability to differentiate into multiple lineages,
including chondrocytes, osteocytes, adipocytes, myocytes and
astrocytes, and are a potential source of stem cells for cellular
and genetic therapy (3,4). MSCs also exist in other tissues,
including adipose tissue, umbilical cord, fetal liver, muscle and
lung (3,5–8).
MSCs are capable of expanding more than 104-fold in
culture without loss of their multilineage differentiation
potential.
The immunosuppressive function of MSCs has been
reported in several studies (9–12).
Koç et al reported that when allogeneic MSCs were infused
along with allogeneic bone marrow into patients with metachromatic
leukodystrophy or Hurler's syndrome, there was no evidence of
alloreactive T cells and no incidence of GvHD (13). MSCs have also been used to prevent
or treat autoimmune diseases, including experimental autoimmune
encephalomyelitis and collagen-induced arthritis (14,15).
MSCs have a tropism for tumors (16) and several studies have reported
contradictory results concerning the effect of MSCs on tumor
growth. Hall et al (17)
demonstrated that the co-culturing of ALL cell lines with
VCAM-1-overexpressing stromal cells enhanced the survival of the
leukemic cells in a PI-3 kinase-dependent manner, compared with
co-culturing with stromal cells expressing only endogenous VCAM-1.
Djouad et al (18) revealed
that MSCs exhibit side effects related to systemic
immunosuppression that favor induced tumor growth in vivo.
Conversely, MSCs have been reported to be anti-tumorigenic in a
mouse model of Kaposi's sarcoma by inhibiting AKT activity
(19). Tumorigenesis is always
associated with chronic inflammation. Therefore, it is essential to
observe the effects of MSCs on tumor growth in an inflammatory
environment.
In this study, we used the RM-1 prostate cancer cell
line to investigate the effect of MSCs on tumor growth in an
inflammatory environment. The incidence and development of prostate
cancer is often accompanied by an inflammatory microenvironment.
Therefore, it is important to determine the mechanism of the
inflammatory cytokine-induced immunosuppressive effect of MSCs in
prostate cancer cells.
Materials and methods
Reagents
Recombinant mouse IL-1α was from Peprotech, Inc. (La
Jolla, CA, USA). Anti-mouse CD34, CD45, CD90, CD105 and CD29
antibodies were from eBioscience (San Diego, CA, USA).
Cells and animals
MSCs were generated from bone marrow flushed out of
the tibias and femurs of 4–6-week-old mice. The cells were cultured
in DMEM medium supplemented with 10% fetal bovine serum (FBS), 2 mM
glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin
(Invitrogen, Carlsbad, CA, USA). Non-adherent cells were removed
after 72 h and adherent cells were maintained with medium
replenishment every 3 days.
The murine prostate cancer cell line RM-1 was
cultured at 37°C with 5% of CO2 in RPMI-1640 with 10%
FBS, supplemented with 2 mM L-glutamine, 100 U/ml penicillin and
100 μg/ml streptomycin. Cells were subcultured every 3 days when
they reached 70–80% confluence.
Male Balb/c and C57BL/6 mice, 6–8 weeks old, were
purchased from the Shanghai Experimental Animal Center of the
Chinese Academy of Sciences, Shanghai, China. Mice were housed in
pathogen-free conditions and all procedures were performed
according to the guidelines of the Committee on Animals of the
Chinese Academy of Sciences.
Multi-differentiation of MSCs
MSCs were cultured with an osteoinductive medium
consisting of DMEM supplemented with 10% FBS, β-mercaptoethanol,
100 μM L-ascorbic acid, 10 nM dexamethasone and 10 mM
β-glycerophosphate for 14 days. The cells were then stained with
alizarin red to reveal calcium deposition, characteristic of
osteoblasts. MSCs were induced to differentiate into adipocytes by
culturing with DMEM supplemented with 0.5 mM
isobutylmethylxanthine, 60 μM indomethacin, 10 nM dexamethasone and
10 μg/ml insulin for 14 days. The formation of adipocytes was
verified by staining for triglycerides with Oil red O to detect
intracellular lipid accumulation.
RM-1 murine prostate cancer model
RM-1 cells and MSCs were prepared either as
single-cell type suspensions (1×106 cells in 200 μl PBS)
or as a mixture of cells (1×106 RM-1 cells and
2×105 MSCs in 200 μl of PBS). RM-1 cells (alone or mixed
with MSCs) were subcutaneously administered in the armpit area of
Balb/c or C57BL/6 mice. The mice were examined every day and tumor
growth was evaluated by measuring the length and width of the tumor
mass. At the end of the experiment, the animals were sacrificed and
the tumors were removed. The tumor masses were weighed and analyzed
histologically.
Conditioned medium
MSCs were stimulated by culturing with IL-1α (20
ng/ml) for 12 h. The culture medium was then abandoned and replaced
with DMEM culture medium without FBS. After culturing for a further
24 h, the conditioned medium was collected, as well as a 0.22-μm
filtrate of the supernatant medium from the MSCs.
Allogeneic implantation of RM-1
cells
RM-1 cells and MSCs were prepared either as
single-cell type suspensions (1×106 cells in 200 μl of
PBS) or as a mixture of cells (1×106 RM-1 cells and
2×105 MSCs in 200 μl PBS). Subcutaneous administration
of RM-1 cells (alone or mixed with MSCs) was performed in the
armpit area of C57/BL6 mice. Tumor incidence was evaluated 3 times
per week.
Mixed lymphocyte reaction (MLR)
Mouse spleens were disaggregated into 10 ml
RPMI-1640 medium to isolate splenocytes. Erythrocytes were lysed
with 0.84% NH4Cl and subsequently washed 3 times with
RPMI-1640. Trypan blue dye exclusion was used to assess cell count
and viability. Splenocytes were incubated with 5 μg/ml concanavalin
A (ConA; Sigma-Aldrich, St. Louis, MO, USA) for 72 h and then
cultured with IL-2 (200 U/ml) for proliferation. Splenocyte
cultures were maintained in RPMI-1640 medium supplemented with 10%
FBS, 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin
and 50 mM of β-ME (complete medium). MSCs were added to the MLR to
provide a 200-μl final volume. Following 3 days of incubation, 1
μCi/well (0.037 MBq/well) 3H-thymidine was added
overnight and thymidine incorporation was measured using a
β-scintillation counter. The data were presented as the percentage
of the relative proliferative response, corresponding to the mean
counts per min (cpm) of a responder stimulator pair in the absence
of MSCs which was attributed a 100% value.
Real-time PCR
MSCs were incubated with IL-1α (20 ng/ml) for 12 h
and the total cell mRNA was collected with TRIzol reagent
(Invitrogen). cDNA was synthesized using M-MLV reverse
transcriptase (Promega Corporation, Madison, WI, USA) and 2 μg
total RNA and oligo dT18-primers. PCR amplification was carried out
using 2-μl aliquots of cDNA. Real-time PCR was performed in
triplicate using the SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.,
Shiga, Japan). The primer sequences for TGF-β were: forward,
5′-TGTCACCGGAGTTGTGCGGC-3′; reverse, 5′-CTCGGCGGCCGGTAGTGAAC-3′.
Total sample RNA was normalized to endogenous β-actin mRNA.
Thermocycler conditions included an initial hold at 50°C for 2 min
and then 95°C for 10 min, followed by a two-step PCR program of
95°C for 15 sec and 60°C for 60 sec repeated for 40 cycles using an
Mx 4000 system (Stratagene, La Jolla, CA, USA) on which data were
collected and quantitatively analyzed. Expression levels of mRNA
were presented as fold changes relative to an untreated
control.
Western blot analysis
Cells were washed with PBS solution, and protein was
then extracted according to an established protocol. Furthermore,
proteins were mixed with Laemmli sample buffer, heated at 65°C for
10 min, loaded (20 μg for each sample), separated by sodium dodecyl
sulfate-polyacrylamide gel (7.5%) electrophoresis under denaturing
conditions and electroblotted onto nitrocellulose membranes. The
nitrocellulose membranes were blocked by incubation in blocking
buffer (1% BSA in Tris-buffered saline-0.1% Tween 20), incubated
with anti-TGF-β-antibody (Abcam, Cambridge, UK), and washed and
incubated with anti-rabbit peroxidase-conjugated secondary antibody
(Invitrogen). Signals were visualized by chemiluminescent
detection. Blots were quantified using Quantity One software from
Bio-Rad (Hercules, CA, USA), and TGF-β expression was normalized to
values in the control group. Equal loading of samples was verified
by Coomassie blue staining of simultaneously run gels. Gels were
run 4 times and the images shown are representative.
Statistical analysis
Statistical analysis of the data was performed using
GraphPad Prism 4 software. The Student's t-test was used to compare
the mean values of the two groups. Data among 3 or more groups were
compared using the one-way analysis of variance, followed by the
Dunnett's post hoc test. Final values were expressed as the mean ±
SEM. P<0.05 was considered to indicate a statistically
significant result.
Results
Pretreatment of MSCs with inflammatory
cytokines promotes the growth of RM-1 prostate cancer cells in
vivo
We identified long spindle-shaped fibroblastic cells
isolated from bone marrow by examining their surface markers and
ability to differentiate. The results demonstrated that these cells
were positive for CD90, CD105 and CD29 and negative for CD34 and
CD45. Furthermore, these cells differentiated into adipocytes and
osteoblast-like cells. The results indicated that the cells
isolated from the bone marrow had properties that were consistent
with those of MSCs (Fig. 1A and
B).
MSCs, which were either pretreated with inflammatory
cytokines IL-1α or not, were co-injected with RM-1 cells into
Balb/c mice. We found that a more rapid growth of the RM-1 cells
that were mixed with non-pretreated MSCs in vivo than that
of the RM-1 cells alone. Compared with the control group, MSCs
pretreated with IL-1α demonstrated a tumor-promoting effect
(Fig. 2). Conditioned media were
collected from the MSCs and IL-1α-pretreated MSCs. The conditioned
media were infused into subcutaneous RM-1 tumor-bearing mice via
tail vein injection. Compared with the control groups, the
conditioned medium from IL-1α-pretreated MSCs significantly
enhanced the tumor growth in vivo (Fig. 2). These results indicate that IL-1α
stimulates MSCs to promote the growth of RM-1 tumors in
vivo.
 | Figure 2Inflammatory cytokine-pretreated MSCs
promoted the growth of RM-1 prostate cancer cells in vivo.
Balb/c mouse MSCs (2×105) were pretreated with IL-1α (20
ng/ml) for 12 h, mixed with RM-1 cells (1×106) and
subcutaneously administered in the armpit area of Balb/c mice. The
mice were sacrificed 14 days following implantation, the tumors
were dissected and their weights were measured. In addition, the
conditioned media CM1, from MSCs, and CM2, from IL-1α-pretreated
MSCs, were collected. The conditioned media were infused via the
tail vein once every 3 days into Balb/c mice that had been
implanted with RM-1 cells. The weights of the tumors were measured
following removal of the tumors from the mice
(*P<0.05). MSCs, mesenchymal stem cells; CM1,
conditioned medium 1; CM2, conditioned medium 2. |
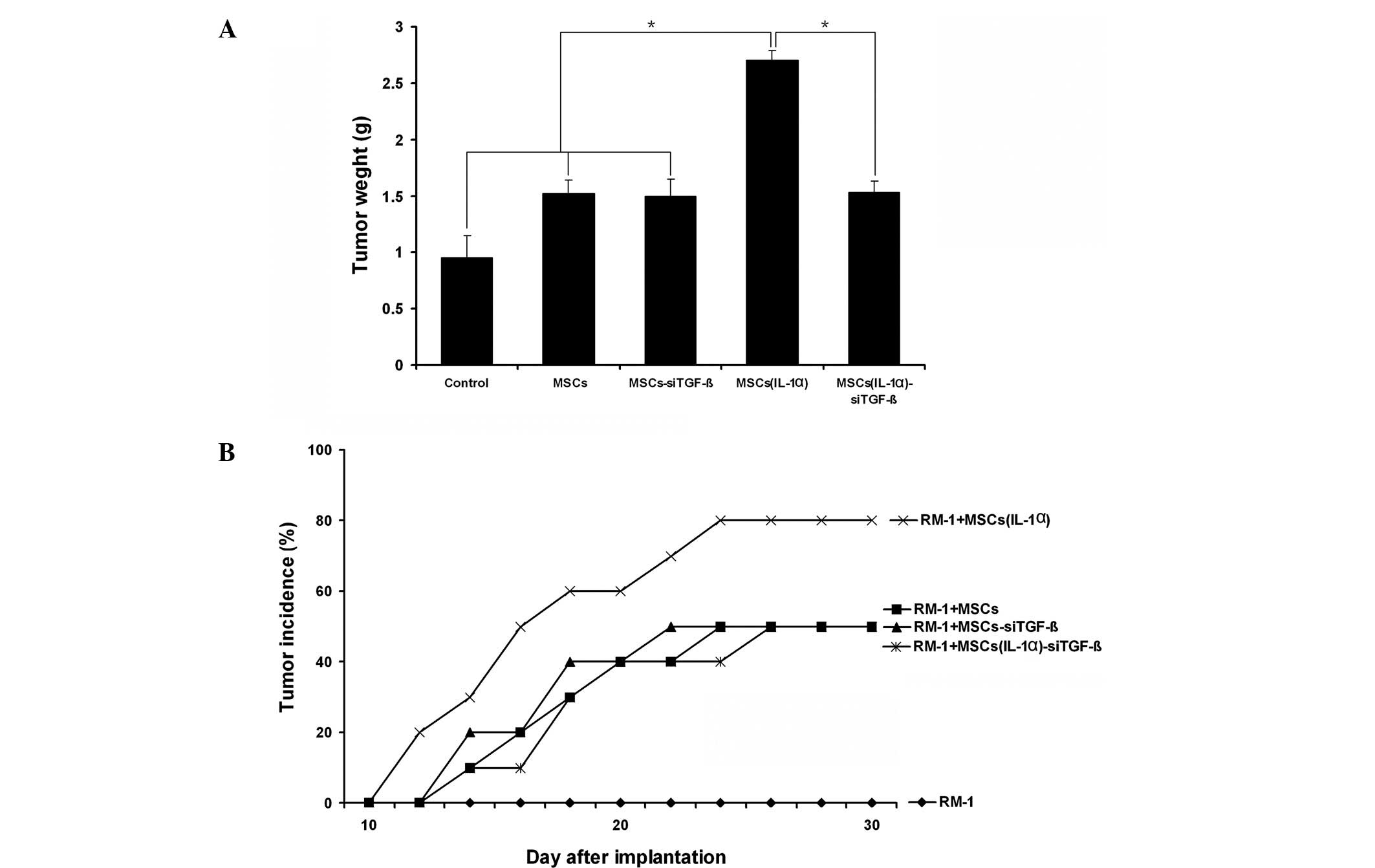
RM-1 prostate cancer cells are not
rejected by C57BL/6 mice when co-injected with MSCs
To determine the immunosuppressive effect of MSCs in
the promotion of tumor growth in vivo, we combined RM-1
cells with MSCs, which were either pre-incubated with IL-1α or not,
and implanted subcutaneously in C57/BL6 mice. The RM-1 cells
developed into tumors when implanted in Balb/c mice; however, these
cells were rejected by the C57/BL6 mice. When RM-1 cells were
co-injected with MSCs, the tumor incidence markedly increased.
Furthermore, compared with the control group, IL-1α-pretreated MSCs
further enhanced the tumor incidence (Fig. 3). In addition, we found that the
only conditioned medium able to increase the RM-1 tumor incidence
in the C57/BL6 mice was that obtained from IL-1α-pretreated MSCs
(Fig. 3). Taken together, the
results suggest that IL-1α induces the immunosuppressive action of
the MSCs, which may help the RM-1 cells to escape from immune
rejection by the C57/BL6 mice.
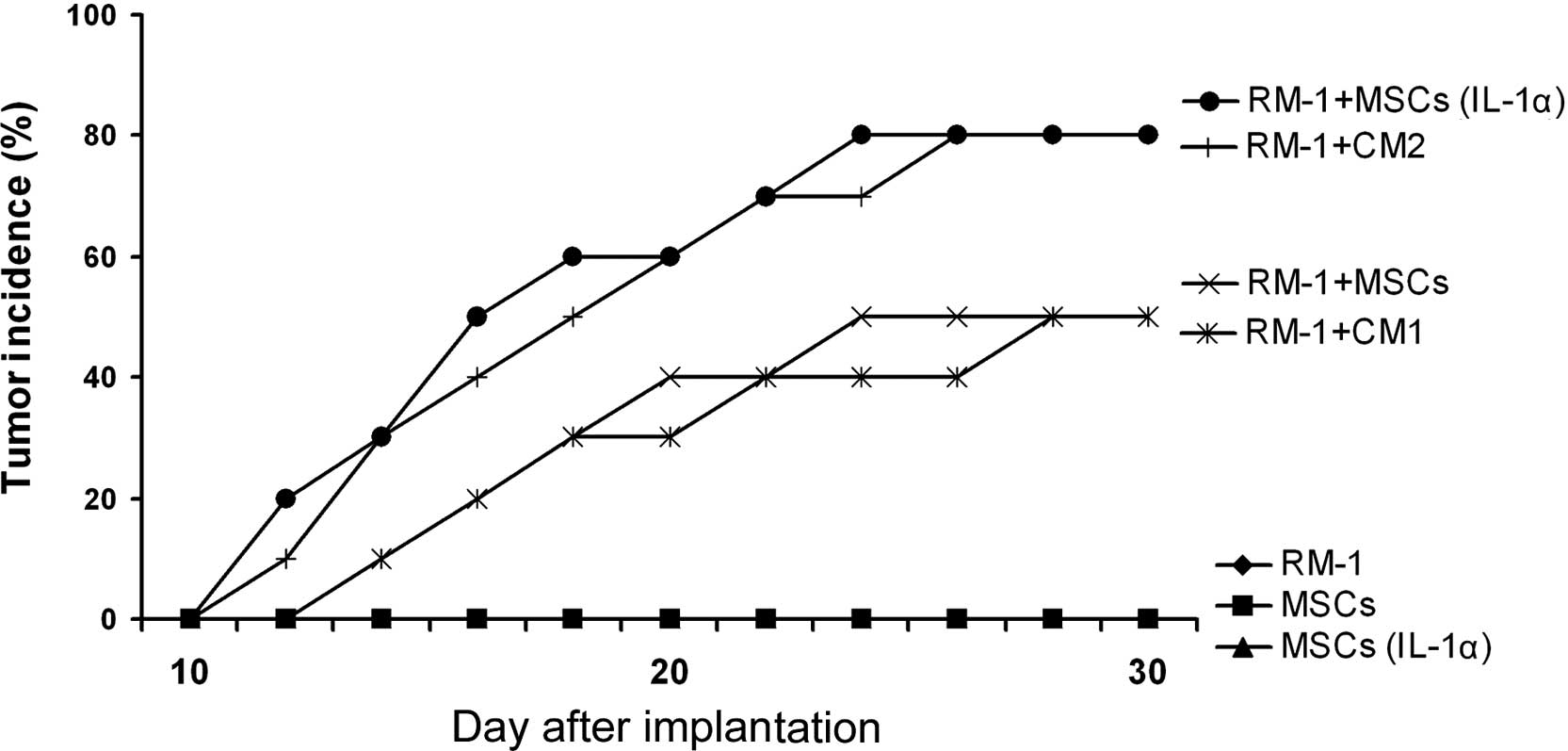 | Figure 3RM-1 prostate cancer cells are not
rejected by C57BL/6 mice when co-injected with MSCs. C57/BL6 mouse
MSCs (2×105) were pretreated with IL-1α (20 ng/ml) for
12 h, mixed with RM-1 cells (1×106) and then
subcutaneously administered in the armpit area of C57BL/6 mice. In
addition, the conditioned media CM1, from MSCs, and CM2, from
IL-1α-pretreated MSCs, were collected. The conditioned media were
infused via the tail vein once every 3 days into Balb/c mice that
had been implanted with RM-1 cells. Tumor incidence was observed to
evaluate the immunosuppressive function of the MSCs that assisted
the RM-1 cells to escape from immunological rejection by the Balb/c
mice. As negative controls, RM-1 cells or MSCs alone were implanted
in the C57/BL6 mice. MSCs, mesenchymal stem cells; CM1, conditioned
medium 1; CM2, conditioned medium 2. |
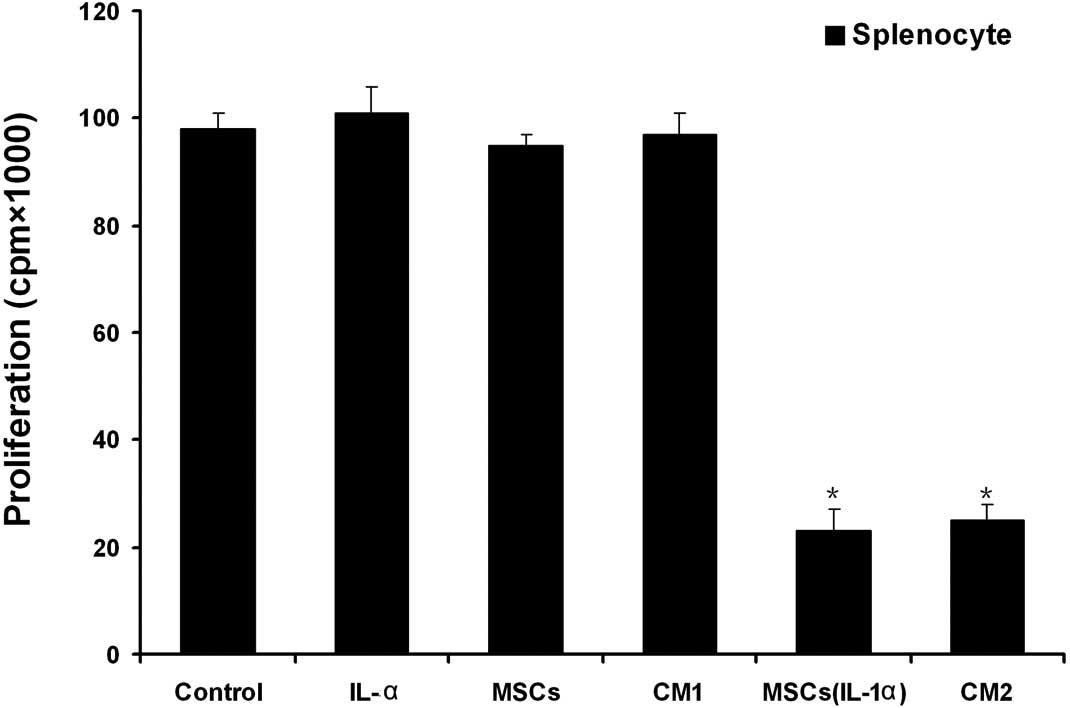
IL-1α induces the immunosuppressive
function of MSCs by upregulating TGF-β
We employed the MLR to examine the immunosuppressive
function of MSCs induced by IL-1α. We activated the splenocytes
from Balb/c mice with Con A for 72 h and then expanded them with
IL-2. The activated splenocytes were co-cultured with MSCs that
were either pretreated with IL-1α or not. The results demonstrated
that the MSCs did not inhibit the proliferation of splenocytes
unless they were pretreated with IL-1α (Fig. 4). In addition, conditioned media
collected from the MSCs were added to the activated splenocyte
culture system and the proliferation of the splenocytes was
examined. The conditioned medium obtained from IL-1α-pretreated
MSCs effectively inhibited the proliferation of the splenocytes
(Fig. 4).
To detect the mechanism by which IL-1α induced the
immunosuppressive function of the MSCs, we examined the production
of immunosuppression-related cytokines in the MSCs following
exposure to IL-1α. Real-time PCR and western blotting were employed
to detect the expression of immunosuppression-related cytokines in
the MSCs. As shown in Fig. 5A and
B, IL-1α effectively upregulated the expression of TGF-β in the
MSCs. To confirm the role of TGF-β in the immunosuppressive
function of the MSCs, TGF-β siRNA was used to inhibit the
expression of TGF-β in the MSCs. In mixed co-cultures of
splenocytes and MSCs pre-stimulated by IL-1α, splenocyte
proliferation was restored to normal levels by TGF-β siRNA
(Fig. 5C). These results suggest
that TGF-β is the key factor that mediates the IL-1α-induced
immunosuppressive effect of the MSCs on splenocyte
proliferation.
Enhancement of RM-1 cell growth in vivo
by MSCs was prevented by TGF-β siRNA
We have demonstrated that IL-1α effectively induces
the ability of MSCs to promote the growth of RM-1 cells in
vivo and that this enhancement may be associated with the
immunosuppressive function of MSCs. We have also shown that the
IL-1α-induced immunosuppressive action of the MSCs was mediated by
TGF-β. Therefore, we used TGF-β siRNA to confirm the role of TGF-β
in the immunosuppressive effect of the MSCs. The results showed
that the promotive effect of MSCs on tumor growth in vivo
induced by IL-1α was inhibited by TGF-β siRNA (Fig. 6A). Furthermore, the enhancement of
RM-1 tumor incidence in the C57/BL6 mice by IL-1α-pretreated MSCs
was reduced following the use of TGF-β siRNA (Fig. 6B). These data suggest that TGF-β is
a key factor in the immunosuppressive action of MSCs that enables
RM-1 cells to escape from immune injury.
Discussion
It has been reported that MSCs are able to
differentiate into osteoblasts, chondrocytes, adipocytes, myotubes,
neural cells and hematopoietic supporting stroma (3,4,20).
MSCs have been recognized to contribute to the regeneration of a
wide variety of organs and to the healing of certain diseases
(21–23). Furthermore, MSCs also are
influential in the treatment of various degenerative diseases and
immune disorders. Therefore, MSCs have been regarded as a potential
therapy for numerous diseases. However, the immunosuppressive
effects of MSCs have been reported in several studies (9–12),
and in certain circumstances, the immunosuppressive effect may
promote tumor growth. Therefore, it is essential to observe the
effect of MSCs on tumor growth in an inflammatory environment.
In this study, we investigated the underlying
mechanism by which MSCs enable prostate cancer cells to escape from
immune surveillance in the inflammatory microenvironment. Firstly,
we demonstrated that in comparison to the control groups, MSCs
pretreated with IL-1α effectively promoted the growth of the mouse
prostate cancer cell line RM-1 in vivo. Furthermore, when
RM-1 prostate cancer cells were co-injected with MSCs pretreated
with IL-1α, tumor incidence significantly increased in allogeneic
recipients. In addition, we investigated the mechanism by which
MSCs enable RM-1 cells to escape from immune injury. The results
revealed that treatment with IL-1α led to the upregulation of TGF-β
in MSCs. The inflammatory cytokine-induced promotive effect of MSCs
on RM-1 cells in vivo was inhibited by TGF-β siRNA. The
results of our study suggest that inflammatory cytokines induce the
immunosuppressive function of MSCs, which enables prostate cancer
cells to escape from immune injury.
Several studies have demonstrated that MSCs are able
to promote tumor growth. Hall et al have shown that the
co-culturing of ALL cell lines with VCAM-1-overexpressing stromal
cells enhanced the survival of the leukemic cells in a PI-3
kinase-dependent manner, compared with co-culturing with stromal
cells expressing only endogenous VCAM-1 (17). Djouad et al revealed that
MSCs exhibited side effects related to systemic immunosuppression
which induced tumor growth in vivo (18). Conversely, MSCs have been reported
to be anti-tumorigenic in a mouse model of Kaposi's sarcoma by
inhibiting AKT activity (19). Liu
et al have shown that IFN-γ and TNF-α are able to induce the
upregulation of VEGF in MSCs, which may be a significant mechanism
for the promotion of tumor growth (24). The results of our study suggest
that inflammatory cytokines, including IL-1α, are key factors that
regulate the actions of MSCs on tumor growth.
There are still a number of problems limiting the
application of MSCs in clinical therapy, particularly the
regulatory effect of the microenvironment. Therefore, it is
necessary to investigate the biological activity of the MSCs in
combination with the microenvironment, in order to improve the
clinical application of MSCs in tissue engineering and regenerative
medicine. Our results suggest that inflammatory cytokines,
including IL-1α, are key factors that induce the immunosuppressive
activity of MSCs and enable the tumor cells to evade immune
surveillance. Therefore, the use of MSCs in cancer therapy should
be carried out with caution.
Acknowledgements
This study was supported by the Science Foundation
for Young Scientists of Guangxi (grant no. 0991075), the Key
Research Project of Guangxi Health Department (grant no. 2011080),
the National Natural Science Foundation (grant nos. 2011080 and
30860329) and the Guangxi Natural Science Foundation (grant no.
2010gxnsfa013240).
References
|
1
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianco P and Gehron Robey P: Marrow
stromal stem cells. J Clin Invest. 105:1663–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anjos-Afonso F and Bonnet D:
Nonhematopoietic/endothelial SSEA-1+ cells define the most
primitive progenitors in the adult murine bone marrow mesenchymal
compartment. Blood. 109:1298–1306. 2007.PubMed/NCBI
|
|
7
|
In 't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetal or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004.
|
|
8
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka
K, Nagai T, Muroi K and Ozawa K: Nitric oxide plays a critical role
in suppression of T-cell proliferation by mesenchymal stem cells.
Blood. 109:228–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rasmusson I, Ringdén O, Sundberg B and Le
Blanc K: Mesenchymal stem cells inhibit lymphocyte proliferation by
mitogens and alloantigens by different mechanisms. Exp Cell Res.
305:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E and Dazzi F: Bone marrow mesenchymal stem cells
inhibit the response of naive and memory antigen-specific T cells
to their cognate peptide. Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002.
|
|
13
|
Koç ON, Day J, Nieder M, Gerson SL,
Lazarus HM and Krivit W: Allogeneic mesenchymal stem cell infusion
for treatment of metachromatic leukodystrophy (MLD) and Hurler
syndrome (MPS-IH). Bone Marrow Transplant. 30:215–222.
2002.PubMed/NCBI
|
|
14
|
Djouad F, Fritz V, Apparailly F,
Louis-Plence P, Bony C, Sany J, Jorgensen C and Noël D: Reversal of
the immunosuppressive properties of mesenchymal stem cells by tumor
necrosis factor alpha in collagen-induced arthritis. Arthritis
Rheum. 52:1595–1603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zappia E, Casazza S, Pedemonte E,
Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti
F, Frassoni F, et al: Mesenchymal stem cells ameliorate
experimental autoimmune encephalomyelitis inducing T-cell anergy.
Blood. 106:1755–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamizo A, Marini F, Amano T, Khan A,
Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et
al: Human bone marrow-derived mesenchymal stem cells in the
treatment of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
17
|
Hall BM, Fortney JE, Taylor L, Wood H,
Wang L, Adams S, Davis S and Gibson LF: Stromal cells expressing
elevated VCAM-1 enhance survival of B lineage tumor cells. Cancer
Lett. 207:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noël D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J,
Zhou H and Chen Y: Mesenchymal stem cells from adult human bone
marrow differentiate into a cardiomyocyte phenotype in vitro. Exp
Biol Med (Maywood). 229:623–631. 2004.PubMed/NCBI
|
|
21
|
D'Agostino B, Sullo N, Siniscalco D, De
Angelis A and Rossi F: Mesenchymal stem cell therapy for the
treatment of chronic obstructive pulmonary disease. Expert Opin
Biol Ther. 10:681–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazzini L, Ferrero I, Luparello V,
Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti
R, Miglioretti M, et al: Mesenchymal stem cell transplantation in
amyotrophic lateral sclerosis: a phase I clinical trial. Exp
Neurol. 223:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loebinger MR, Eddaoudi A, Davies D and
Janes SM: Mesenchymal stem cell delivery of TRAIL can eliminate
metastatic cancer. Cancer Res. 69:4134–4142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX,
Wang CY, Sun K, Jiang GC, Zhao X, Li R, et al: Effects of
inflammatory factors on mesenchymal stem cells and their role in
the promotion of tumor angiogenesis in colon cancer. J Biol Chem.
286:25007–25015. 2011. View Article : Google Scholar : PubMed/NCBI
|