Introduction
Fibronectin (FN), also named fibrinogenase, is an
extracellular macromolecular non-collagenous glycoprotein. FN is
mainly produced by hepatocytes, Kupffer cells and endotheliocytes,
and is present extensively in cell surfaces, extracellular fluid,
connective tissues and the majority of basilar membranes. FN has
multiple biological functions. It is capable of promoting blood
coagulation, enhancing the reticuloendothelial system and promoting
repair in trauma and embryonic formation and development; its
correlations with cell transformation and tumor genesis,
development, invasion and metastasis have obtained widespread
attention. In addition, FN has an impact on such biological
behaviors as cellular adhesion, migration, growth, differentiation
and diffusion (1). According to
the literature, FN in human hepatic blood sinusoids is capable of
promoting the uptake of hepatitis B viruses (HBV) by hepatocytes
(2). However, to the best of our
knowledge, no study on the effect of FN on in vitro-cultured
human fetal hepatocytes infected by HBV has been published to
date.
In this study, the effect of FN on human fetal
hepatocytes infected by HBV was investigated, and is expected to
provide a basis for further exploration into the mechanism of
hepatocellular HBV infection.
Materials and methods
Human fetal hepatocytes
A liver from a 22-week fetus aborted artificially
was obtained, and the mother was tested as negative for HBsAg.
Total liver cell suspension was isolated according to a two-step
perfusion method designed by Wang et al(3). Hepatic tissues were placed on a
culture dish, and perfused vascularly using 37°C perfusate
containing Ca2+ and Mg2+ until the majority
of the liver appeared gray. The tissues were repeatedly infused
with 0.05% collagenase at 37°C (containing 0.1% dispase), cut up,
agitated at 37°C, digested for 30 min and then filtered with 3-ply
aseptic gauze. Collagenase (0.05%; containing 0.1% dispase) was
repeatedly injected into the blood vessel at 37°C, liver tissues
were cut into sections, shocked, digested for 30 min, filtered with
3 layers of sterile gauze, centrifuged at 50 × g for 3 min three
times and liver cells were purified. The cells were cultured with
DMEM containing 10% FBS. The cell concentration was adjusted to
2×105/ml. The cells were then inoculated onto 6-well
culture dishes with and without FN coating, with 2 ml of culture
solution in each well (4×105 cells/well). HEPES, 100
U/ml penicillin and 100 μg/ml streptomycin were added and then
incubated in an atmosphere of 5% CO2 at 37°C. The
culture solution was changed 24 h later. Purified (100 μl) serum
from patients positive for HBV DNA was added into each well with a
quantity ratio between cells and viruses of 1:10. The mixture was
incubated at 37°C for 16 h (overnight) and then the supernatant was
discarded. Cells were washed six times with PBS (pH 7.4) (the
washing mixture from the sixth wash was retained for detection).
The cells were continuously cultured with DMEM medium containing
10% FBS. The day of washing was day 0 after infection, and HBsAg,
HBeAg and HBV DNA in supernatants were detected every 24 h
following infection. Two wells of cells with and without FN
coating, respectively, were digested and collected with digestive
fluid containing 0.05% trypsin and 0.03% EDTA every 24 h from day
1–10 after infection, in which one well was used for
immunohistochemistry following fixation with paraformaldehyde and
the other was stored at -70°C for the extraction of DNA. The cells
without infected serum were used as a control. Cell survival rates
were determined using the trypan blue staining method. This study
was conducted in accordance with the declaration of Helsinki and
with approval from the Ethics Committee of Southwest Hospital,
Third Military Medical University. Written informed consent was
obtained from all participants.
FN coating of culture dishes
Aseptic double-distilled water (5 ml) was added into
5 mg FN, then the solution was left to dissolve. Two hundred
microliters of the solution was extracted, followed by the addition
of 800 μl Hanks’ buffer salt solution, and then the sample was
mixed vigorously. Culture dishes were coated at 2
μg/cm2. FN was well spread on the culture dishes with a
sterile rubber brush, stored for 4 h at 37°C and then washed three
times with culture medium.
Serum infected by HBV and its
purification
Serum infected by HBV was obtained from patients
with chronic hepatitis B in the Department of Infectious Diseases,
Anning Branch of Lanzhou General Hospital, Lanzhou Military Area
Command. In these patients, HBsAg (+), HBeAg (+) and anti-HBc (+)
were detected by ELISA, 5×108 copies/ml of HBV DNA was
detected by fluorescent quantitative PCR, and negative results were
found for hepatitis A, C, D, E and G virus detection. Serum was
purified according to the method described by Mabit et
al(4). Specifically, 30%
sucrose was added into serum and then ultracentrifuged at
23×104 × g for 18 h. The supernatant was removed and the
precipitation was dissolved in 1X TNE solution. Fluorescent
quantitative PCR showed that there were ~5×107 copies/ml
of HBV DNA.
Morphological observation
The morphological change and adhesion of the cells
were observed under an inverted phase-contrast microscope every 24
h after culture.
ELISA
HBsAg and HBeAg in supernatants were detected by the
ELISA method according to the manufacturer’s instructions (Shanghai
Kehua Bio-Engineering, Co., Ltd., Shanghai, China).
Immunohistochemistry
HBcAg in nuclei was detected by immunohistochemistry
according to the manufacturer’s instructions (Fuzhou Maixin
Biotechnology Development, Co., Ltd., Fuzhou, China).
PCR
The extraction of HBV DNA in cells occurred by
digestion with proteinase K, followed by extraction by phenol,
chloroform and isoamyl alcohol and ethanol precipitation.
Fluorescent quantitative PCR was adopted for the detection of HBV
DNA. The forward primer was 5′-TGTGTCTGCGGC GTTTTATC-3′ (378–397,
20 bp) and the reverse primer was 5′-GTTTAAATGTATACCCAGAGAC-3′
(816–837, 22 bp). The length of PCR products was 460 bp. The
amplification conditions were as follows: 35 cycles of 95°C for 45
sec, 54°C for 40 sec and 72°C for 1 min, followed by a final
extension at 72°C for 5 min.
HBV cccDNA was detected by nested PCR (5), in which the outer primers crossed
over HBV genomic nicks. The outer forward primer of cccDNA was
5′-CCTCTGC CGATCCATCTGCGGAAC-3′ (1255–1279, 25 bp) and the outer
reverse primer was 5′-CTGCGAGGCGAGGGAGTTC TTCTTC-3′ (2376–2400, 25
bp). The length of PCR products was 1139 bp. The amplification
conditions were as follows: 35 cycles of 95°C for 40 sec and 72°C
for 3 min, followed by a final extension at 72°C for 5 min.
The inner positive primer of cccDNA was 5′-CTGAAT
CCCGCGGACGACCC-3′ (1441–1460, 21 bp) and the inner negative primer
was 5′-ACCCAAGGCACAGCTTGGAGG-3′ (1867–1889, 23 bp). The length of
PCR products was 449 bp. The amplification conditions were as
follows: 35 cycles of 95°C for 40 sec, 67°C for 40 sec and 72°C for
1 min, followed by a final extension at 72°C for 5 min.
As mung bean nuclenase (MBN) at a low concentration
was sensitive to single-stranded DNA, while insensitive to
double-stranded DNA, it was applied in the identification of
cccDNA. Cells without infected serum were used as a negative
control, while HepG2.2.15 cells were used as a positive
control.
Results
Dynamic morphological changes
Cells inoculated onto non-coated dishes grew well,
showing a larger cell volume, clear nuclei, active cell
proliferation, and were thin and flat irregular polygons in shape.
Most of the cells maintained their normal morphological structure
within 15 days of culture, with the exception of some that began to
lose their normal morphology from day 7 or 8. After culture (15
days), the majority of the cells died and were replaced by other
heterogeneous cells, which grew excessively.
Compared to the cells on the non-coated dishes 6 h
after inoculation, cells on the coated dishes displayed stronger
adhesion and adherence abilities, a more spreading shape, a larger
cell volume, more granules in the cytoplasm, earlier apoptosis and
a shorter time span to maintain their normal morphology. Twelve
days later, almost all the cells had lost their normal
morphology.
Replication and expression of HBV in
hepatocytes
Fluorescent quantitative PCR showed that the
solution kept from the sixth wash after the inoculation of positive
HBV DNA from serum was negative for HBV DNA. Detection of the
series of stored supernatants following HBV infection by ELISA
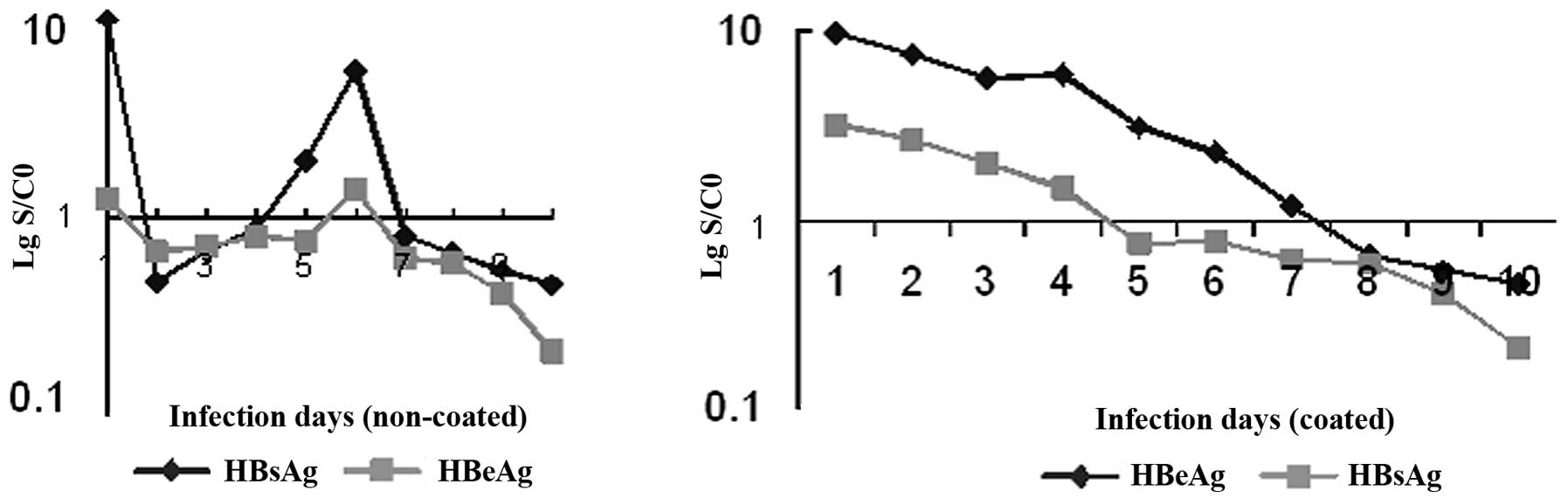
showed that HBsAg and HBeAg in the non-coated cells were positive
on day 1, were negative from day 2 with a subsequent increase in
the HBsAg signal-to-cutoff (S/CO) value, reappeared positive on day
5–6, and then became negative once again with a decrease of the
S/CO value; HBsAg in the coated cells was found to be positive on
day 1 and became negative until day 7; but HBeAg in the coated
cells displayed the same variation compared to the non-coated cells
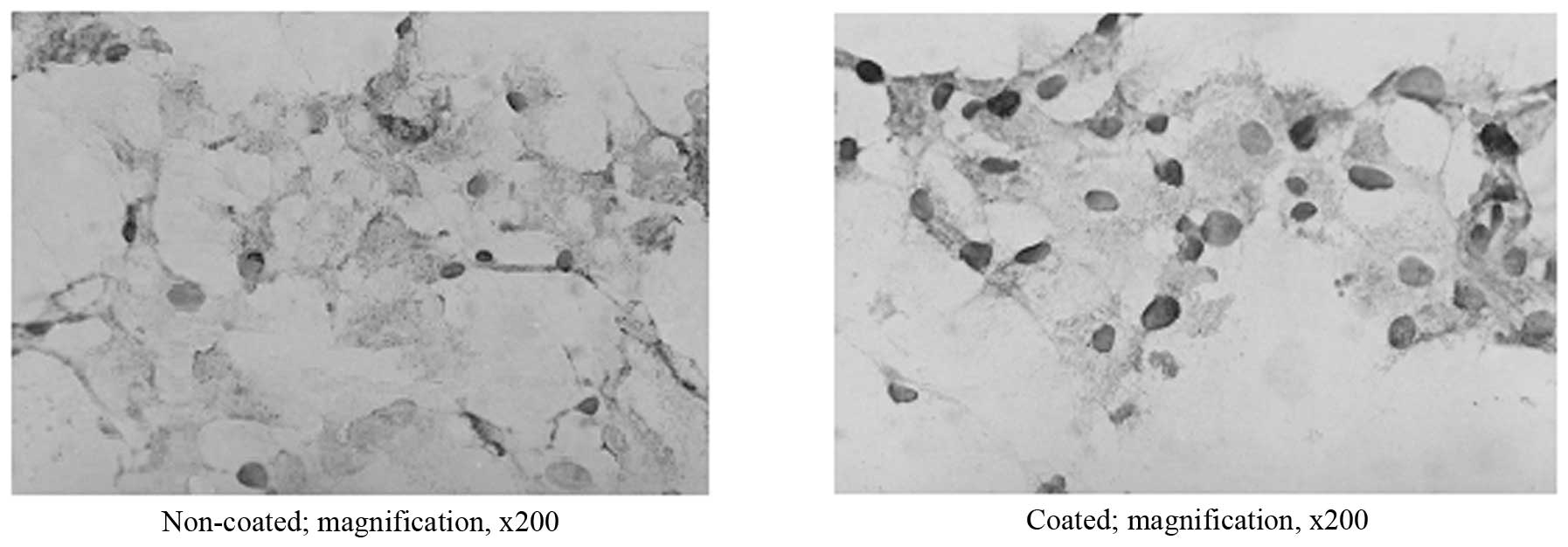
(Fig. 1). Immunohistochemical
detection showed that HBcAg in the nuclei of the non-coated cells
was positive from day 2 with a positive rate of ≥15%, and the
nuclei and part of the cytoplasm and intercellular substance were
stained; HBcAg in the nuclei of the coated cell was found to be
positive from day 1 with a positive rate of >90% and the
majority of the staining occurred in the nuclei. In addition, with
the prolongation of infection time, the number of infected cells
did not increase in any of the groups (Fig. 2). Fluorescent quantitative PCR
showed that HBV DNA in the non-coated cells was detected from day
5, and the copy number increased from then on and began to decrease
from day 9; HBV DNA in the coated cells was detected from day 1
with a higher titer compared to the non-coated cells, and the copy
number began to drop gradually one week later (Table I).
 | Table IHBV DNA in cells and supernatants by
fluorescent quantitative PCR. |
Table I
HBV DNA in cells and supernatants by
fluorescent quantitative PCR.
| Infection day | HBV DNA (non-coated
cells) | HBV DNA (coated
cells) |
|---|
| 1 |
4.231×104 |
4.231×105 |
| 2 | 0 |
5.612×104 |
| 3 | 0 |
7.568×105 |
| 4 |
3.459×103 |
3.523×105 |
| 5 |
2.312×104 |
4.875×105 |
| 6 |
7.117×104 |
7.431×104 |
| 7 |
1.983×104 |
1.566×105 |
| 8 |
6.233×103 |
5.421×104 |
| 9 |
1.573×103 |
1.573×103 |
| 10 | 0 |
2.135×103 |
Nested PCR showed that HBV cccDNA in the non-coated
cells appeared from day 8 and the results were negative from day
10; HBV cccDNA in the coated cells was positively detected from day
2 and turned negative from day 9. The detection of cccDNA was not
influenced by the action of MBN on the templates (Fig. 3).
Discussion
FN is distributed among all the extracellular spaces
in the liver, including the blood sinusoids and the basilar
membranes. Hepatocytes, hepatic endotheliocytes, Ito cells and
Kupffer cells are all capable of synthesizing FN. According to its
different distribution areas, FN may be divided into cellular and
plasmic FN. Cellular FN exists in the extracellular matrix in an
insoluble form, while plasmic FN exists in the blood plasma in a
soluble form (1). FN is a protein
that possesses multiple biological functions. According to the
literature, FN in the blood sinusoids in the human liver may
promote the uptake of HBV by hepatocytes (2). However, to date, the molecular
mechanism of HBV infection in human hepatocytes remains unknown.
Thus, in this study, the effect of FN on human fetal hepatocytes
infected by HBV was investigated, with the aim of further
elucidating the mechanism of HBV infection in hepatocytes.
FN may be expressed in tissue culture cells of
healthy individuals and rats, whereas it will decrease or even
disappear on the surface of cells in which there is some
transformation, or on the surface of induced or spontaneous tumor
cells. FN has the following effects on cultured cells: i) The
adhesive attraction effect. FN is capable of promoting the mutual
adhesion ability between cells and the adherence ability between
cells and supporting materials (6,7). The
mechanisms underlying its adhesive attraction remain unclear.
Presumably, one mechanism may be that specific gangliosides or
related sialoglycoconjugates are the receptors of FN on the surface
of cells, and another may be that FN is an extensible
biomacromolecule that binds with plastics, collagens, gelatins,
fibers and proteoglycans. ii) The effects on cell growth,
proliferation and differentiation. Bitterman et al
discovered that FN could promote the proliferation of human diploid
fibroblasts (8). A study on the
effect of FN in a rat liver cell line showed that normal
hepatocytes could not grow in medium without serum, but those after
transformation could grow, indicating that those transformants
could synthesize FN for their own growth requirements (9). Studies on FN receptors on the cell
surface also indicate that there is a correlation between FN and
cell growth. Spiegelman and Ginty (10) proved that FN inhibited the
differentiation of 3T3 adipose cells. The mechanism underlying the
promotion effect of FN on cell growth and proliferation may be
associated with the direct influence of FN on cells or with the
method by which FN activates target cells to produce more growth
factors. The mechanism of the inhibitory effect of FN on cell
differentiation may be correlated with the influence of FN on
cytoskeletons. iii) The effect on morphological changes of cells.
It has been proven that FN may lead to morphological changes of
fibroblasts. The added purified FN in the culture system could make
transformed fibroblasts appear more spread and flatter in
morphology (such as normal cells), and such an effect could also be
found when it was added to the alveolar epithelial cell line and
the breast cancer cell line (11).
iv) The migration and diffusion effects. The content of FN is
directly proportional to the migration and diffusion of cells. v)
The invasion and metastasis effects. FN may inhibit the invasion
and metastasis of tumor cells.
In this study, the results revealed that cells
coated with FN, compared to those not coated, displayed stronger
adhesion and adherence abilities, a more spreading appearance, a
larger cell volume and a faster cell proliferation rate, which is
consistent with reports in the related literature. In addition, our
results showed that the granules in the cytoplasm increased, the
time of cell death was advanced and the time span for normal
morphological maintenance was shortened. The possible reason may be
that FN is capable of speeding up cell proliferation at the early
stage of cell culture. When cells proliferate to a certain degree,
proliferation begins to slow down and finally stops due to the
contact inhibition characteristics of cells. After some time of
maintenance, cells begin to die and shed off.
This study also found that FN coating advanced HBV
infection and increased the number of infected cells. The
hepatocytes coated with FN were infected with serum with HBV after
24 h of culture and detected by ELISA. Results showed that HBsAg
was positive from day 1 and the titer began to decrease gradually
after 7 days, while positive HBsAg in the non-coated cells could
only be detected on day 5 or 6. Immunohistochemistry results showed
that HBcAg in the coated cells appeared positive from day 1 after
infection with a positive rate of >90%, while HBcAg in the
non-coated cells appeared positive from day 2 after infection, with
a positive rate of 15%. Results by fluorescent quantitative PCR
showed that HBV DNA in the coated cells was detected from day 1,
the copy number was maintained at a high level and the high level
began to gradually drop one week later, while HBV DNA in non-coated
cells was detected from day 5 with a relatively low copy number.
Results by nested PCR showed that cccDNA in the coated cells
appeared positive from day 2 and was negative from day 10, while in
the non-coated cells cccDNA appeared positive from day 8 and was
negative two days later. Our results showed that the infectivity of
the cells disappeared with the prolongation of infection time,
which may be a result of the cells dying in the late stage of
culture and a decrease in the number of infected cells. The
infection time of the coated cells was not prolonged compared to
that of the non-coated cells, which may be correlated with the
advanced time of cell death and the shortened cell survival time.
Among the different methods adopted in this study,
immunohistochemistry was the method able to detect HBV infection
the earliest, showing a high sensitivity. Mung bean nuclease was
used for the identification of cccDNA in this study, displaying
good specificity.
A possible mechanism underlying the promoting effect
of FN on HBV infection in hepatocytes may be that FN improves
cell-cell and cell-supporting material adhesion and adherence
abilities, and promotes cell growth and proliferation, which is of
great help for HBV infection. Another possible mechanism may be
that FN mediates HBV infection of hepatocytes directly. According
to Budkowska et al(2), FN
in human hepatic blood sinusoids could bind with HBV via antigenic
determinants coded by the HBV S2 area, and such binding could
accelerate the uptake of HBV by hepatocytes, while recombinant
HBsAg granules could not bind with FN in the liver due to lack of
antigenic determinants coded by the HBV S2 area. It is very
possible that FN has the same effect in vitro to accelerate
HBV infection in fetal hepatocytes. However, further studies are
still required for confirmation.
References
|
1
|
Kornblihtt AR, Pesce CG, Alonso CR, et al:
The fibronectin genes as a model for splicing and transcription
studies. FASEB J. 10:248–257. 1996.PubMed/NCBI
|
|
2
|
Budkowska A, Bedossa P, Groh F, Louise A
and Pillot J: Fibronectin of human liver sinusoids binds hepatitis
B virus: identification by an anti-idiotypic antibody bearing the
internal image of the pre-S2 domain. J Virol. 69:840–848. 1995.
|
|
3
|
Wang YM, Chen GZ, Dong JH, Yuan LP, Liu GD
and Ding J: An in vitro perfusion method for the isolation
of hepatocytes. Chin J Dig. 14:175–178. 1994.(In Chinese).
|
|
4
|
Mabit H, Dubanchet S, Capel F, Dauguet C
and Petit MA: In vitro infection of human hepatoma cells
(HepG2) with hepatitis B virus (HBV): spontaneous selection of a
stable HBV surface antigen-producing HepG2 cell line containing
intergrated HBV DNA sequences. J Gen Virol. 75:2681–2689. 1994.
View Article : Google Scholar
|
|
5
|
Lu X, Block TM and Gerlich WH:
Protease-induced infectivity of hepatitis B virus for a human
hepatoblastoma cell line. J Virol. 70:2277–2285. 1996.PubMed/NCBI
|
|
6
|
Hynes RO and Yamada KM: Fibronectins:
Multilfunctional modular glycoproteins. J Cell Biol. 95:369–377.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D’Ardenne AJ and Barnard NJ: Paucity of
fibronectin in invasive lobular carcinama of breast. J Pathol.
157:219–224. 1989.PubMed/NCBI
|
|
8
|
Bitterman PB, Rennard SI, Adelberg S and
Crystal RG: Role of fibronectin as a growth factor for fibroblasts.
J Cell Biol. 97:1925–1932. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Junker JL and Heine UI: Effect of adhesion
factors fibronectin, laminin, and type IV collagen on spreading and
growth of transformed and control rat liver epithelial cells.
Cancer Res. 47:3802–3807. 1987.PubMed/NCBI
|
|
10
|
Spiegelman BM and Ginty CA: Fibronectin
modulation of cell shape and lipogenie gene expression in
3T3-adipocytes. Cell. 35:657–666. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steele JG, Savolainen TA and Smith GJ:
Expression of fibronectin on clonally related transformed and
control sublines from an epithelial cell strain and a tumor line of
mouse alveolus. Cancer Res. 48:4933–4940. 1988.PubMed/NCBI
|