Introduction
Coronary heart disease (CHD) occurs with high
incidence and results in many deaths from ischemic events (1). CHD may be attributed to the
interaction of genetic and environmental factors (2). CHD pathogenesis proceeds when
vascular endothelial injury leads to blood lipid deposition in the
smooth endarterium, causing the formation of white atherosclerotic
plaques. These plaques continue to grow, forming a thrombus that
may either block blood flow directly or dislodge and travel through
the circulation. These actions may result in myocardial infarction
or stroke.
While a number of genetic and environmental factors
that promote CHD have been identified, relatively few factors are
known to reduce the risk of the disease. One, nitric oxide (NO), is
considered to be an endogenous anti-atherosclerotic plaque factor
(3). NO is produced in the body by
nitric oxide synthase (NOS) (4,5). NOS
activity, and therefore NO synthesis, may be reduced by an
endogenous competitive inhibitor, asymmetric dimethylarginine
(ADMA) (6). ADMA, however, becomes
inactivated via hydrolysis by dimethylarginine
dimethylaminohydrolases (DDAHs); DDAH has two subtypes, including
DDAH2 which is mainly expressed in vascular endothelial cells
(7). Changes in DDAH activity or
expression may affect the synthesis of NO by affecting ADMA levels
(8). DDAH activity and/or
expression may be affected by polymorphisms within the gene;
therefore, polymorphisms in DDAH may be associated with
CHD.
Two polymorphisms in DDAH have been
identified: rs805305 and rs2272592 (9,10).
In the present study, we determined the frequency of these
polymorphisms in CHD patients and healthy individuals to
investigate whether these polymorphisms may predispose an
individual to CHD.
Materials and methods
Participants
A total of 180 patients who were diagnosed in The
First Affiliated Hospital of Zhengzhou University from October 2008
to May 2011 were enrolled in the study. CHD was confirmed by
coronary angiography. The study population included 96 males and 84
females, aged 37–87 years (mean age 54.37±11.05 years). Another 180
healthy individuals who received a physical examination in our
hospital from January 2010 to June 2011 were selected as a control
group; these included 100 males and 80 females, aged 35–87 years
(mean age 52.36±14.25 years). No statistically significant
difference was observed in age or gender between the two groups.
History of hypertension, diabetes, smoking and alcohol use was
surveyed for patients in the two groups; the body mass index (BMI)
and blood glucose, triglyceride and cholesterol levels were also
measured for the two groups. The study was approved by the ethics
committee of the First Affiliated Hospital of Zhengzhou University.
All patients provided informed consent.
DDAH2 genotype identification at the
rs805305 locus
The polymerase chain reaction-restriction fragment
length polymorphism (PCR-RFLP) method was used to genotype
DDAH2 at the rs805305 locus. A 5-ml sample of venous blood
was taken from each patient and genomic DNA was isolated from each
sample by phenol-chloroform-isoamyl alcohol extraction. The target
fragment that potentially contained the polymorphism was amplified
by PCR using the following primers (Takara, Dalian, China):
forward, 5′-CCTTCTCGTTCGGGTATTCAG-3′; and reverse,
5′-TCCAGACCTTCCGCTCCT-3′. The reaction mix included 2 μl genomic
DNA, 2.5 μl 10X PCR buffer (Promega, Madison, WI, USA), 1.5 μl 25
mM dNTP (Promega), 0.25 μl 2.5 U/ml Taq DNA polymerase (Promega),
1.5 μl 25 mM MgCl2 (Promega), 0.5 μl forward primer (100
pmol/l), 0.5 μl reverse primer (100 pmol/l) and water (to a final
volume of 25 μl). The thermal cycling conditions were as follows:
pre-denaturation at 94°C for 5 min; 30 cycles of denaturation at
94°C for 60 sec, annealing at 58°C for 60 sec and extension at 72°C
for 60 sec; and elongation at 72°C for 5 min. The amplified PCR
product (2 μl) was digested with 2 μl SmaI (Promega) in 2 μl
10X buffer (with deionized water to a final volume of 20 μl) in a
30°C water bath for 4 h. The reaction product (5 μl) was separated
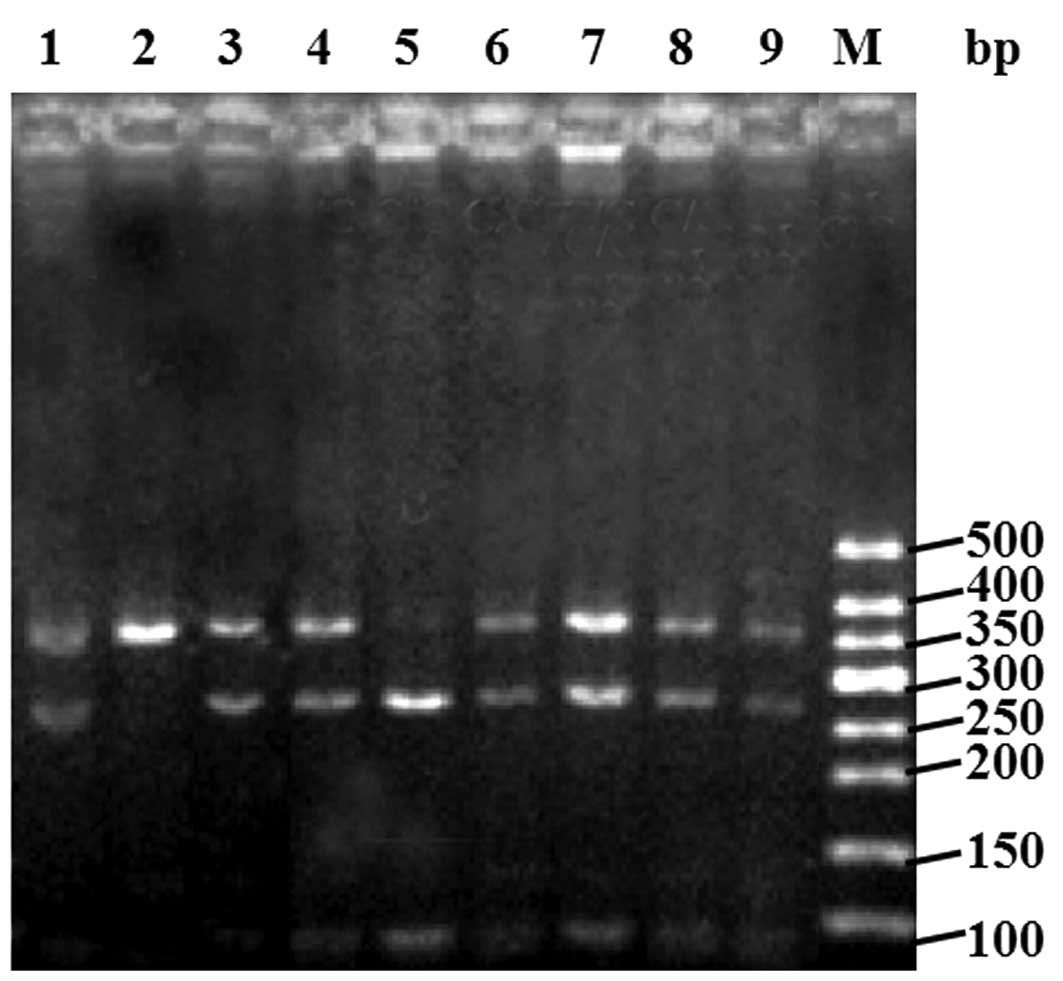
by agarose gel electrophoresis. The expected band sizes are as
follows (see Fig. 1): the GG
genotype (no SmaI restriction site) produces one band of 341
bp; the CC genotype product produces two bands, 254 and 87 bp; and
the CG genotype produces 3 bands, 341, 254 and 87 bp.
DDAH2 genotype identification at the
rs2272592 locus
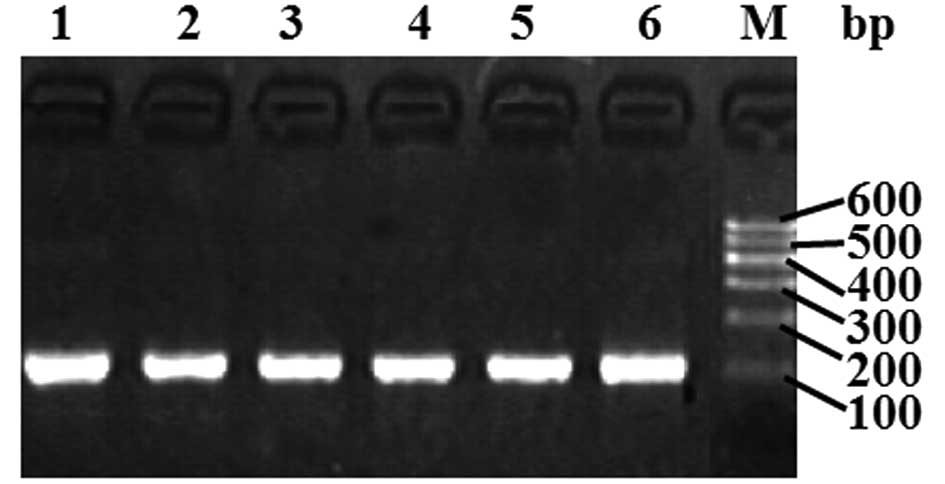
The ligase detection reaction (LDR) (11) was used to detect the target gene
sequence containing the SNP. The sequence of the forward primer was
5′-GGGTGGGATGAGTAGGACAA-3′, and that of the reverse primer was
5≥-ACTGACCCACCC CTCATTC-3≥. The reaction mix components were the
same as for the preceding reaction. The thermal cycling conditions
were as follows: 94°C for 5 min; 30 cycles of 94°C for 45 sec, 56°C
for 45 sec and 72°C for 45 sec; and 72°C for 5 min. The products
were verified by agarose gel electrophoresis (see Fig. 2). The products were then subjected
to LDR with the following probe sequences: rs2272592
reverse_modify, P-TTTAAAGTGTGGGCGACATGGAATGTTTTTTTTTTT
TTTTTTTTTTTTT-FAM; rs2272592 reverse_C, TTTTTT
TTTTTTTTTTTTTTTTTTATCAAGTGTTTTTACGCAT GGGGG, LDR length 98 bp; and
rs2272592 reverse_T, TTTTTTTTTTTTTTTTTTTTTTTTTTATCAAGTGTTTT
TACGCAATGGGGA, LDR length 100 bp. The reaction mix contained 1 μl
1X buffer, 1 μl 12.5 pmol/μl each probe mix, 1 μl 2 units ligase, 1
μl PCR product and water to a final volume of 10 μl. The thermal
cycling conditions were as follows: 95°C for 2 min; 35 cycles of
94°C for 30 sec; and 50°C for 2 min. The reaction products were
sequenced (ABI sequencer, Foster City, CA, USA), and Genemapper
software was used to analyze the genotype at rs2272592. The results
revealed three genotypes at this locus, GG, GA and AA.
Statistical analysis
SPSS 13.0 for Windows statistical software was used
to perform statistical analysis. A χ2 test was applied
to numerical data and a t-test was applied to measurement data.
P<0.05 was considered to indicate a statistically significant
result.
Results
Comparison of medical history and
biochemical indexes between the CHD patients and healthy
individuals
Analysis of the medical histories and biochemical
indices between the patients with CHD and the healthy individuals
revealed higher systolic blood pressure and blood triglyceride and
glucose levels in the CHD patients than in the control group; these
differences were statistically significant (P<0.05).
Additionally, the percentages of patients with CHD who had a
history of hypertension, diabetes, smoking or alcohol use were
56.11, 27.22, 47.78 and 52.78%, respectively. Each of these
percentages was significantly higher than the corresponding value
in the control group (P<0.05; Table
I).
 | Table IClinical history of patients with CHD
and healthy individuals. |
Table I
Clinical history of patients with CHD
and healthy individuals.
| Experimental group
(n=180) | Control group
(n=180) | P-value |
|---|
| BMI
(kg/m2) | 25.36±3.65 | 24.11±3.54 | <0.05 |
| SBP (mmHg) | 131.66±21.09 | 127.66±18.85 | >0.05 |
| DBP (mmHg) | 82.84±20.87 | 80.66±18.45 | >0.05 |
| Triglyceride
(mmol/l) | 1.37±0.12 | 1.20±0.13 | <0.05 |
| Cholesterol
(mmol/l) | 4.20±0.42 | 4.14±0.38 | >0.05 |
| LDL-C (mmol/l) | 2.46±0.22 | 2.45±0.25 | >0.05 |
| HDL-C (mmol/l) | 1.08±0.15 | 1.08±0.14 | >0.05 |
| Glucose (mmol/l) | 6.02±1.02 | 5.61±0.79 | <0.05 |
| History |
| Hypertension
(no/yes) | 79/101 | 108/72 | <0.05 |
| Diabetes
(no/yes) | 131/49 | 169/11 | <0.05 |
| Smoking
(no/yes) | 94/86 | 114/66 | <0.05 |
| Drinking
(no/yes) | 85/95 | 162/18 | <0.05 |
DDAH2 genotype frequency does not differ
at the rs805305 locus
The allele frequencies for the polymorphisms in
DDAH2 at rs805305 were in Hardy-Weinberg equilibrium for the
experimental and control groups (P>0.05). The genotype
frequencies of GG, CG and CC were, respectively, 17.78, 65.56 and
16.67% in patients with CHD and 23.33, 55.56 and 21.11% in the
control group; these distributions were not significantly
different. The frequencies of G and C alleles were 50.56 and 49.44%
in the experimental group and 51.11 and 48.89%, respectively, in
the control group; again, these distributions were not
significantly different (Table
II).
 | Table IIDimethylarginine
dimethylaminohydrolase (DDAH2) rs805305 genotype and allele
frequency distribution. |
Table II
Dimethylarginine
dimethylaminohydrolase (DDAH2) rs805305 genotype and allele
frequency distribution.
| Genotype | Allele |
|---|
|
|
|
|---|
| Group | GG | CG | CC | GG+CG | G | C |
|---|
| Experimental | 32 | 118 | 30 | 150 | 182 | 178 |
| Control | 42 | 100 | 38 | 142 | 184 | 176 |
| χ2 | | 3.78 | | 1.16 | 0.02 |
| P | | >0.05 | | >0.05 | >0.05 |
DDAH2 genotype does not differ at the
rs2272592 locus
The allele frequencies for the polymorphisms in
DDAH2 at rs2272592 were in Hardy-Weinberg equilibrium for
the experimental and control groups (P>0.05). The genotype
frequencies of GG, GA and AA were 63.89, 33.89 and 2.22%,
respectively, in the experimental group and 71.11, 27.22 and 1.67%,
respectively, in the control group; these differences were not
statistically significant. The frequencies of G and A alleles were
81.94 and 18.06%, respectively, in the experimental group and 84.72
and 15.28%, respectively, in the control group; again, these
distributions were not statistically different (Table III).
 | Table IIIDimethylarginine
dimethylaminohydrolase (DDAH2) rs2272592 genotype and allele
frequency distribution. |
Table III
Dimethylarginine
dimethylaminohydrolase (DDAH2) rs2272592 genotype and allele
frequency distribution.
| Genotype | Allele |
|---|
|
|
|
|---|
| Group | GG | GA | AA | GA+AA | G | A |
|---|
| Experimental | 115 | 61 | 4 | 65 | 295 | 65 |
| Control | 128 | 49 | 3 | 52 | 305 | 55 |
| χ2 | | 2.15 | | 2.09 | 1.01 |
| P | | >0.05 | | >0.05 | >0.05 |
Discussion
Studies have shown that NO, a fat-soluble molecule,
is able to rapidly diffuse into target cells, including vascular
smooth muscle cells and neurons; additionally, NO is considered to
be a messenger molecule with wider effects in the body (12). NO is produced by endothelial cells
and it diffuses into and relaxes vascular smooth muscle cells,
causing hemangiectasis and blood pressure decline (13). Furthermore, NO is able to inhibit
smooth muscle cell proliferation by preventing platelet aggregation
and reducing the formation and growth of atherosclerotic plaques.
Endogenous NO is mainly generated via L-arginine conversion and a
reaction catalyzed by NOS (14);
defects in the metabolism of NO are considered to contribute to the
pathogenesis of CHD.
Due to the ability of the endogenous molecule ADMA
to inhibit NOS activity, it is very important in the pathogenesis
of CHD (15). However, since ADMA
is hydrolyzed by DDAH to L-citrulline and dimethylamine in the
body, it may lose its activity. DDAH, therefore, may effectively
reduce the concentration of ADMA and increase the activity of NOS,
thereby increasing NO synthesis. DDAH has two subtypes, DDAH1,
which is mainly expressed in the nervous system, and DDAH2, which
is mainly expressed in the cardiovascular system (16). Gene knockouts have shown that a
lack of DDAH expression in DDAH null mice results in an increased
plasma ADMA concentration and reduced NO concentration, along with
significantly increased blood pressure (17). Therefore, individuals with reduced
DDAH2 activity may carry a CHD risk factor.
The results of this study demonstrated, as expected,
that BMI and blood triglyceride and glucose levels were all
significantly higher in patients with CHD than in healthy
individuals; similarly, histories of hypertension, diabetes,
smoking or alcohol use were all more common among CHD patients.
Indeed, hypertension, diabetes, smoking and alcohol use are known
risk factors for CHD (18).
However, although DDAH2 has generated interest as a
potential risk factor for CHD, our analysis of two polymorphic loci
in the DDAH2 gene did not uncover any differences in the
distribution of alleles between healthy individuals and patients
with CHD. Therefore, polymorphism at rs805305 or rs2272592 is not
associated with CHD. Notably, a study by Jones et
al(19) suggested that
polymorphisms in the DDAH2 promoter affected DDAH2
expression in vascular endothelial cells and that polymorphism at
the DDAH2 promoter 871 locus was correlated with changes in
ADMA levels in the body. However, Maas et al(10) reported no correlation between
polymorphisms at the rs805304 or rs805305 loci of the DDAH2
promoter and CHD, supporting our findings. The differences between
our findings and those reported by Jones et al may result
from two characteristics of our study: a smaller sample size and
the possibility that participants in our study may not accurately
represent the population distribution of the region.
In conclusion, the results from this study reveal
that hypertension, diabetes, smoking and alcohol consumption are
all risk factors for CHD, but polymorphisms in DDAH2 at
rs805305 and rs2272592 are not associated with CHD.
Acknowledgements
This study was supported by the Key Science and
Technology Project of Henan Province (No. 112102310180).
References
|
1
|
Gaziano TA, Bitton A, Anand S,
Abrahams-Gessel S and Murphy A: Growing epidemic of coronary heart
disease in low- and middle-income countries. Curr Probl Cardiol.
35:72–115. 2010.PubMed/NCBI
|
|
2
|
Tearney GJ, Regar E, Akasaka T, et al:
Consensus standards for acquisition, measurement, and reporting of
intravascular optical coherence tomography studies: a report from
the International Working Group for Intravascular Optical Coherence
Tomography Standardization and Validation. J Am Coll Cardiol.
59:1058–1072. 2012. View Article : Google Scholar
|
|
3
|
Kolovou G and Giannakopoulou V:
Endothelial nitric oxide synthase gene variants and coronary heart
disease. Angiology. 63:84–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Niroomand F, Liu Z, Zankl A, Katus
HA, Jahn L and Tiefenbacher CP: Expression of nitric oxide related
enzymes in coronary heart disease. Basic Res Cardiol. 101:346–353.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia CQ, Ning Y, Liu TT and Liu ZL:
Association between G894T mutation in endothelial nitric oxide
synthase gene and premature coronary heart disease. Zhonghua Liu
Xing Bing Xue Za Zhi. 26:51–53. 2005.(In Chinese).
|
|
6
|
Celik M, Iyisoy A, Celik T, Yilmaz MI,
Yuksel UC and Yaman H: The relationship between L-arginine/ADMA
ratio and coronary collateral development in patients with low
glomerular filtration rate. Cardiol J. 19:29–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palm F, Onozato ML, Luo Z and Wilcox CS:
Dimethylarginine dimethylaminohydrolase (DDAH): expression,
regulation, and function in the cardiovascular and renal systems.
Am J Physiol Heart Circ Physiol. 293:H3227–H3245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gad MZ, Hassanein SI, Abdel-Maksoud SM, et
al: Assessment of serum levels of asymmetric dimethylarginine,
symmetric dimethylarginine and L-arginine in coronary artery
disease. Biomarkers. 15:746–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abhary S, Burdon KP, Kuot A, Javadiyan S,
Whiting MJ, Kasmeridis N, Petrovsky N and Craig JE: Sequence
variation in DDAH1 and DDAH2 genes is strongly and additively
associated with serum ADMA concentrations in individuals with type
2 diabetes. PLoS One. 5:e94622010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maas R, Erdmann J, Lüneburg N, et al:
Polymorphisms in the promoter region of the dimethylarginine
dimethylaminohydrolase 2 gene are associated with prevalence of
hypertension. Pharmacol Res. 60:488–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YZ, Xiao JH, Liu LG, Ye CY, Shen HY,
Xu TM and Zhu KZ: Simultaneous detection of hepatitis B virus
genotypes and mutations associated with resistance to lamivudine,
adefovir, and telbivudine by the polymerase chain reaction-ligase
detection reaction. Braz J Infect Dis. 15:560–566. 2011. View Article : Google Scholar
|
|
12
|
Alasbahi RH and Melzig MF: Forskolin and
derivatives as tools for studying the role of cAMP. Pharmazie.
67:5–13. 2012.PubMed/NCBI
|
|
13
|
Houston M: The role of magnesium in
hypertension and cardiovascular disease. J Clin Hypertens
(Greenwich). 13:843–847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anthony S, Leiper J and Vallance P:
Endogenous production of nitric oxide synthase inhibitors. Vasc
Med. (Suppl 1)10:S3–S9. 2005. View Article : Google Scholar
|
|
15
|
Maas R: Pharmacotherapies and their
influence on asymmetric dimethylargine (ADMA). Vasc Med. (Suppl
1)10:S49–S57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tatematsu S, Wakino S, Kanda T, et al:
Role of nitric oxide-producing and -degrading pathways in coronary
endothelial dysfunction in chronic kidney disease. J Am Soc
Nephrol. 18:741–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leiper J, Nandi M, Torondel B, et al:
Disruption of methylarginine metabolism impairs vascular
homeostasis. Nat Med. 13:198–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zand Parsa AF, Ziai H and Haghighi L: The
impact of cardiovascular risk factors on the site and extent of
coronary artery disease. Cardiovasc J Afr. 23:197–199.
2012.PubMed/NCBI
|
|
19
|
Jones LC, Tran CT, Leiper JM, Hingorani AD
and Vallance P: Common genetic variation in a basal promoter
element alters DDAH2 expression in endothelial cells. Biochem
Biophys Res Commun. 310:836–843. 2003. View Article : Google Scholar : PubMed/NCBI
|