Introduction
Glioma is one of the most common malignant tumors
affecting the central nervous system. In the United States, 22,000
people per year are diagnosed with primary brain tumors (1). Despite a wide range of treatments,
including surgery, radiotherapy and chemotherapy, the majority of
therapies eventually fail (2). The
characteristics of the disease are a short survival period and a
high recurrence rate and mortality. Human glioma is defined by the
World Health Organization as the most common and malignant type of
central nervous system tumor. There is increasing evidence that a
variety of cancers, including human glioma, are driven by certain
tumor-initiating cells that retain stem cell-like properties. In
previous years, researchers have proposed that glioma stem-like
cells (GSLCs) not only have similar features to neural stem cells
in a number of aspects but also have a certain relationship with
embryology (3). Non-Chinese
researchers consider GSLCs to be the cause of the occurrence and
recurrence of glioma. GSLCs are also the main source of the
tolerance of glioma to radiotherapy and chemotherapy (4). Therefore, GSLCs have a guiding
significance for individual programs of glioma clinical treatment.
At present, there are three main methods for separating and
identifying GSLCs: serum-free suspension clone formation (5), a CD133 immunomagnetic bead sorting
method (6) and flow cytometry of
the side population (SP) (7).
Temozolomide (TMZ), a DNA-alkylating agent (8), has potent antitumor activity. TMZ is
a commonly used neuro-oncology drug. Drug resistance limits the
clinical therapeutic effect of this alkylating agent and is one of
the primary reasons for the failure of glioma chemotherapy.
Therefore, there is an urgent and important requirement for the
discovery of new chemotherapy drugs with high selectivity, low
toxicity and potent effects for use in the field of brain
science.
It has been reported that the growth of tumor cells
is inhibited by genistein (9),
quercetin (10), resveratrol
(11), curcumin (12), chrysin (13), apigenin (14), luteolin (15) and casticin (16). Studies have also shown that the
acetoacetate extract of Vitex negundo seed (EVn-50)
(17), neolignan (VB-1) (18) and 8-bromo-7-methoxychrysin (BrMChR)
(19) are able to inhibit tumor
cell growth and promote cell apoptosis. Vitexicarpin, an active
component of Vitex trifolia(20) has been reported to induce the
apoptosis of breast tumor cells. EFV-3 is an extract of Fructus
Viticis. In our study, U251 cells were incubated with
serum-free medium. Following the formation of neurosphere-like
cells, the active drugs, which are from 12 different classes, were
screened by MTT assay. We then observed the effects of these drugs
on the number of tumor balls, to provide experimental data for the
study of human glioma.
Materials and methods
Reagents
Dulbecco’s minimum essential medium (DMEM) and
serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium
were obtained from Hyclone (Logan, UT, USA). Fetal calf serum (FBS)
was purchased from Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd. (Hangzhou, China). Trypsin was purchased from
Beyotime Biotech (Shanghai, China). Dimethyl sulfoxide (DMSO) was
purchased from Genview (USA).
3-(4,5-Dimethylthiazo-2-yl)-2,5-diphenyltetrazolium (MTT),
genistein, quercetin, resveratrol, curcumin, chrysin, apigenin,
luteolin and casticin were purchased from Sigma (St. Louis, MO,
USA). EVn-50, VB-1, BrMChR and EFV-3 were gifts from Professor
Jianguo Cao (Hunan Normal University College of Medicine). Typan
blue, insulin, penicillin and streptomycin were purchased from
Beijing Dingguo Changsheng Biotech Co., Ltd. (Beijing, China). Cell
culture plates and ultra-low attachment plates were purchased from
Corning Inc. (Acton, MA, USA). Epidermal growth factor (EGF) and
basic fibroblast growth factor (bFGF) were purchased from Protein
Specialists (Ness-Ziona, Israel). Genistein, quercetin,
resveratrol, curcumin, chrysin, apigenin, luteolin, casticin,
EVn-50, VB-1, BrMChR and EFV-3 were dissolved in DMSO. All drug
solutions dissolved in DMSO were stored at −20°C.
Cell culture and treatment
The U251 cells were obtained from the College of
Life Science of Hunan Normal University and were maintained in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2. When the cells were near to 80% confluence, they
were passaged with 0.25% trypsin. The Ethics Committee of Hunan
Normal University approved the study.
Formation of GSLCs
The U251 cells, near 80% confluence and in good
condition, were digested with trypsin and then washed with PBS
three times. The U251 cells were seeded in 6-well ultra-low
attachment plates at 2×103 cells/ml in the serum-free
stem cell culture medium (DMEM/F12) in the presence of 20 ng/ml EGF
and bFGF, 4 μg/ml insulin, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2. Fresh stem cell medium was added every 3 days. Cell
growth and cloning ball formation was observed under an inverted
microscope after 10 days. The cell culture medium was collected and
centrifuged for 5 min at 800 rpm. The cells were digested with
trypsin and incubated for 10 days as described above.
Screening active drugs with MTT
assay
The U251 GSLCs were seeded in 96-well ultra-low
attachment plates at a density of 5×103 cells/well and
U251 cells were seeded in 96-well plates at the same density. After
24 h, the U251 GSLCs and U251 cells were treated with the 12 drugs.
Each drug was tested in three concentrations (10, 20 and 40 μM) and
each concentration was used in three parallel wells. After
treatment of the cells for 48 h, 10 μl MTT was added to each well
and the cells were cultured for 4 h at 37°C. DMSO (150 μl) was
added to each well. The absorbance at a wavelength of 490 nm
(A490) was measured using an enzyme-linked immunosorbent
instrument (ELx800, Bio-Tek, Winooski, VT, USA). The experiments
were divided into zero setting, control and experimental groups.
The relative cell proliferation inhibition rate (IR) = (1 − average
A490 of the experimental group/average A490
of the control group) ×100%. The experiment was repeated three
times.
Effect on the number of tumor balls
The U251 GSLCs were seeded in 6-well plates. When
the cells were near 80% confluence, the active drugs were applied
to treat the cells at a final concentration of 10 μmol/l. The cells
were digested with trypsin after 48-h treatment and then washed
with PBS three times. The cells were seeded in 96-well ultra-low
attachment plates at a density of 5× cells/well. The drug-treated
cells were seeded in 96-well ultra-low attachment plates at
densities of 5×102 and 1×103 cells/well. Stem
cell medium was added every 2 days. The numbers of tumor balls were
counted after 7 days.
Results
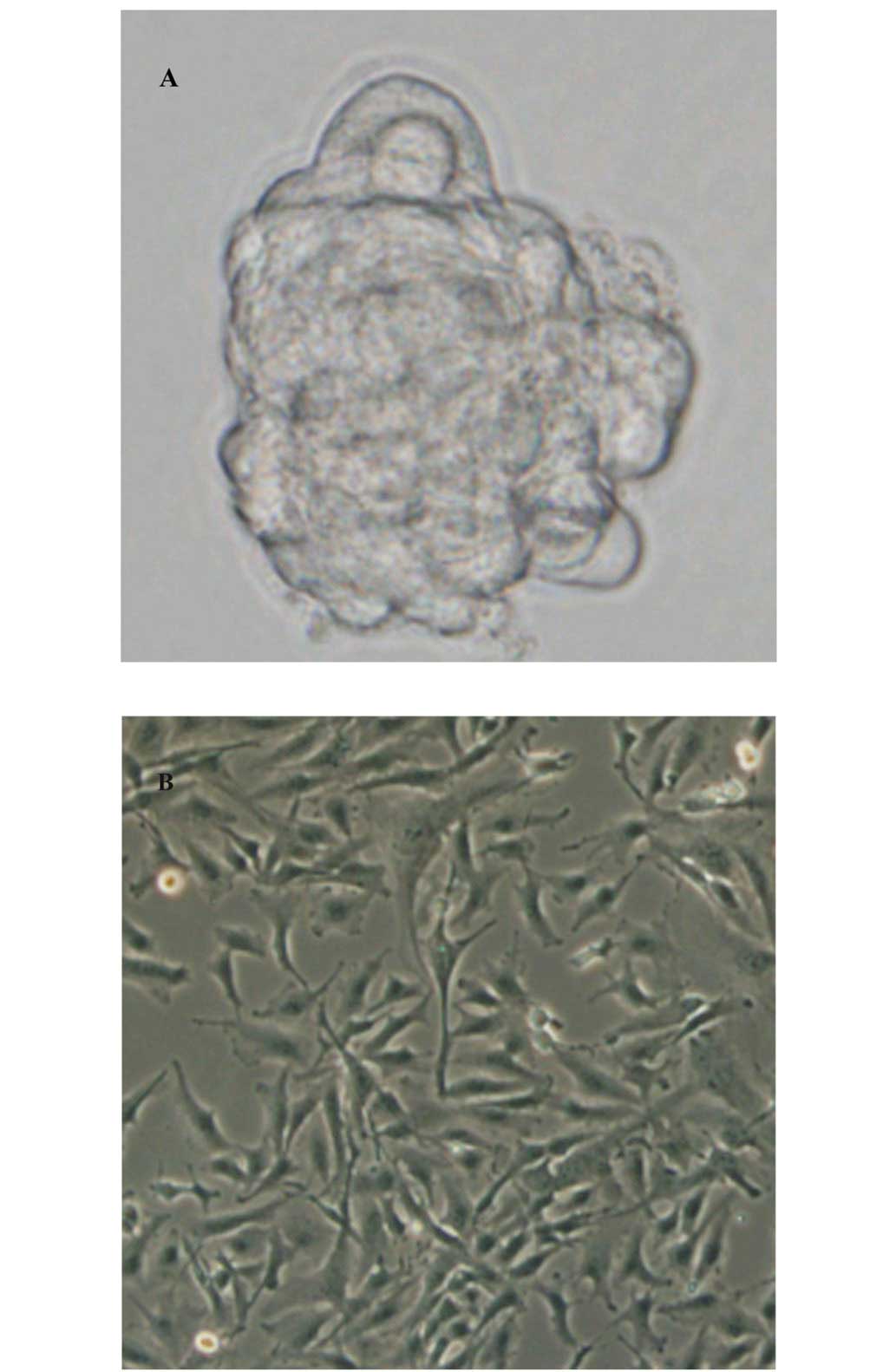
Formation of GSLCs
The U251 cells were adherent to the plastic surface
of the cell culture plates (Fig.
1B), while the human brain glioma cell lines grew in a globular
form in the serum-free stem cell culture medium and successfully
formed U251 GSLCs (Fig. 1A). The
U251 GSLCs gathered into spherical cell masses of various sizes,
suspended in the stem cell medium. The clone balls comprised dozens
to hundreds of cells, which had a strong refraction. However, there
remained a small number of cells grown in an adherent state. With
the prolongation of the time of incubation, we observed the tumor
balls gradually becoming larger, with a very clear cell shape and
outline, smooth edges, no distinct processes and regular shape. The
cells were passaged once every 10 days and the growth process was
repeated. The U251 GSLCs had the capacity to proliferate and grow
and were able to be passaged continuously.
MTT assay screening of active drugs
The inhibitory rates of drugs from 12 different
classes on tumor stem cell-like cells and tumor cells were compared
by MTT assay. The results revealed that 6 of the drugs had higher
inhibitory rates on the U251 GSLCs than on the U251 cells; these
drugs were curcumin, chrysin, apigenin, luteolin, casticin and
BrMChR (Fig. 2).
 | Figure 2MTT assay screening of active drugs.
1, genistein; 2, quercetin; 3, resveratrol; 4, curcumin; 5,
chrysin; 6, apigenin; 7, luteolin; 8, casticin; 9, EVn-50; 10,
VB-1; 11, BrMChR; 12, EFV-3. MTT assay showed that the inhibition
rate of active drugs from 6 classes to U251 GSLCs is higher than
U251 cells, including curcumin, chrysin, apigenin, luteolin,
casticin and BrMChR. GSLCs, glioma stem-like cells; MTT,
3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium; EVn-50,
acetoacetate extract of Vitex negundo seed; VB-1, neolignan;
BrMChR, 8-bromo-7-methoxychrysin; EFV-3, extract of Fructus
Viticis. |
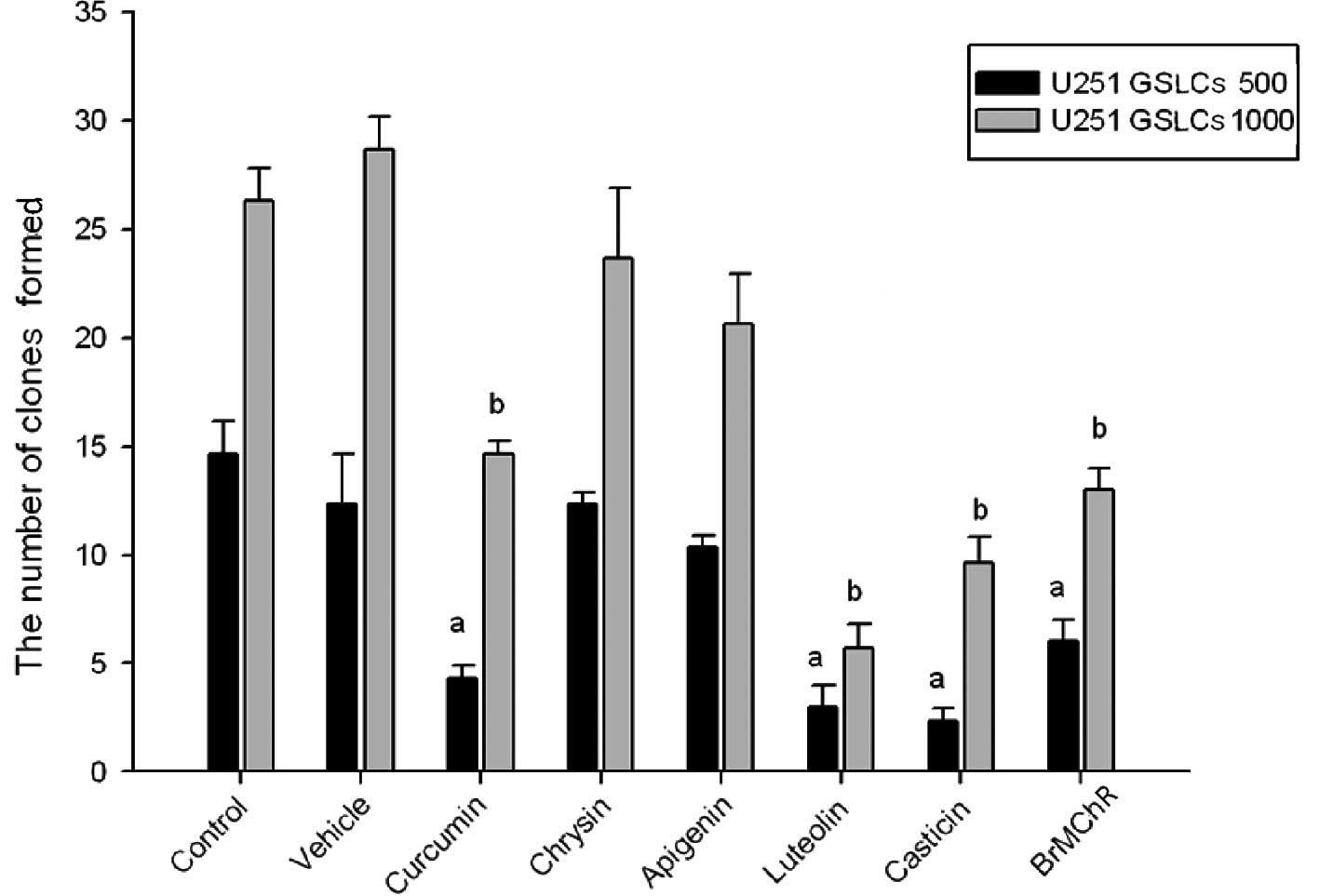
Effect on the number of tumor balls
As shown in Fig. 3,
U251 GSCLs treated with 6 active drugs were seeded in 96-well
ultra-low attachment plates. The number of tumor balls in the
control samples gradually increased over 7 days. The number of
tumor balls was decreased by treatment with 4 of the drugs after 7
days, specifically curcumin, luteolin, casticin and BrMChR; all
P<0.05 compared with the control group.
Discussion
Glioma is a common type of adult malignancy with
high morbidity and mortality. Although clinical treatments with
surgery, chemotherapy, radiotherapy and biological therapy, are
presently used, the tumors recur without exception (21–24).
The disease progresses rapidly following recurrence and seriously
threatens the life and health of the patients (25,26).
Within the past decades, accumulating evidence from a number of
biological systems, including the blood (27), breast (28) and brain (6,29),
has indicated that the transformation of cancer stem-like cells may
induce the formation of tumors. One novel treatment strategy under
investigation is to make cancer stem-like cells differentiate into
non-dividing cells. If successful, patients with brain tumors would
be able to lead a normal life. The tumor would stop growing due to
the terminal differentiation of the cells (30). A number of sorting technologies for
stem cell-like cells, including immunomagnetic beads and SP sorting
methods, are becoming widely used in the study of cancer stem
cell-like cells. However, these sorting methods have a number of
limitations.
In our study, serum-free suspension clone formation
successfully induced the formation of U251 GSLCs. Purified U251
GSLCs could be suspended in serum-free medium. Active drugs of 6
classes (curcumin, chrysin, apigenin, luteolin, casticin and
BrMChR) were identified by the screening of drugs from 12 classes
by MTT assay. Four of the drugs were able to affect the number of
tumor balls; these were curcumin, luteolin, casticin and BrMChR
(all P<0.05).
We have preliminarily identified methods for
culturing stem cell-like cells and used the cells in the screening
of active drugs. The immunofluorescence identification of relevant
molecular markers of GSLCs and animal experiments in vivo
are subjects of our future studies. Only by performing these
studies are we likely to find the most active drug, to contribute
further to the investigation of human glioma.
Acknowledgements
This project was supported by the Provincial Science
and Technology Plan of Hunan, China (No. 2010FJ3017), the Research
Foundation of Education Bureau of Hunan Province, China (No.
11B081), the Chinese Traditional Medicine Administration of Hunan
Province, China (No. 2009102)and the Excellent Talent Program of
Hunan Normal University (2011).
References
|
1
|
2010, CBTRUS Statistical Report. Primary
brain and central nervous system tumors diagnosed in the United
States in 2004–2006. Central Brain Tumor Registry of the United
States. http://www.cbtrus.org/reports/reports.html.
Accessed March 2010
|
|
2
|
Gong X, Schwartz PH, Linskey ME and Bota
DA: Neural stem/progenitors and glioma stem-like cells have
differential sensitivity to chemotherapy. Neurology. 76:1126–1134.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bearzatto A, Szadkowski M, MaePherson P,
et al: Epgenetieregulation of the MGMT and hMSH6 DNA repair genes
in cells resistant to methylating Agents. Cancer Res. 60:3262–3270.
2000.PubMed/NCBI
|
|
4
|
Lavon I, Fuchs D, Zrihan D, et al: Novel
mechanism whereby nuclear factor kappa B mediates DNA damage repair
through regulation of O(6)-methylguanine-DNA-methyltrans-ferase.
Cancer Res. 67:8952–8959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inagaki A, Soeda A, Oka N, et al:
Long-term maintenance of brain tumor stem cell properties under at
non-adherent and adherent culture conditions. Biochem Biophys Res
Commun. 361:586–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris MA, Yang H, Low BE, et al: Cancer
stem cells are enriched in the side population cells in a mouse
model of glioma. Cancer Res. 68:10051–10059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostermann S, Csajka C, Buclin T, et al:
Plasma and cerebrospinal fluid population pharmacokinetics of
temozolomide in malignant glioma patients. Clin Cancer Res.
10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi W, Weber CR, Wasland K and Savkovic SD:
Genistein inhibits proliferation of colon cancer cells by
attenuating a negative effect of epidermal growth factor on tumor
suppressor FOXO3 activity. BMC Cancer. 11:2192011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong JH, An JY, Kwon YT, Rhee JG and Lee
YJ: Effects of low dose quercetin: cancer cell-specific inhibition
of cell cycle progression. J Cell Biochem. 106:73–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukui M, Yamabe N, Kang KS and Zhu BT:
Growth-stimulatory effect of resveratrol in human cancer cells. Mol
Carcinog. 49:750–759. 2010.PubMed/NCBI
|
|
12
|
Ravindran J, Prasad S and Aggarwal BB:
Curcumin and cancer cells: how many ways can curry kill tumor cells
selectively? AAPS J. 11:495–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: the role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mak P, Leung YK, Tang WY, Harwood C and Ho
SM: Apigenin suppresses cancer cell growth through ERbeta.
Neoplasia. 8:896–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Cao J, Tian L, Liu F and Sheng X:
Induction of apoptosis by casticin in cervical cancer cells through
reactive oxygen species-mediated mitochondrial signaling pathways.
Oncol Rep. 26:1287–1294. 2011.PubMed/NCBI
|
|
17
|
Xiao JW, Zhuang YZ, Cao JG, et al: Effect
of Semen Viticis negundo extract EVn-50 on cell proliferation and
apoptosis of human breast cancer cell line MCF-7. Chin J Cancer
Prev Treat. 16:175–178. 2009.
|
|
18
|
Zeng FX, Zhou YJ, Tang AQ, et al:
Induction of apoptosis in ovarian cancer CoC1 cells by neolignan
VB-1. J Hunan Normal Univ (Med Sci). 8:40–42. 2011.
|
|
19
|
Zheng X, Meng WD, Xu YY, et al: Synthesis
and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett.
13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song YC, Zhang X, Lei GY and Dang CX:
Vitexicarpin affects proliferation and apoptosis in mutated p53
breast cancer cell. Zhonghua Yi Xue Za Zhi. 90:703–707. 2010.(In
Chinese).
|
|
21
|
Chang SM, Theodosopoulos P, Lamborn K, et
al: Temozolomide in the treatment of recurrent malignant glioma. J
Cancer. 100:605–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tatter SB: Recurrent malignant glioma in
adults. Curr Treat Options Oncol. 3:509–524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidinger M, Linzmayer L, Becherer A, et
al: Psychometric and quality-of-life assessment in long-term
glioblastoma survivors. J Neurooncol. 63:55–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corn BW, Wang M, Fox S, et al: Health
related quality of life and cognitive status in patients with
glioblastoma multiforme receiving escalating doses of conformal
three dimensional radiation on RTOG 98-03. J Neurooncol.
95:247–257. 2009. View Article : Google Scholar
|
|
27
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taylor MD, Poppleton H, Fuller C, et al:
Radial glia cells are candidate stem cells of ependymoma. Cancer
Cell. 8:323–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piccirillo SG, Reynolds BA, Zanetti N, et
al: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|